Abstract
Rehabilitation might improve bone health in breast cancer (BC) patients, but the effects on bone biomarkers are still debated. Thus, this meta-analysis of randomized controlled trials (RCTs) aims at characterizing the impact of rehabilitation on bone health biomarkers in BC survivors. On 2 May 2022, PubMed, Scopus, Web of Science, Cochrane, and PEDro were systematically searched for RCTs assessing bone biomarker modifications induced by physical exercise in BC survivors. The quality assessment was performed with the Jadad scale and the Cochrane risk-of-bias tool for randomized trials (RoBv.2). Trial registration number: CRD42022329766. Ten studies were included for a total of 873 patients. The meta-analysis showed overall significant mean difference percentage decrease in collagen type 1 cross-linked N-telopeptide (NTX) serum level [ES: −11.65 (−21.13, −2.17), p = 0.02)] and an increase in bone-specific alkaline phosphatase (BSAP) levels [ES: +6.09 (1.56, 10.62). According to the Jadad scale, eight RCTs were considered high-quality studies. Four studies showed a low overall risk of bias, according to RoBv.2. The significant effects of rehabilitation on bone biomarkers suggested a possible implication for a precision medicine approach targeting bone remodeling. Future research might clarify the role of bone biomarkers monitoring in rehabilitation management of cancer treatment induced bone-loss.
1. Introduction
Breast cancer (BC) is the most common malignancy in women, with an increasing incidence worldwide [1]. In the last years, the mortality rate related to BC significantly decreased due to the advances in screening programs, early diagnosis, and therapeutical interventions [2]. However, in response to the progressive increase in BC survivors, the prevalence of long terms disabling consequences in these women is steadily increasing, along with the growing need for therapeutic intervention addressing physical and psychosocial impairment that characterizes the so-called “survivorship issues” in BC women [3,4,5].
In this scenario, cancer treatment-induced bone loss (CTIBL) is a common consequence of cancer treatments affecting several BC survivors [6,7,8]. Hormonal therapy (HT) is the gold standard adjuvant therapy for postmenopausal women with hormone receptor (HR)-positive non-metastatic BC [6,7,8]. However, HT negatively affects bone mineral density (BMD) due to residual serum endogenous estrogen levels deprivation, leading to a significant increase in fragility fracture risk [8,9,10,11]. Concurrently, chemotherapy has been related to an unspecific increase in bone resorption, while corticosteroids drug administration has been widely documented to have detrimental consequences on bone health due to a reduction in both bone formation and osteoblast and osteocyte viability [12,13]. Therefore, several pharmacological approaches have been proposed to counter CTIBL, with growing evidence emphasizing the need for precise risk stratification to better guide clinicians in anti-resorptive drug prescription to preserve bone health and reduce the risk of fragility fractures [14,15,16]. On the other hand, lifestyle medicine plays a pivotal role in the multicomponent management of bone and muscle health status in non-metastatic BC survivors, with several relevant guidelines recommending the implementation of a comprehensive CTIBL management, including a calcium-enriched diet, oral supplementation of 1000–2000 IU of vitamin D3 daily, and physical exercise to counteract a potential osteosarcopenia [17,18,19].
More in-detail, physical exercise might prevent bone loss, increase BMD, and reduce fall risk due to the well-known improvement in physical function, balance control, and muscle strength [20]. In this context, hip and trunk muscles are considered as main targets for physical training aiming at stimulating exercise-induced osteogenic effects [21]. To date, several studies supported the role of rehabilitation and physical exercise in improving bone health and quality of life in post-menopausal osteoporotic women [22,23]. More in-detail, the recent systematic review and meta-analysis performed by Kemmler et al. [22] underlined that different exercise modalities might positively affect BMD at the lumbar spine, femoral neck, or total hip site in postmenopausal women [22]. However, to date, the role of rehabilitation in preventing and managing CTIBL is far from being fully understood, whereas recent research is now focusing on the implementation of a precision medicine approach to rehabilitation interventions in accordance with the recent trend of biomarker-based treatment of cancer patients [24,25]. Thus, despite the mechanisms underpinning CTIBL being far from understood in detail, targeting specific molecular modifications might be considered a promising therapeutical approach in the precision medicine management of bone health in BC survivors. On the other hand, evidence supporting precise monitoring of biological effects of rehabilitation interventions is still lacking, not only in cancer patients, but also in other fields of medicine. Moreover, to the best of our knowledge, no previous systematic reviews assessed the effects of different exercise modalities on bone biomarkers in BC survivors.
Therefore, the aim of this systematic review and meta-analysis was to assess the impact of physical rehabilitation interventions on bone biomarker modifications in non-metastatic BC patients. This might potentially guide physicians and future research to more precise monitoring of bone health and CTIBL treatment in these women.
2. Methods
2.1. Registration
This systematic review of randomized controlled trials (RCTs) was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [26]. Preliminary research on the international prospective register of systematic reviews (PROSPERO) was performed to evaluate if other similar works were in progress. No similar review was identified, thus the study was submitted to PROSPERO and accepted on 2 May 2022 (available at https://www.crd.york.ac.uk/prospero, accessed on 16 December 2022, registration number CRD42022329766).
2.2. Search Strategy
Five databases on medical sciences and physical and rehabilitation medicine were systematically searched on 10 May 2022. Two investigators independently searched PubMed/Medline, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), Physiotherapy Evidence Database (PEDro), and Web of Science (WOS). Duplicates were excluded independently by two investigators. Further details of the search strategy are reported in Table 1.

Table 1.
Search strategy.
2.3. Selection Criteria
Review question was characterized by the following PICO model [27]:
- (P) Participants: adult women (18 years and older) with non-metastatic BC.
- Intervention: any rehabilitation treatment administered before, during, or after chemotherapy and/or radiotherapy treatments.
- (C) Comparator: any comparator including pharmacological, non-pharmacological, or no treatment.
- (O) Outcome: primary outcomes were bone metabolic biomarkers. Secondary outcomes were other bone health outcomes, including bone mineral density or trabecular bone score.
RCTs were considered eligible if published in international peer-reviewed journals. The exclusion criteria were the following: (i) language other than English; (ii) studies involving animals; (iii) pregnancy; (iv) clinical instability; and (v) conference abstracts, masters, or doctorate theses.
