Neuroendocrine Differentiation of Lung Cancer Cells Impairs the Activation of Antitumor Cytotoxic Responses in Mice
Abstract
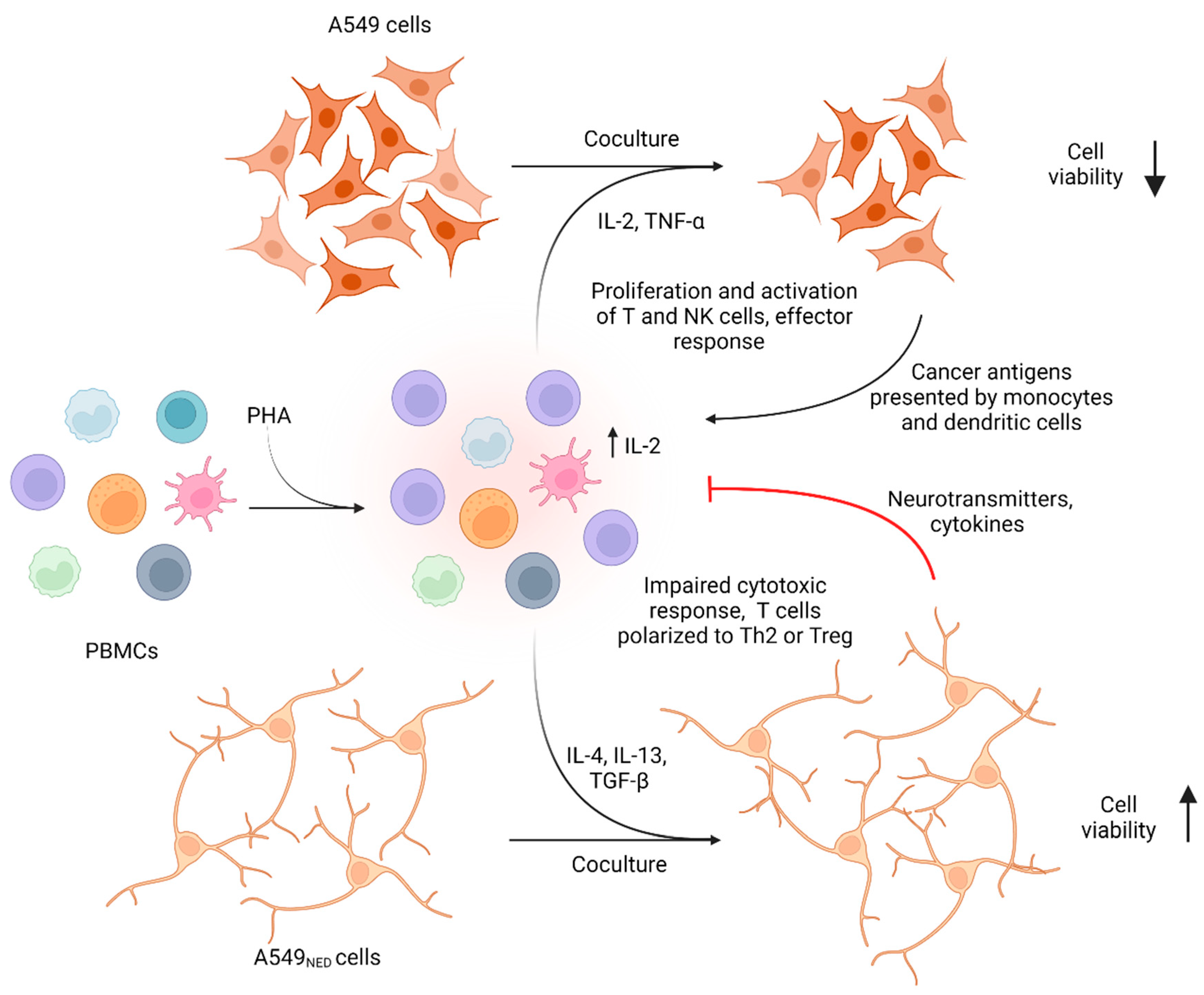
1. Introduction
2. Results
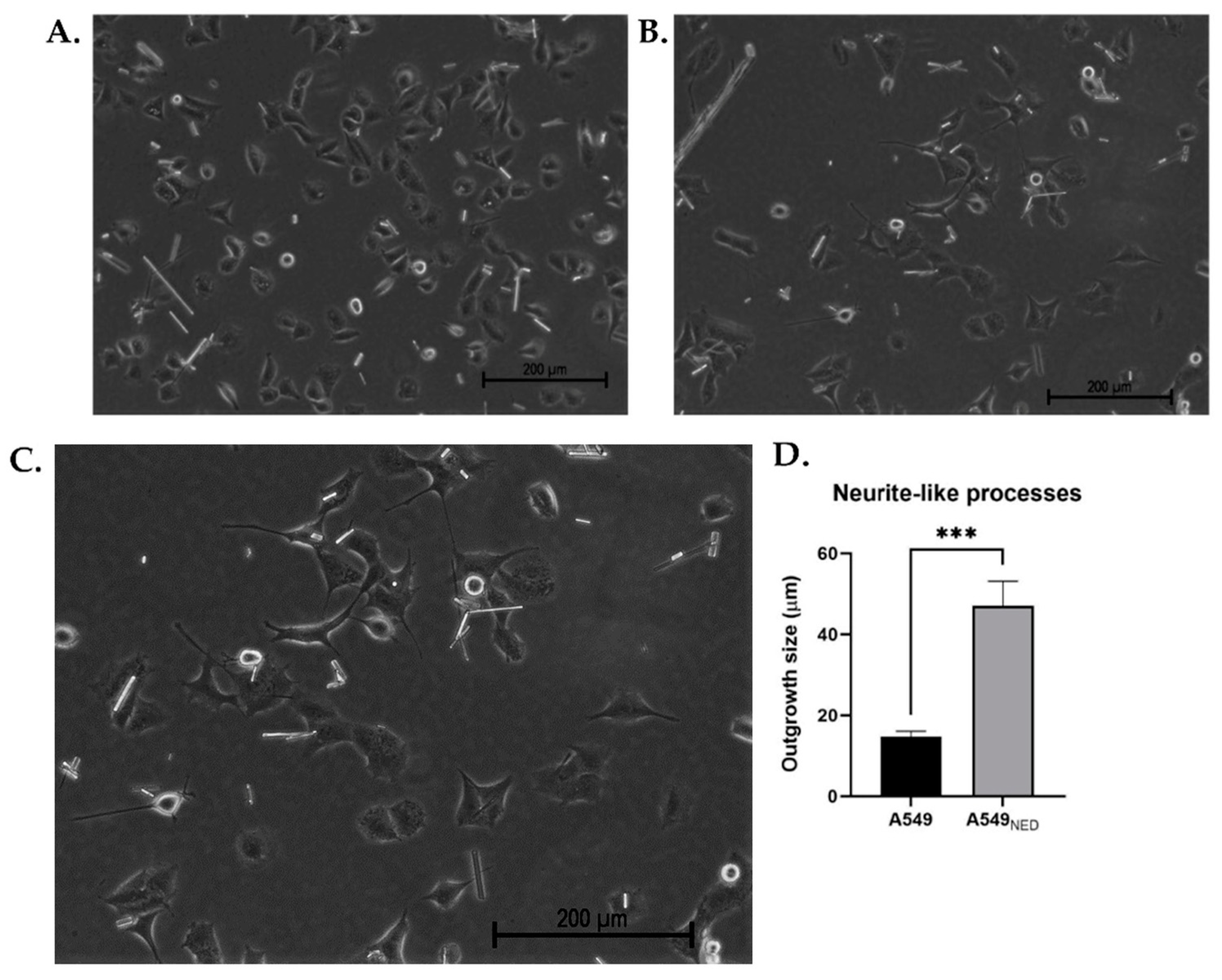
2.1. Neuroendocrine Differentiation of A549 Lung Cancer Cells Is Induced by Treatment with cAMP-Elevating Agents
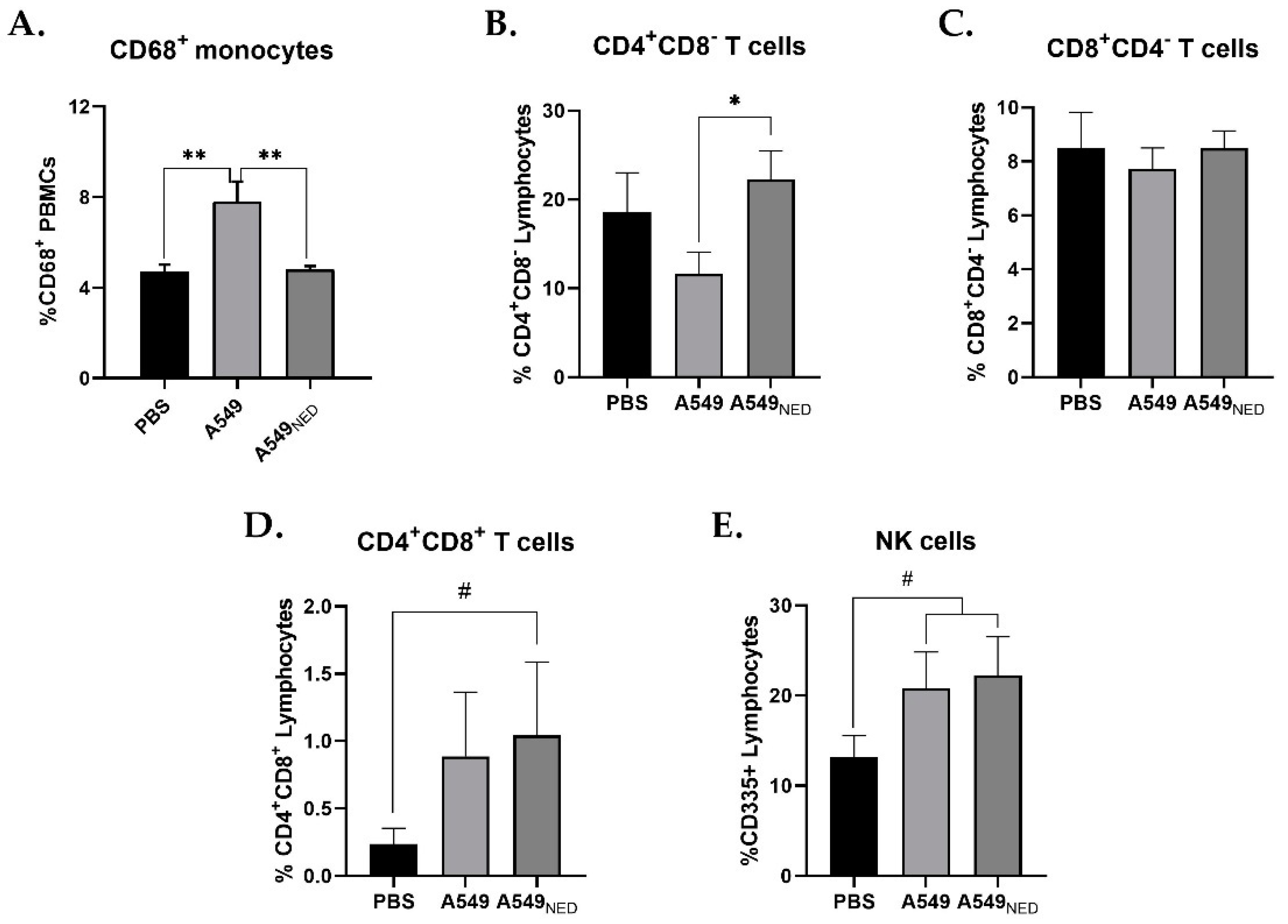
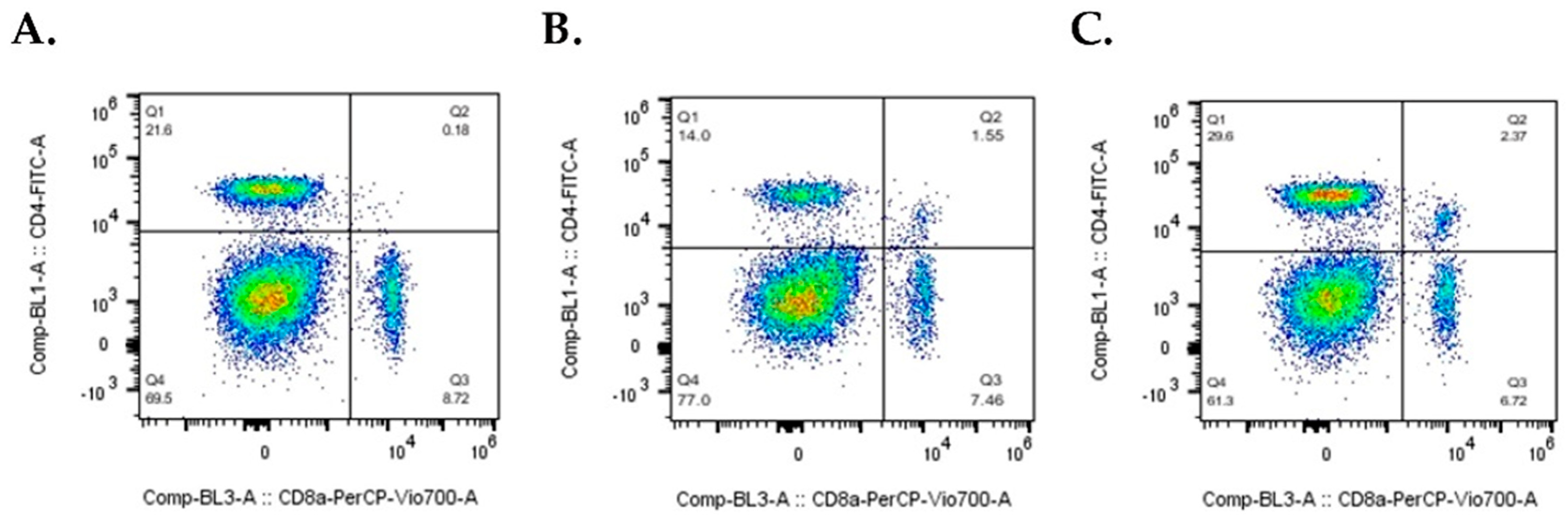
2.2. CD68+ Monocyte Levels Increase and CD4+ T Cells Decrease in Mice Immunized with A549 Cells, whereas Double Positive CD4+CD8+ T Cells Increase in Mice Immunized with A549NED Cells
2.3. IL-2 Increases in Mice Immunized with A549 Cells, whereas IL-10 Decreases in Mice Immunized with A549NED Cells and IFN-γ Stays the Same in All Groups
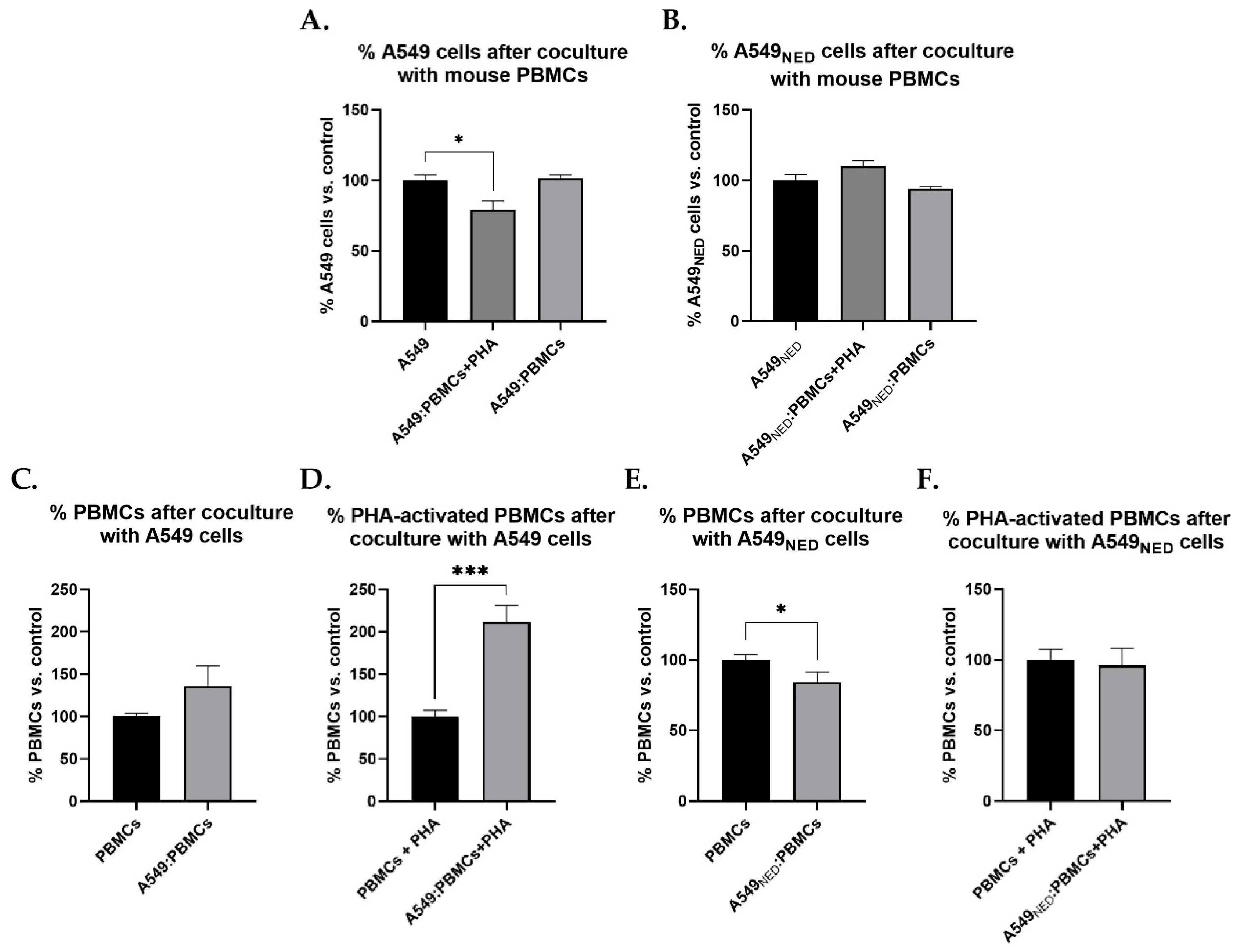
2.4. PBMCs Exert Cytotoxic Activity on A549 Cells, but They Lose This Capacity when Confronted with A549NED Cells
2.5. PBMCs Proliferate in Response to A549 Cells but Die when Cocultured with A549NED Cells
3. Discussion
4. Materials and Methods
4.1. Neuroendocrine Differentiation of Lung Adenocarcinoma Cells
4.2. BALB/c Mice Immunization
4.3. PBMC Extraction and Analysis with Flow Cytometry
4.4. Serum Isolation and Analysis
4.5. Co-Cultures between PBMCs and Cancer Cells
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Raina, V. Lung cancer: Prevalent trends & emerging concepts. Indian J. Med. Res. 2015, 141, 5–7. [Google Scholar] [CrossRef]
- Myers, D.J.; Wallen, J.M. Lung Adenocarcinoma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519578/ (accessed on 18 July 2021).