2.4. Study Screening and Eligibility Assessment
After duplication removal, two investigators independently reviewed the title and abstracts of the retrieved records to choose relevant articles. Discordances between the two authors were solved by collegial discussion. A third reviewer was asked if consensus was not possible. All the reports that met the inclusion and exclusion criteria were screened in full text by the same investigators, and the records that met the eligibility criteria were included in the data extraction. Any disagreements between the two investigators were discussed with a third reviewer to reach consensus.
2.5. Data Extraction and Synthesis
All data were assessed and extracted independently from full-text documents into Word by two authors. Any disagreement between the two reviewers was solved by collegial discussion among the Authors. In case of disagreement, a third author was asked. All the data extracted were summarized in tables.
Data synthesis was performed for the following data: (1) authors; (2) journal; (3) publication year; (4) nationality; (5) participants characteristics [number, mean age and age range, Body Mass Index (BMI)]; (6) tumor characteristics; (7) treatment characteristics; (8) interventions’ characteristics (type of rehabilitative treatment, number of sessions, intensity, duration of intervention); (9) comparator; (10) outcomes; and (11) main findings.
2.6. Meta-Analysis
The meta-analysis was performed by Revman 5.4.0 (The Cochrane Collaboration, 2020, Boston, MA, USA). Changes in serum markers were displayed as mean difference percentage (MD%) and standard deviation (SD). The heterogeneity among comparisons was estimated by the Chi-squared and I2 statistic tests. An I2 > 75% determined significant heterogeneity across the articles. In the event of considerable heterogeneity, a random-effects model was adopted to determine the pooled estimates with the effect size (ES) and 95% confidence interval (CI). Missing means and SDs were estimated from medians, ranges, and interquartile ranges (IQRs) using the method introduced by Hozo et al. [28].
2.7. Quality Assessment and Risk of Bias
The quality of the studies included was assessed independently by two authors, according to the Jadad scale [29]. Discordances were solved by discussion between the authors or by asking a third reviewer. The items assessed were the following (i) random sequence generation; (ii) appropriate randomization; (iii) blinding of participants or personnel; (iv) blinding of outcome assessors; and (v) withdrawals and dropouts. A Jadad score between 3 to 5 points was considered high quality.
The Cochrane risk-of-bias tool for randomized trials (RoBv.2) [30] was implemented for risk of bias assessment. The following domains were assessed by RoBv.2: (i) randomization process; (ii) deviations from the intended interventions; (iii) missing outcome data; (iv) measurements of the outcome; and (v) selection of the reported results. According to these items, bias was classified as low, high, or having some concerns.
3. Results
Through our search strategy, 352 records were identified from the five databases. After duplication removal, 249 studies were assessed for eligibility and screened for title and abstract. Therefore, 220 records were excluded, and 29 full-text records were assessed for eligibility. Nineteen records were excluded for inconsistency with the eligibility criteria (two were only abstracts, eight studies did not assess relevant bone biomarkers, five studies were not RCT, two studies were RCT protocols, one study did not assess a homogeneous sample of BC patients, and one study did not assess rehabilitation intervention). The studies assessed in full text and the reasons for exclusions are presented in detail in Supplementary Table S1. Lastly, 10 studies were included in the present work [31,32,33,34,35,36,37,38,39,40]. Figure 1 shows the PRISMA 2020 flow diagram of the search process in detail.
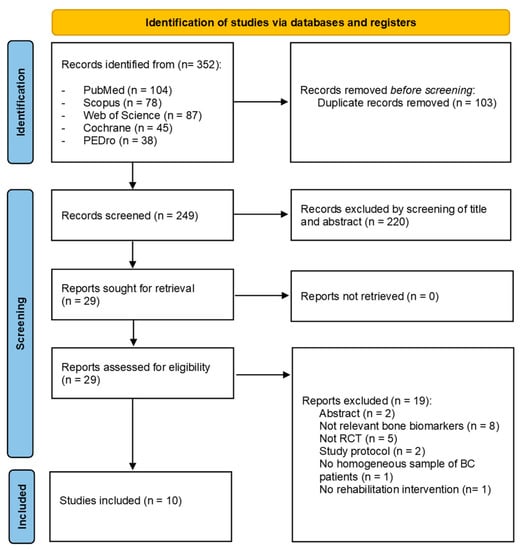
Figure 1.
PRISMA 2020 flow diagram.
3.1. Study Characteristics
The RCTs included were published between 2010 [36,38] and 2018 [31,32,33,35]. The nationalities of the studies included in this review were as follows: seven studies (70%) were conducted in the USA [33,35,36,37,38,39,40], one (10%) was conducted in Australia [31], one (10%) was conducted in Brazil [32], and one (10%) was conducted in South Korea [34]. All the characteristics of the included studies are shown in detail in Table 2.

Table 2.
Main characteristics of the study population.
3.2. Participants
In the present review, 873 subjects (100% females) were assessed in the included studies. More in-detail, 442 BC patients were included in the intervention groups, while 431 BC patients were included in the control groups. The ages of the subjects included ranged from 45.2 ± 5.9 years [37] to 66.6 ± 9.6 years [32]. The body composition was assessed by BMI, and it ranged from 23.3 ± 4.3 kg/m2 [34] to 33.5 ± 5.5 kg/m2 [33]. However, it should be noted that one study [38] reported the number of patients per range of age (≤60, >60 years) and BMI (≤25, >25 kg/m2).
The cancer stages ranged from 0 [34,35,36,39] to IIIB [36], but it should be noted that one study [31] did not characterize the cancer stage, although including only non-metastatic BC patients.
Breast cancer surgery was characterized by three studies as mastectomy and breast conservative surgery. None of the studies included characterized axillary surgery [31,32,33,34,35,36,37,38,39,40]. More in-detail, mastectomy prevalence ranged between 52.2% [3] and 57% [5] in interventional groups, while in control groups it ranged between 20% [4] and 41.2% [2].
Radiation therapy administration ranged between 60.9% [34] and 100% [35] in intervention groups and between 61.1% [40] and 100% [35] in control groups. However, 4fourstudies did not report radiation therapy administration [32,36,37,38].
Chemotherapy administration ranged between 54.5% [35] and 100% [37] in intervention groups, while it ranged between 59.3% [39] and 100% [37] in control groups. Five studies did not characterize chemotherapy administration [31,33,36,38,40].
Hormonal therapy was administered to 100% of study participants in three studies [31,32,35]. Among the other studies, hormone therapy administrations in the intervention groups ranged between 42% [36] and 78.3% [34], while in the control group it ranged between 53.7% [39] and 85% [34]. On the other hand, two studies did not characterize endocrine therapy administration [33,38].
Table 2 shows further details on cancer stage and cancer treatments received in each study included.
3.3. Control Groups
Control groups included BC patients that underwent usual care, vitamin supplementation, pharmacological treatment, stretching and relaxation exercises, and/or psychosocial support therapy. More in-detail, rehabilitation treatment was compared to usual care in three studies [31,33,35], standard treatment combined with psychosocial support in one study [36], stretching and relaxation techniques in three studies [32,39,40], monthly health newsletter in one study [37], and pharmacological intervention with risedronate, calcium, and vitamin D administration in one study [38].
The groups have been characterized in detail in Table 3.

Table 3.
Main characteristics of the study interventions.
3.4. Rehabilitation Therapy Interventions
In the present review, the rehabilitation therapy intervention included resistance exercise training (RET), combined exercise training (CET—aerobic exercise training combined with RET), RET combined with impact exercise training (IET), Thai Chi Chuan, and whole-body vibration (WBV) training. More in-detail, five studies [32,33,34,35,37] assessed CET protocols, making CET the most studied training modality. Only one study focused on the effects of RET [38], while two studies assessed RET combined with IET [39,40]. The remaining two studies assessed the effects of WBV training [31] and Thai Chi Chuan exercise programs [36]. Interestingly, Peppone et al.’s (2018) study assigned the BC patients to three different control groups, assessing separately the efficacy of CET in addition to oral vitamin supplementation, only CET, and only oral vitamin supplementation. Five of the training protocols were supervised by operators [31,32,33,36,37], while three studies assessed training protocols performed with initial supervision followed by home-based sessions [38,39,40]. Lastly, 2 studies assessed home-based protocols [34,35]. All the studies assessed exercise protocols in BC patients after chemotherapy and/or radiotherapy [31,32,33,34,35,36,37,38,39,40].
3.5. Primary Outcome—Bone Biomarkers Modifications
In the present review were included RCTs assessing the biological effect of different rehabilitation programs in terms of modification of concentration of the markers described below.
- Collagen type 1 cross-linked N-telopeptide (NTX) was assessed in four studies [33,35,36,38], but significant changes were reported only in one study [38]. In particular, Waltman et al. [38] reported significant changes (p < 0.05) in both the intervention group (RET + risedronate, calcium, and Vitamin D) and the control group (risedronate, calcium, and vitamin D).
- Urinary NTX excretion normalized to creatinine (NTX/Cr) was assessed in three studies [31,34,37]. However, only Tabatabai et al. [37] reported significant changes (p < 0.05) in both intervention (CET) and control groups (monthly health newsletter).
- Procollagen type I N-terminal propeptide (P1NP) was assessed in two studies [31,37], reporting significant changes in one study [37]. More in-detail, Tabatabai et al. [37] reported a significant decrease (p < 0.05) in both intervention and control groups.
- Collagen Type I C-Telopeptide (CTX) was assessed in three studies [32,33,37]. Interestingly, Tabatabai et al. [41] reported a significant decrease in both groups (p < 0.05). However, no significant between-group differences were reported in the studies considered [32,33,37].
- Osteocalcin was assessed in five papers [32,33,37,39,40]; out of these, two studies [32,33] reported a significant increase (both p < 0.05) in the intervention group after CET, while Winters-Stone et al., 2011, [39] reported a significative inter-group difference after RET combined with IET (p = 0.01). Lastly, Tabatabai et al. [37] reported a significant decrease in both intervention (CET) and control groups.
- Bone-specific alkaline phosphatase (BSAP) was assessed in four papers [33,35,36,38]; among these studies, Dieli-Conwright et al. [33] reported a significant increase in serum concentration in the CET intervention group compared to intervention; concurrently, Waltman et al. [38] reported a reduction in both the intervention group (RET + risedronate, calcium, and Vitamin D) and control group (risedronate, calcium, and Vitamin D). The remaining studies did not report a significant modification of BSAP values (p > 0.05).
- Deoxypyridinoline change in serum level was assessed in two studies [39,40], although neither reported a significative intra- or intergroup difference (p > 0.05).
- Receptor activator of nuclear factor (RANK) was assessed by Dieli-Conwright et al. [33], but the study did not report significant changes (p > 0.05).
- Receptor activator of nuclear factor ligand (RANKL) was assessed by Dieli-Conwright et al. [33], without reporting significant changes (p > 0.05).
Figure 2 graphically summarized the bone biomarkers proposed in the current literature to assess the effects of different exercise modalities.
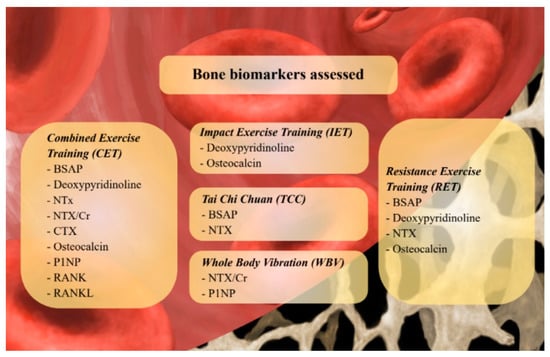
Figure 2.
Bone biomarkers proposed in the current literature to assess the effects of different exercise modalities. Abbreviations: BSAP: Bone Specific Alcaline Phosphatase; CTX: Cross-linked Collagen Type I C-telopeptide; NTX: Crosslinked Collagen Type I N-telopeptide; NTX/Cr: Cross-linked Collagen Type I N-telopeptide/creatinine ratio; RANK(L): Receptor Activator of Nuclear Factor KB (Ligand); P1NP: Procollagen Type 1 intact N-terminal.
3.6. Secondary Outcomes—Bone Mineral Density
Most of the papers [32,33,34,37,38,39,40] included in our study assessed BMD; further details are reported below:
- Whole body BMD was assessed in two studies [32,33,37], without reporting significant changes in intergroup analysis.