- Roviello, G. The distinctive nature of adenocarcinoma of the lung. Onco Targets Ther. 2015, 8, 2399–2406. [Google Scholar] [CrossRef][Green Version]
- Sardenberg, R.A.; Mello, E.S.; Younes, R.N. The lung adenocarcinoma guidelines: What to be considered by surgeons. J. Thorac. Dis. 2014, 6 (Suppl. 5), S561–S5617. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, R.I.; Mulshine, J.L.; Steinberg, S.M.; Funa, K.; Matthews, M.J.; Cotelingam, J.D.; Gazdar, A.F. Neuroendocrine Differentiation in Endocrine and Nonendocrine Lung Carcinomas. Am. J. Clin. Pathol. 1998, 90, 641–652. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, J.; Huang, J. Neuroendocrine differentiation in prostate cancer. Am. J. Transl. Res. 2009, 1, 148–162. [Google Scholar] [PubMed]
- Li, Q.; Zhang, C.S.; Zhang, Y. Molecular aspects of prostate cancer with neuroendocrine differentiation. Chin. J. Cancer Res. 2016, 28, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.C.; Cambio, A.J.; Yang, J.C.; Ok, J.H.; Lara, P.N.; Evans, C.P. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate Cancer Prostatic Dis. 2007, 10, 6–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terry, S.; Beltran, H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol. 2014, 4, 60. [Google Scholar] [CrossRef]
- Komiya, A.; Suzuki, H.; Imamoto, T.; Kamiya, N.; Nihei, N.; Naya, Y.; Ichikawa, T.; Fuse, H. Neuroendocrine differentiation in the progression of prostate cancer. Int. J. Urol. 2009, 16, 37–44. [Google Scholar] [CrossRef]
- Hu, C.D.; Choo, R.; Huang, J. Neuroendocrine differentiation in prostate cancer: A mechanism of radioresistance and treatment failure. Front. Oncol. 2015, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Mendieta, I.; Nuñez-Anita, R.E.; Pérez-Sánchez, G.; Pavón, L.; Rodríguez-Cruz, A.; García-Alcocer, G.; Berumen, L.C. Effect of A549 neuroendocrine differentiation on cytotoxic immune response. Endocr. Connect. 2018, 7, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Carnaghi, C.; Rimassa, L.; Garassino, I.; Santoro, A. Clinical significance of neuroendocrine phenotype in non-small-cell lung cancer. Ann. Oncol. 2001, 12, S119–S123. [Google Scholar] [CrossRef] [PubMed]
- Kriegsmann, K.; Zgorzelski, C.; Muley, T.; Christopoulos, P.; Thomas, M.; Winter, H.; Eichhorn, M.; Eichhorn, F.; von Winterfeld, M.; Herpel, E.; et al. Role of Synaptophysin, Chromogranin and CD56 in adenocarcinoma and squamous cell carcinoma of the lung lacking morphological features of neuroendocrine differentiation: A retrospective large-scale study on 1170 tissue samples. BMC Cancer 2021, 21, 486. [Google Scholar] [CrossRef] [PubMed]
- Cerasuolo, M.; Paris, D.; Iannotti, F.A.; Melck, D.; Verde, R.; Mazzarella, E.; Ligresti, A. Neuroendocrine Transdifferentiation in Human Prostate Cancer Cells: An Integrated Approach Neuroendocrine Transdifferentiation and Prostate Cancer. Cancer Res. 2015, 75, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front. Oncol. 2020, 10, 1399. [Google Scholar] [CrossRef]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, B.D.; et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: A role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [CrossRef]
- Shigeta, K.; Kosaka, T.; Kitano, S.; Yasumizu, Y.; Miyazaki, Y.; Mizuno, R.; Shinojima, T.; Kikuchi, E.; Miyajima, A.; Tanoguchi, H.; et al. High Absolute Monocyte Count Predicts Poor Clinical Outcome in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel Chemotherapy. Ann. Surg. Oncol. 2016, 23, 4115–4122. [Google Scholar] [CrossRef]
- Feng, F.; Zheng, G.; Wang, Q.; Liu, S.; Liu, Z.; Xu, G.; Wang, F.; Guo, M.; Lian, X.; Zhang, H. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Hanna, R.N.; Cekic, C.; Sag, D.; Tacke, R.; Thomas, G.D.; Nowyhed, H.; Herrley, E.; Rasquinha, N.; McArdle, S.; Wu, R.; et al. Patrolling monocytes control tumor metastasis to the lung. Science 2015, 350, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Kanti Barman, P.; Kumar Thatoi, P.; Tripathy, R.; Kumar Das, B.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.; Prenen, H.; Van Delm, W.; Di Matteo, M.; Wenes, M.; Delamarre, E.; Mazzone, M. Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut 2015, 65, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Imam, R.; Chang, Q.; Black, M.; Yu, C.; Cao, W. CD47 expression and CD163+ macrophages correlated with prognosis of pancreatic neuroendocrine tumor. BMC Cancer 2021, 21, 320. [Google Scholar] [CrossRef]