- Lumbar spine BMD was assessed in seven studies [32,33,34,37,38,39,40]; more in-detail, Winters-Stone et al., 2011, [39] underlined significant changes (p < 0.01) in the intergroup analysis after RET combined with IET intervention after 12 months. Similarly, Waltman et al. [38] reported a percentage mean difference significant both in the intervention group (RET + risedronate, calcium, and vitamin D) and the control group (risedronate, calcium, and vitamin D) (both p < 0.05). Interestingly, Tabatabai et al. [37] reported a significant mean decrease in the control group, which received only a monthly health newsletter (p = 0.03).
- Total Hip BMD was assessed in seven studies [32,33,34,37,38,39,40]. Waltman et al. [38] reported significant changes in both intervention group (RET + risedronate, calcium, and Vitamin D) and control group (risedronate, calcium, and Vitamin D).
- Trochanter BMD was assessed in four studies [32,33,39,40], but no significant changes were reported.
- Femoral neck BMD was assessed in six studies [33,34,37,38,39,40], without showing significant changes.
- Radius (33% length) BMD was assessed by Waltman et al. [38], without reporting significant changes.
- Total radius BMD was assessed by Waltman et al. [38], with no significant changes after the intervention.
Table 4 summarized the primary and secondary outcomes of the present review.

Table 4.
Main results of the studies included.
3.7. Meta-Analysis
A meta-analysis was performed to underline the effects of different exercise interventions on bone metabolism biomarkers of non-metastatic BC patients, showing an overall significant MD% decrease in NTX serum level [ES: −11.65 (−21.13, −2.17), p = 0.02)] and an increase in BSAP levels [ES: +6.09 (1.56, 10.62), p = 0.008)]. On the other hand, no significant differences were found for urinary NTX, CTX, and osteocalcin markers. Percentage differences between intervention and control group provided by Waltman et al. [38] were used to adapt the data related to the combined intervention (exercise combined with risedronate versus risedronate alone). A random-effects model was adopted since the low number of RCTs included and the high heterogeneity of rehabilitation intervention (for further details see Figure 3).
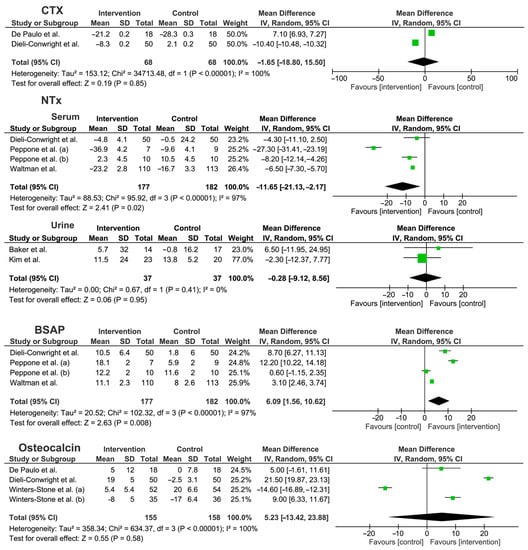
Figure 3.
Meta-analysis of the studies included [31,32,33,34,35,36,38,39,40]. Abbreviations BSAP: bone specific alkaline phosphatase; CI: confidence interval; CTX: C-telopeptides of type I collagen; NTX: crosslinked N-telopeptides of type I collagen; SD: standard deviation.
The study by Tabatabai et al. [37] was excluded from the meta-analysis because numerical data were not reported.
3.8. Quality Assessment and Risk of Bias
According to the Jadad scale, eight (80%) RCTs were considered high-quality studies [31,33,34,35,36,37,39,40]. Lower quality was found in two (30%) studies [32,38] due to missing information about randomization methods or blindness of data assessors. On the other hand, it should be noted that blindness of participants and personnel was not achievable in all the studies included due to the intrinsic nature of the rehabilitative treatment. Table 5 showed in detail the score of each subitem of the Jadad scale for the RCTs included.

Table 5.
Quality assessment of the studies included in the present systematic review.
The risk of bias was assessed by RoBv.2, reporting 4 studies [31,34,39,40] (40%) with a low overall risk of bias. Two studies (20%) [33] showed some concerns in the second domain for deviations from intended interventions due to the missing appropriate analysis for effect of assignment to intervention. These concerns lead to an overall medium risk of bias. Lastly, 1 study [33] (10%) showed a high risk of bias for exclusion of five women from analysis after the intervention, resulting in a high overall risk of bias (see Figure 4).
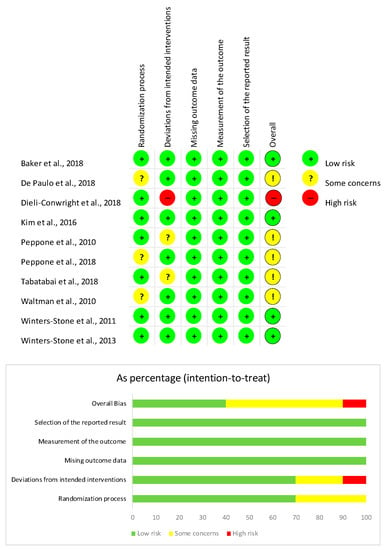
Figure 4.
Risk of bias of the studies included [31,32,33,34,35,36,37,38,39,40] according to the RoB2.
4. Discussion
In recent years, the long-term management of BC survivors has gained a rising interest in both clinical and research settings, considering the growing prevalence of cancer disabling sequelae affecting these women. Several papers highlight the need for structured and tailored rehabilitation intervention to improve both physical and psychosocial well-being of BC women [41,42]. In this scenario, CTIBL is widespread disabling condition in cancer patients and physical exercise plays a pivotal role in its prevention due to the multifaceted effects on the whole musculoskeletal system, improving both BMD and reducing the risk of falling in patients at high risk of fragility fracture [43,44,45]. However, to date, several questions are still open about the precise biological effects of physical exercise on bone metabolism and health since the complex multilevel interactions characterizing CTIBL in non-metastatic BC survivors. In light of these considerations, this meta-analysis of RCTs assessed the effects of different exercise modalities on currently available bone biomarkers, providing a broad overview about the evidence supporting biomarker implementation in the clinical setting in order to guide physicians in a precise prescription of individualized rehabilitation plans.