- Orozco-Morales, M.; Avilés-Salas, A.; Hernández-Pedro, N.; Catalán, R.; Cruz-Rico, G.; Colín-González, A.L.; Dosal-Mancilla, E.; Barrios-Bernal, P.; Arrieta, O. Clinicopathological and Prognostic Significance of CD47 Expression in Lung Neuroendocrine Tumors. J. Immunol. Res. 2021, 2021, 6632249. [Google Scholar] [CrossRef]
- Qu, S.; Jiao, Z.; Lu, G.; Xu, J.; Yao, B.; Wang, T.; Wang, J.; Yao, Y.; Yan, X.; Wang, T.; et al. Human lung adenocarcinoma CD47 is upregulated by interferon-γ and promotes tumor metastasis. Mol. Ther. Oncolytics 2022, 25, 276–287. [Google Scholar] [CrossRef]
- Affandi, A.J.; Olesek, K.; Grabowska, J.; Nijen Twilhaar, M.K.; Rodríguez, E.; Saris, A.; Zwart, E.S.; Nossent, E.J.; Kalay, H.; de Kok, M.; et al. CD169 Defines Activated CD14+ Monocytes with Enhanced CD8+ T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840. [Google Scholar] [CrossRef]
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical murine hematopathology: A comparative review and implications for research. Comp. Med. 2015, 65, 96–113. [Google Scholar]
- Espinoza-Delgado, I.; Bosco, M.C.; Musso, T.; Gusella, G.L.; Longo, D.L.; Varesio, L. Interleukin-2 and human monocyte activation. J. Leukoc. Biol. 1995, 57, 13–19. [Google Scholar] [CrossRef]
- Bosco, M.C.; Curiel, R.E.; Zea, A.H.; Malabarba, M.G.; Ortaldo, J.R.; Espinoza-Delgado, I. IL-2 signaling in human monocytes involves the phosphorylation and activation of p59hck. J. Immunol. 2000, 16, 4575–4585. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Denardo, D.G.; Coussens, L.M. Polarized immune responses differentially regulate cancer development. Immunol. Rev. 2008, 222, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Bohner, P.; Chevalier, M.F.; Cesson, V.; Rodrigues-Dias, S.C.; Dartiguenave, F.; Burruni, R.; Tawadros, T.; Valerio, M.; Lucca, I.; Nardelli-Haefliger, D.; et al. Double Positive CD4+CD8+ T Cells Are Enriched in Urological Cancers and Favor T Helper-2 Polarization. Front. Immunol. 2019, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Pommier, A.; Lucas, B.; Prévost-Blondel, A. Crucial role of inflammatory monocytes in antitumor immunity. Oncoimmunology 2013, 2, e26384. [Google Scholar] [CrossRef]
- Lee, H.L.; Jang, J.W.; Lee, S.W.; Yoo, S.H.; Kwon, J.H.; Nam, S.W.; Bae, S.H.; Choi, J.Y.; Han, N.I.; Yoon, S.K. Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci. Rep. 2019, 9, 3260. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef]
- Kalia, V.; Sarkar, S. Regulation of Effector and Memory CD8 T Cell Differentiation by IL-2-A Balancing Act. Front. Immunol. 2018, 9, 2987. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.E.; Ohlén, C.; Nelson, B.H.; Greenberg, P.D. Enhanced signaling through the IL-2 receptor in CD8+ T cells regulated by antigen recognition results in preferential proliferation and expansion of responding CD8+ T cells rather than promotion of cell death. Proc. Natl. Acad. Sci. USA 2002, 99, 3001–3006. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, N.; Zhou, L.; Wang, J.; Zhou, Y.; Zhang, T.; Fang, Y.; Deng, J.; Gao, Y.; Liang, X.; et al. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021, 22, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8+ T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef] [PubMed]