Interestingly, our meta-analysis showed significant effects in terms of NTX serum level [ES: −11.65 (−21.13, −2.17), p = 0.02)]. However, it should be noted that the results of individual studies were not significant in the majority of the RCT included. This limitation might be partly related to the small sample of the studies considered and the low effect size, given that the results of the pooled sample showed significant differences in terms of NTX. NTX is one of the most important biomarkers to assess bone resorption [46,47,48]. Its levels in bloodstream reflect the liberation of peptides produced by degradation of osteoid (composed mostly of collagen); in this context, its serum levels might quantify the rate of bone resorption [49], also considering the role that might play in repairing bone and nerves [50,51]. Moreover, the recent systematic review by Migliorini et al. [52] found a significant association between NTX serum level and lower spine and hip BMD, suggesting that a NTX serum level might reflect an increased bone turnover, leading to a reduction in both BMD and T-score. Interestingly, the qualitative synthesis identified one study [38] reporting significant changes in NTX serum levels after RET intervention, suggesting that RET might be the most promising modality in inducing NTX serum level modifications. However, it should be noted that urinary NTX excretion did not show significant changes in the meta-analysis [37].
In recent years, the International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) identified NTX and CTX as the most promising bone biomarkers in the clinical setting of bone pathological conditions, including osteoporosis [53,54]. Despite positive results being reported in NTX serum level modifications, the results of our meta-analysis did not show significant differences in terms of CTX modifications after physical exercise programs. However, few studies [37,38] assessed CTX serum levels in BC women undergoing physical exercise programs. Therefore, these data might be significantly affected by the low number of studies currently available in the literature.
Considering bone deposition biomarkers, BSAP is a bone marker of bone formation that showed a significant increase after exercise therapy interventions in BC patients (BSAP levels [ES: +6.09 (1.56, 10.62) p = 0.008)]. Approximately 50% of BSAP is produced from the skeletal system in subjects with normal liver function. However, no studies assessing BSAP reported the liver function status of study participants. Thus, to date, CET or RET seems to be the exercise modality most supported in improving blood levels of BSAP in BC survivors [33,38].
Similarly, osteocalcin is selectively secreted by osteoblast and is considered a bone marker to assess bone anabolic activity [55]. In particular, γ-carboxylate osteocalcin has great affinity for hydroxyapatite and is commonly stored in bone tissues [56]. Osteocalcin decarboxylation promotes its endocrine activity as bone-derived hormone, with recent studies highlighting its role in glucose metabolism [55,56,57]. Our results highlighted a significant improvement in terms of osteocalcin serum level in two studies [32,33] assessing CET or RET combined with IET. On the other hand, the meta-analysis did not show significant benefits of exercise in terms of osteocalcin.
In the last two decades, increasing interest has been raised in both RANK and RANK-L pathways, two crucial pharmacological targets in the management of osteoporosis. More in-detail, RANK is a transmembrane receptor involved in the signaling pathway regulating osteoclast differentiation and activation. To date, this pathway is the main target of the monoclonal antibody Denosumab, exerting its antiresorptive action by blocking the interaction between RANK and RANK-L, with consequent inhibition of osteoclast activity [16,58]. On the other hand, RANK is not monitored in the current clinical practice, and there is a lack of studies in terms of modifications after physical exercise training in BC survivors [33].
In addition, RANK monitoring might be crucially affected by the pharmacological therapies commonly administered to prevent CTIBL [59,60,61]. Therefore, RANK and RANK-L should not be considered bona fide biomarkers to assess the biological effects of physical exercise in BC patients. Lastly, no evidence supports their integration in a precision medicine approach focusing on bone health management in BC survivors.
Taken together, our findings showed positive results of certain specific bone biomarkers reflecting the effects of physical exercise on bone health in BC survivors. However, conflicting data were reported about BMD modifications induced by physical exercise in these patients. More in-detail, three studies showed positive results in terms of lumbar spine BMD improvement after physical exercise interventions [37,38,39]. These findings might be probably related to the trabecular structure of vertebra that is metabolically more active and might be more sensible to mechanical stimuli promoting bone formation at the lumbar spine level [62,63]. In addition, RET alone or combined with IET might be the most promising therapeutic approach to improve lumbar spine BMD [37,38,39]. Unfortunately, few studies included in the present work assess T-score or Z-score, probably due to the short-term follow-up period and the non-pharmacological intervention that might provide little changes related to the short terms follow-up and instrumental errors, highlighting another gap of knowledge in the current. In this scenario, previous studies suggested that a multimodal approach, including different exercise modalities, might be the most suitable option to improve bone health in patients with osteoporosis [64,65,66]. Moreover, the recent systematic review by Marini et al. [65] suggested RET and IET as the most promising exercise modalities to reduce the risk of fracture.
On the other hand, several controversies are still open about the macroscopical effects of physical exercise on BMD, and previous systematic reviews and meta-analyses reported insufficient evidence to support a superior effect of one specific exercise modality [67,68]. However, it should be noted that the currently available literature focused on standardized exercise programs without focusing on the biological effects of physical exercise in an individualized rehabilitation plan. Moreover, our systematic review did not identify studies considering a precision medicine approach based on bone remodeling biomarkers to tailor physical exercise programs to the patient’s characteristics.
Taken together, our findings underlined that bone biomarkers might be significantly affected by physical exercise and could be possibly implemented in monitoring tailored rehabilitation interventions aimed at treating CTIBL in BC survivors. To the best of our knowledge, this is the first systematic review focusing on the effects of different exercise modalities on bone biomarkers in non-metastatic BC survivors. In the era of precision medicine, a biomarker-based approach might have a role in improving the comprehensive rehabilitation management of these women, including not only physical exercise, but also antiresorptive drugs in patients at high risk of fracture to maximize outcomes and reduce the disability and socio-sanitary costs of fragility fractures [69,70,71]. In addition, due to the widely documented effects of physical exercise on oxidative stress and inflammation, a precise multitarget rehabilitation intervention might not only improve bone health, but also have potential interaction with malignant transformation and tumor progression pathways in BC patients [72,73,74,75,76].