- Rha, M.S.; Shin, E.C. Activation or exhaustion of CD8+ T cells in patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Hadad, U.; Thauland, T.J.; Martinez, O.M.; Butte, M.J.; Porgador, A.; Krams, S.M. NKp46 Clusters at the Immune Synapse and Regulates NK Cell Polarization. Front. Immunol. 2015, 6, 495. [Google Scholar] [CrossRef]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Groth, A.; Klöss, S.; von Strandmann, E.P.; Koehl, U.; Koch, J. Mechanisms of tumor and viral immune escape from natural killer cell-mediated surveillance. J. Innate Immun. 2011, 3, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Cai, G.; Kastelein, R.A.; Hunter, C.A. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur. J. Immunol. 1999, 29, 2658–2665. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef]
- Park, Y.J.; Song, B.; Kim, Y.S.; Kim, E.K.; Lee, J.M.; Lee, G.E.; Kim, J.O.; Kim, Y.J.; Chang, W.S.; Kang, C.Y. Tumor microenvironmental conversion of natural killer cells into myeloid-derived suppressor cells. Cancer Res. 2013, 73, 5669–5681. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Oft, M. IL-10: Master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol. Res. 2014, 2, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.; Karki, R.; Sundaram, B.; Kancharana, B.; Lee, S.; Samir, P.; Kanneganti, T.D. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. ImmunoHorizons 2021, 5, 568–580. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Potts, R.C.; Brown, R.A.; Robertson, A.J.; Beck, J.S. Mechanisms of phytohaemagglutinin (PHA) stimulation of normal human lymphocytes: ‘trigger’ ‘push’ or both? Cell Prolif. 1982, 15, 131–137. [Google Scholar] [CrossRef]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Gerosa, F.; Mingari, M.C.; Moretta, L. Interleukin-2 production in response to phytohemagglutinin is not necessarily dependent upon the T3-mediated pathway of T-cell activation. Clin. Immunol. Immunopathol. 1986, 40, 525–531. [Google Scholar] [CrossRef]
- Futakuchi, M.; Lami, K.; Tachibana, Y.; Yamamoto, Y.; Furukawa, M.; Fukuoka, J. The Effects of TGF-β Signaling on Cancer Cells and Cancer Stem Cells in the Bone Microenvironment. Int. J. Mol. Sci. 2019, 20, 5117. [Google Scholar] [CrossRef]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and Their Receptors in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, T.H.; Appell, K.C.; Berg, L.J. Cell cycle progression following naive T cell activation is independent of Jak3/common gamma-chain cytokine signals. J. Immunol. 2009, 183, 4493–4501. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Bloch, W.; Kieven, M.; Lövenich, L.; Lehmann, J.; Holthaus, M.; Theurich, S.; Schenk, A. Serotonin Shapes the Migratory Potential of NK Cells—An in vitro Approach. Int. J. Sports Med. 2017, 38, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garcés-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavón, L. Immunomodulatory effects mediated by serotonin. J. Immunol. Res. 2015, 2015, 354957. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Stefulj, J.; Jakopec, S.; Osmak, M.; Jernej, B. Serotonin and apoptosis: Studies on rat lymphocytes. Neuroimmunomodulation 2002, 10, 132–133. [Google Scholar] [CrossRef]
- Coley, J.S.; Calderon, T.M.; Gaskill, P.J.; Eugenin, E.A.; Berman, J.W. Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PLoS ONE 2015, 10, e0117450. [Google Scholar] [CrossRef]
- Arreola, R.; Alvarez-Herrera, S.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutierrez, E.O.; Garcés-Alvarez, M.E.; de la Cruz-Aguilera, D.L.; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 3160486. [Google Scholar] [CrossRef]
- Capellino, S.; Claus, M.; Watzl, C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell. Mol. Immunol. 2020, 17, 705–711. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Liu, Z.; Cao, B.B.; Peng, Y.P.; Qiu, Y.H. Dopamine receptors modulate cytotoxicity of natural killer cells via cAMP-PKA-CREB signaling pathway. PLoS ONE 2013, 8, e65860. [Google Scholar] [CrossRef] [PubMed]