Besides these considerations, we are aware that this study is not free from limitations. More in-detail, the low number of studies included, and the small sample size might limit the strengths of our conclusions. On the other hand, our results reflect the papers currently available about this topic in five different databases and put to light a gap of knowledge in the current literature. However, it should be noted that the sample size assessed allows us to obtain significant results in quantitative synthesis. On the other hand, the heterogeneity of the study population, exercise characteristics, and bone biomarkers might represent the main limitations of the present review. To reduce potential bias related to this issue, we provided a detailed qualitative synthesis to characterize the heterogeneity of the studies. Moreover, meta-analysis has been performed in subgroup analysis for bone biomarkers, limiting the potential implications of their heterogeneity. Lastly, only the study by Waltman et al. [38] assessed the effects of physical exercise in a comprehensive rehabilitation approach to CTIBL, including also pharmacological treatments. In this context, it should be noted that antiresorptive drugs should be integrated into the bone health management of BC survivors receiving AIs in accordance with the most recent guidelines [10,17,18,77]. Given the antiresorptive drugs’ effects on bone metabolisms, further good quality studies are needed to better characterize the impact of physical exercise on bone biomarkers in BC patients treated with antiresorptive drugs for preventing CTIBL.
However, our findings might be a catalyst for a deeper understanding of biological processes regulating the multilevel interaction between physical exercise, bone remodeling, and CTIBL. Future research should focus on the precise characterization of physical exercise programs, highlighting the biological differences induced by a comprehensive rehabilitation plan.
5. Conclusions
Physical exercise is one of the main non-pharmacological interventions counteracting CTIBL in non-metastatic BC survivors. However, to date, no previous systematic review assessed the effects of physical exercise on circulating bone biomarkers, and the effects of different exercise modalities on bone biomarkers are still debated.
Taken together, the results of the meta-analysis suggested significant effects of rehabilitation in terms of NTX and BSAP levels modifications, even though the heterogeneity of the study results might limit the strength of our conclusions. However, our data might have potential implications for the prescription of physical exercise targeting bone remodeling in patients with non-metastatic BC. Future research might clarify the role of bone biomarker monitoring in the comprehensive management of CTIBL to optimize the synergistic role of non-pharmacological and pharmacological approaches in promoting bone health in BC survivors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24020921/s1.
Author Contributions
Conceptualization, A.d.S., L.L. and M.I.; methodology, A.d.S., L.L. and M.I.; formal analysis, N.M.; data curation, A.d.S., L.L. and M.I.; writing—original draft preparation, A.d.S., L.L. and N.M.; writing—review and editing, A.A., N.F. and M.I.; visualization, A.F., D.C., S.M. and A.T.; supervision, A.d.S., N.F. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Nardin, S.; Mora, E.; Varughese, F.M.; D’Avanzo, F.; Vachanaram, A.R.; Rossi, V.; Saggia, C.; Rubinelli, S.; Gennari, A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Bernetti, A.; Bai, A.V.; Segatori, L.; Monti, M.; Maggi, G.; Ippolitoni, G.; Tinelli, L.; Santilli, V.; Paoloni, M. The sequelae of mastectomy and quadrantectomy with respect to the reaching movement in breast cancer survivors: Evidence for an integrated rehabilitation protocol during oncological care. Support Care Cancer. 2021, 29, 899–908. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Losco, L.; Cisari, C.; Gennari, A.; Boldorini, R.; Fusco, N.; Cigna, E.; Invernizzi, M. Axillary web syndrome in women after breast cancer surgery referred to an Oncological Rehabilitation Unit: Which are the main risk factors? A retrospective case-control study. Eur Rev Med Pharmacol Sci. 2020, 24, 8028–8035. [Google Scholar] [CrossRef]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F.; Committee, E.G. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v8–v30. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J. Clin. Oncol. 2014, 32, 2255–2269. [Google Scholar] [CrossRef]
- Diana, A.; Carlino, F.; Giunta, E.F.; Franzese, E.; Guerrera, L.P.; Di Lauro, V.; Ciardiello, F.; Daniele, B.; Orditura, M. Cancer Treatment-Induced Bone Loss (CTIBL): State of the Art and Proper Management in Breast Cancer Patients on Endocrine Therapy. Curr. Treat. Options Oncol. 2021, 22, 45. [Google Scholar] [CrossRef]
- Hadji, P.; Coleman, R.E.; Wilson, C.; Powles, T.J.; Clezardin, P.; Aapro, M.; Costa, L.; Body, J.J.; Markopoulos, C.; Santini, D.; et al. Adjuvant bisphosphonates in early breast cancer: Consensus guidance for clinical practice from a European Panel. Ann. Oncol. 2016, 27, 379–390. [Google Scholar] [CrossRef]
- Migliaccio, S.; Francomano, D.; Romagnoli, E.; Marocco, C.; Fornari, R.; Resmini, G.; Buffa, A.; Di Pietro, G.; Corvaglia, S.; Gimigliano, F.; et al. Persistence with denosumab therapy in women affected by osteoporosis with fragility fractures: A multicenter observational real practice study in Italy. J. Endocrinol. Invest. 2017, 40, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A. Bone Loss and Fracture Risk Associated with Cancer Therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Kobza, A.O.; Herman, D.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Understanding and Managing Corticosteroid-Induced Osteoporosis. Open Access Rheumatol. Res. Rev. 2021, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.M.; Heisey, R.; Srighanthan, J. Breast cancer and osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 532–538. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Gennari, A.; Cisari, C.; Pasqua, S.; Foglio Bonda, P.L.; Invernizzi, M.; Migliario, M. Bone health, vitamin D status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents 2021, 35, 397–402. [Google Scholar] [CrossRef]
- de Sire, A.; Lippi, L.; Venetis, K.; Morganti, S.; Sajjadi, E.; Curci, C.; Ammendolia, A.; Criscitiello, C.; Fusco, N.; Invernizzi, M. Efficacy of Antiresorptive Drugs on Bone Mineral Density in Post-Menopausal Women With Early Breast Cancer Receiving Adjuvant Aromatase Inhibitors: A Systematic Review of Randomized Controlled Trials. Front. Oncol. 2021, 11, 829875. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, O.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Dhesy-Thind, S.; Fletcher, G.G.; Blanchette, P.S.; Clemons, M.J.; Dillmon, M.S.; Frank, E.S.; Gandhi, S.; Gupta, R.; Mates, M.; Moy, B.; et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 2062–2081. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Mullooly, M.; Bennett, K.; Crown, J. Vitamin D Supplementation: Does It Have a Preventative or Therapeutic Role in Cancer? Nutr Cancer. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Letizia Mauro, G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435. [Google Scholar] [CrossRef]
- Kemmler, W.; Shojaa, M.; Kohl, M.; von Stengel, S. Effects of Different Types of Exercise on Bone Mineral Density in Postmenopausal Women: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 409–439. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; Kim, J.; Fusco, N. Editorial: Quality of Life in Breast Cancer Patients and Survivors. Front. Oncol. 2020, 10, 620574. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Guerini-Rocco, E.; Viale, G.; Fumagalli, C.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Malapelle, U.; Fusco, N. Immunotherapy in Breast Cancer Patients: A Focus on the Use of the Currently Available Biomarkers in Oncology. Anticancer Agents Med. Chem. 2022, 22, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Althouse, S.K.; Olsen, T.P.; Miller, K.D.; Sledge, J.S. A Personalized, Dynamic Physical Activity Intervention Is Feasible and Improves Energetic Capacity, Energy Expenditure, and Quality of Life in Breast Cancer Survivors. Front. Oncol. 2021, 11, 626180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Baker, M.K.; Peddle-McIntyre, C.J.; Galvão, D.A.; Hunt, C.; Spry, N.; Newton, R.U. Whole Body Vibration Exposure on Markers of Bone Turnover, Body Composition, and Physical Functioning in Breast Cancer Patients Receiving Aromatase Inhibitor Therapy: A Randomized Controlled Trial. Integr. Cancer Ther. 2018, 17, 968–978. [Google Scholar] [CrossRef] [PubMed]
- de Paulo, T.R.S.; Winters-Stone, K.M.; Viezel, J.; Rossi, F.E.; Simões, R.R.; Tosello, G.; Freitas, I.F.J. Effects of resistance plus aerobic training on body composition and metabolic markers in older breast cancer survivors undergoing aromatase inhibitor therapy. Exp. Gerontol. 2018, 111, 210–217. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, Y.U.; Kim, S.J.; Hong, S.; Han, M.S.; Choi, E. The Effect on Bone Outcomes of Adding Exercise to Supplements for Osteopenic Breast Cancer Survivors: A Pilot Randomized Controlled Trial. Cancer Nurs. 2016, 39, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Peppone, L.J.; Ling, M.; Huston, A.J.; Reid, M.E.; Janelsins, M.C.; Puzas, J.E.; Kamen, C.; Del Giglio, A.; Asare, M.; Peoples, A.R.; et al. The effects of high-dose calcitriol and individualized exercise on bone metabolism in breast cancer survivors on hormonal therapy: A phase II feasibility trial. Support. Care Cancer 2018, 26, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Peppone, L.J.; Mustian, K.M.; Janelsins, M.C.; Palesh, O.G.; Rosier, R.N.; Piazza, K.M.; Purnell, J.Q.; Darling, T.V.; Morrow, G.R. Effects of a structured weight-bearing exercise program on bone metabolism among breast cancer survivors: A feasibility trial. Clin. Breast Cancer 2010, 10, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, L.S.; Bloom, J.; Stewart, S.; Sellmeyer, D.E. A Randomized Controlled Trial of Exercise to Prevent Bone Loss in Premenopausal Women with Breast Cancer. J. Women’s Health 2019, 28, 87–92. [Google Scholar] [CrossRef]
- Waltman, N.L.; Twiss, J.J.; Ott, C.D.; Gross, G.J.; Lindsey, A.M.; Moore, T.E.; Berg, K.; Kupzyk, K. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: A 24-month randomized controlled trial. Osteoporos. Int. 2010, 21, 1361–1369. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.; Bennett, J.A.; Leo, M.C.; Naik, A.; Schwartz, A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: A randomized, controlled trial. Breast Cancer Res. Treat. 2011, 127, 447–456. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Nail, L.M.; Bennett, J.A.; Leo, M.C.; Torgrimson-Ojerio, B.; Luoh, S.W.; Schwartz, A. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: A randomized controlled trial. Osteoporos. Int. 2013, 24, 1637–1646. [Google Scholar] [CrossRef]
- de Sire, A.; Lippi, L.; Ammendolia, A.; Cisari, C.; Venetis, K.; Sajjadi, E.; Fusco, N.; Invernizzi, M. Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor-Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study. J. Pers. Med. 2021, 11, 1369. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Bernetti, A.; Bai, A.V.; Capobianco, S.V.; Bonifacino, A.; Maggi, G.; Ippolitoni, G.; Tinelli, L.; Santilli, V.; Agostini, F. The recovery of reaching movement in breast cancer survivors: Two different rehabilitative protocols in comparison. Eur. J. Phys. Rehabil. Med. 2021, 57, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Moayyeri, A. The association between physical activity and osteoporotic fractures: A review of the evidence and implications for future research. Ann. Epidemiol. 2008, 18, 827–835. [Google Scholar] [CrossRef]
- de Kam, D.; Smulders, E.; Weerdesteyn, V.; Smits-Engelsman, B.C. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: A systematic review of randomized controlled trials. Osteoporos. Int. 2009, 20, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Suominen, H. Muscle training for bone strength. Aging Clin. Exp. Res. 2006, 18, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hlaing, T.T.; Compston, J.E. Biochemical markers of bone turnover–uses and limitations. Ann. Clin. Biochem. 2014, 51, 189–202. [Google Scholar] [CrossRef]