- Stolk, R.F.; van der Pasch, E.; Naumann, F.; Schouwstra, J.; Bressers, S.; van Herwaarden, A.E.; Gerretsen, J.; Schambergen, R.; Ruth, M.M.; van der Hoeven, J.G.; et al. Norepinephrine Dysregulates the Immune Response and Compromises Host Defense during Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Xiu, F.; Stanojcic, M.; Jeschke, M.G. Norepinephrine inhibits macrophage migration by decreasing CCR2 expression. PLoS ONE 2013, 8, e69167. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, T.; Hori, Y.; Koga, Y.; Toshima, H.; Hara, A.; Yokoyama, M.M. Norepinephrine inhibits human natural killer cell activity in vitro. Int. J. Neurosci. 1991, 58, 127–131. [Google Scholar] [CrossRef]
- Martino, M.; Rocchi, G.; Escelsior, A.; Fornaro, M. Immunomodulation Mechanism of Antidepressants: Interactions between Serotonin/Norepinephrine Balance and Th1/Th2 Balance. Curr. Neuropharmacol. 2012, 10, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, A.; Bimonte, S.; Palma, G.; Luciano, A.; Rea, D.; Giudice, A.; Scognamiglio, G.; La Mantia, E.; Franco, R.; Perdonà, S.; et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int. J. Oncol. 2015, 47, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Balboa, D.; Saarimäki-Vire, J.; Borshagovski, D.; Survila, M.; Lindholm, P.; Galli, E.; Eurola, S.; Ustinov, J.; Grym, H.; Huopio, H.; et al. Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes. eLife 2018, 7, e38519. [Google Scholar] [CrossRef]
- Reuter, J. Subcutaneous Injection of Tumor Cells. Bio-Protocol 2011, 1, e166. [Google Scholar] [CrossRef]


| Neurotransmitter | Target Cell | Effect | Reference |
|---|---|---|---|
| Serotonin | Monocytes | Loss of ability to secrete proinflammatory cytokines such as TNF-α and IL-1β | [64] |
| Macrophages | Suppresses IFN-γ-mediated phagocytosis as well as their ability to present antigens | [64] | |
| NK cells | Increased proliferative and migratory capacity | [64,65] | |
| T cells | Apoptosis, inhibition of PHA-mediated proliferation, reduced activation capacity, Th0 polarization towards Tregs | [66,67,68] | |
| Dopamine * | Monocytes | Increased migration and adhesion | [69] |
| Macrophages | Increased phagocytic activity | [70] | |
| NK cells | Increased cytotoxic activity against cancer cells | [71,72] | |
| T cells | Inhibition of apoptosis, increased cytokine expression (TNF-α, IL-10), increased migration and extravasation of CTLs | [70] | |
| Norepinephrine | Monocytes | Impaired metabolism and cytokine production | [73] |
| Macrophages | Impaired migration | [74] | |
| NK cells | Decreased activity | [75] | |
| T cells | Th2 polarization of immune response | [76] | |
| Cancer cells | Increased migratory potential | [77] |
| Gene | Sequence | Product (bp) |
|---|---|---|
| Syn | Fwd: 5′-AGACAGGGAACACATGCAAG-3′ | 123 |
| Rev: 5′-TCTCCTTAAACACGAACCACAG-3′ | ||
| CgA 1 | Fwd: 5′-AACCGCAGACCAGAGGACCA-3′ | 102 |
| Rev: 5′-GTCTCAGCCCCGCCGTAGT-3′ | ||
| GAPDH | Fwd: 5′-TTGCCCTCAACGACCACTTT-3′ | 120 |
| Rev: 5′-TGGTCCAGGGGTCTTACTCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fosado, R.; Soto-Hernández, J.E.; Núñez-Anita, R.E.; Aceves, C.; Berumen, L.C.; Mendieta, I. Neuroendocrine Differentiation of Lung Cancer Cells Impairs the Activation of Antitumor Cytotoxic Responses in Mice. Int. J. Mol. Sci. 2023, 24, 990. https://doi.org/10.3390/ijms24020990
Fosado R, Soto-Hernández JE, Núñez-Anita RE, Aceves C, Berumen LC, Mendieta I. Neuroendocrine Differentiation of Lung Cancer Cells Impairs the Activation of Antitumor Cytotoxic Responses in Mice. International Journal of Molecular Sciences. 2023; 24(2):990. https://doi.org/10.3390/ijms24020990
Chicago/Turabian StyleFosado, Ricardo, Jazmín E. Soto-Hernández, Rosa Elvira Núñez-Anita, Carmen Aceves, Laura C. Berumen, and Irasema Mendieta. 2023. "Neuroendocrine Differentiation of Lung Cancer Cells Impairs the Activation of Antitumor Cytotoxic Responses in Mice" International Journal of Molecular Sciences 24, no. 2: 990. https://doi.org/10.3390/ijms24020990
APA StyleFosado, R., Soto-Hernández, J. E., Núñez-Anita, R. E., Aceves, C., Berumen, L. C., & Mendieta, I. (2023). Neuroendocrine Differentiation of Lung Cancer Cells Impairs the Activation of Antitumor Cytotoxic Responses in Mice. International Journal of Molecular Sciences, 24(2), 990. https://doi.org/10.3390/ijms24020990