- Bauer, D.C.; Garnero, P.; Harrison, S.L.; Cauley, J.A.; Eastell, R.; Ensrud, K.E.; Orwoll, E. Biochemical Markers of Bone Turnover, Hip Bone Loss, and Fracture in Older Men: The MrOS Study. J. Bone Miner. Res. 2009, 24, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Christenson, R.H. Biochemical markers of bone metabolism: An overview. Clin. Biochem. 1997, 30, 573–593. [Google Scholar] [CrossRef]
- Taylor, A.K.; Lueken, S.A.; Libanati, C.; Baylink, D.J. Biochemical markers of bone turnover for the clinical assessment of bone metabolism. Rheum. Dis. Clin. N. Am. 1994, 20, 589–607. [Google Scholar] [CrossRef]
- Nair, A.; Chuang, S.C.; Lin, Y.S.; Chen, C.H.; Fang, T.C.; Chiu, H.C.; Lien, C.H.; Chen, S.J. Characterization of collagen response to bone fracture healing using polarization-SHG. Sci. Rep. 2022, 12, 18453. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Molinero-Mourelle, P.; Ferrillo, M.; Cobo-Vázquez, C.; Sanchez-Labrador, L.; Ammendolia, A.; Migliario, M.; de Sire, A. Type I Collagen-Based Devices to Treat Nerve Injuries after Oral Surgery Procedures. A Systematic Review. Appl.Sci. 2021, 11, 3927. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Spiezia, F.; Peretti, G.M.; Tingart, M.; Giorgino, R. Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: A systematic review. J. Orthop. Surg. Res. 2021, 16, 351. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Krege, J.; Lane, N.; Leary, E.; Libanati, C.; Miller, P.; Myers, G.; Silverman, S.; Vesper, H.W.; Lee, D.; et al. National Bone Health Alliance Bone Turnover Marker Project: Current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos. Int. 2012, 23, 2425–2433. [Google Scholar] [CrossRef]
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Ferron, M. Gamma-carboxylation regulates osteocalcin function. Oncotarget 2015, 6, 19924–19925. [Google Scholar] [CrossRef]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.M.; Schoppet, M.; Schaefer, J.R.; Hofbauer, L.C. Novel aspects on RANK ligand and osteoprotegerin in osteoporosis and vascular disease. Calcif. Tissue Int. 2004, 74, 103–106. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger-Zeinitzer, E.; et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): Disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 2019, 20, 339–351. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Ellis, G.K.; Bone, H.G.; Chlebowski, R.; Paul, D.; Spadafora, S.; Smith, J.; Fan, M.; Jun, S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008, 26, 4875–4882. [Google Scholar] [CrossRef]
- Sandino, C.; McErlain, D.D.; Schipilow, J.; Boyd, S.K. Mechanical stimuli of trabecular bone in osteoporosis: A numerical simulation by finite element analysis of microarchitecture. J. Mech. Behav. Biomed. Mater. 2017, 66, 19–27. [Google Scholar] [CrossRef]
- Ott, S.M. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol. 2018, 47, 373–375. [Google Scholar] [CrossRef]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2011, 7, Cd000333. [Google Scholar] [CrossRef] [PubMed]
- Marini, S.; Barone, G.; Masini, A.; Dallolio, L.; Bragonzoni, L.; Longobucco, Y.; Maffei, F. Current Lack of Evidence for an Effect of Physical Activity Intervention Combined with Pharmacological Treatment on Bone Turnover Biomarkers in People with Osteopenia and Osteoporosis: A Systematic Review. J. Clin. Med. 2021, 10, 3442. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Dalla Via, J.; Duckham, R.L.; Fraser, S.F.; Helge, E.W. Exercise for the prevention of osteoporosis in postmenopausal women: An evidence-based guide to the optimal prescription. Braz. J. Phys. 2019, 23, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Kast, S.; Shojaa, M.; Kohl, M.; Von Stengel, S.; Gosch, M.; Jakob, F.; Kerschan-Schindl, K.; Kladny, B.; Klöckner, N.; Lange, U.; et al. Effects of different exercise intensity on bone mineral density in adults: A comparative systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 1643–1657. [Google Scholar] [CrossRef] [PubMed]
- Massini, D.A.; Nedog, F.H.; De Oliveira, T.P.; Almeida, T.A.F.; Santana, C.A.A.; Neiva, C.M.; Macedo, A.G.; Castro, E.A.; Espada, M.C.; Santos, F.J.; et al. The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1129. [Google Scholar] [CrossRef]
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Invernizzi, M.; Baricich, A.; Lippi, L.; Ammendolia, A.; Grassi, F.A.; Leigheb, M. Optimization of transdisciplinary management of elderly with femur proximal extremity fracture: A patient-tailored plan from orthopaedics to rehabilitation. World J. Orthop. 2021, 12, 456–466. [Google Scholar] [CrossRef]
- Muschitz, C.; Hummer, M.; Grillari, J.; Hlava, A.; Birner, A.H.; Hemetsberger, M.; Dimai, H.P. Epidemiology and economic burden of fragility fractures in Austria. Osteoporos. Int. 2022, 33, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Saarto, T.; Sievänen, H.; Kellokumpu-Lehtinen, P.; Nikander, R.; Vehmanen, L.; Huovinen, R.; Kautiainen, H.; Järvenpää, S.; Penttinen, H.M.; Utriainen, M.; et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos. Int. 2012, 23, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Vehmanen, L.; Sievänen, H.; Kellokumpu-Lehtinen, P.; Nikander, R.; Huovinen, R.; Ruohola, J.; Penttinen, H.M.; Utriainen, M.; Tokola, K.; Blomqvist, C.; et al. Five-year follow-up results of aerobic and impact training on bone mineral density in early breast cancer patients. Osteoporos. Int. 2021, 32, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Longobucco, Y.; Masini, A.; Marini, S.; Barone, G.; Fimognari, C.; Bragonzoni, L.; Dallolio, L.; Maffei, F. Exercise and Oxidative Stress Biomarkers among Adult with Cancer: A Systematic Review. Oxidative Med. Cell. Longev. 2022, 2022, 2097318. [Google Scholar] [CrossRef]
- Delrieu, L.; Touillaud, M.; Pérol, O.; Morelle, M.; Martin, A.; Friedenreich, C.M.; Mury, P.; Dufresne, A.; Bachelot, T.; Heudel, P.-E.; et al. Impact of Physical Activity on Oxidative Stress Markers in Patients with Metastatic Breast Cancer. Oxidative Med. Cell. Longev. 2021, 2021, 6694594. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef]
- Eisen, A.; Somerfield, M.R.; Accordino, M.K.; Blanchette, P.S.; Clemons, M.J.; Dhesy-Thind, S.; Dillmon, M.S.; D’Oronzo, S.; Fletcher, G.G.; Frank, E.S.; et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: ASCO-OH (CCO) Guideline Update. J. Clin. Oncol. 2022, 40, 787–800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).