The Anaesthetics Isoflurane and Xenon Reverse the Synaptotoxic Effects of Aβ1–42 on Megf10-Dependent Astrocytic Synapse Elimination and Spine Density in Ex Vivo Hippocampal Brain Slices
Abstract
1. Introduction
2. Results
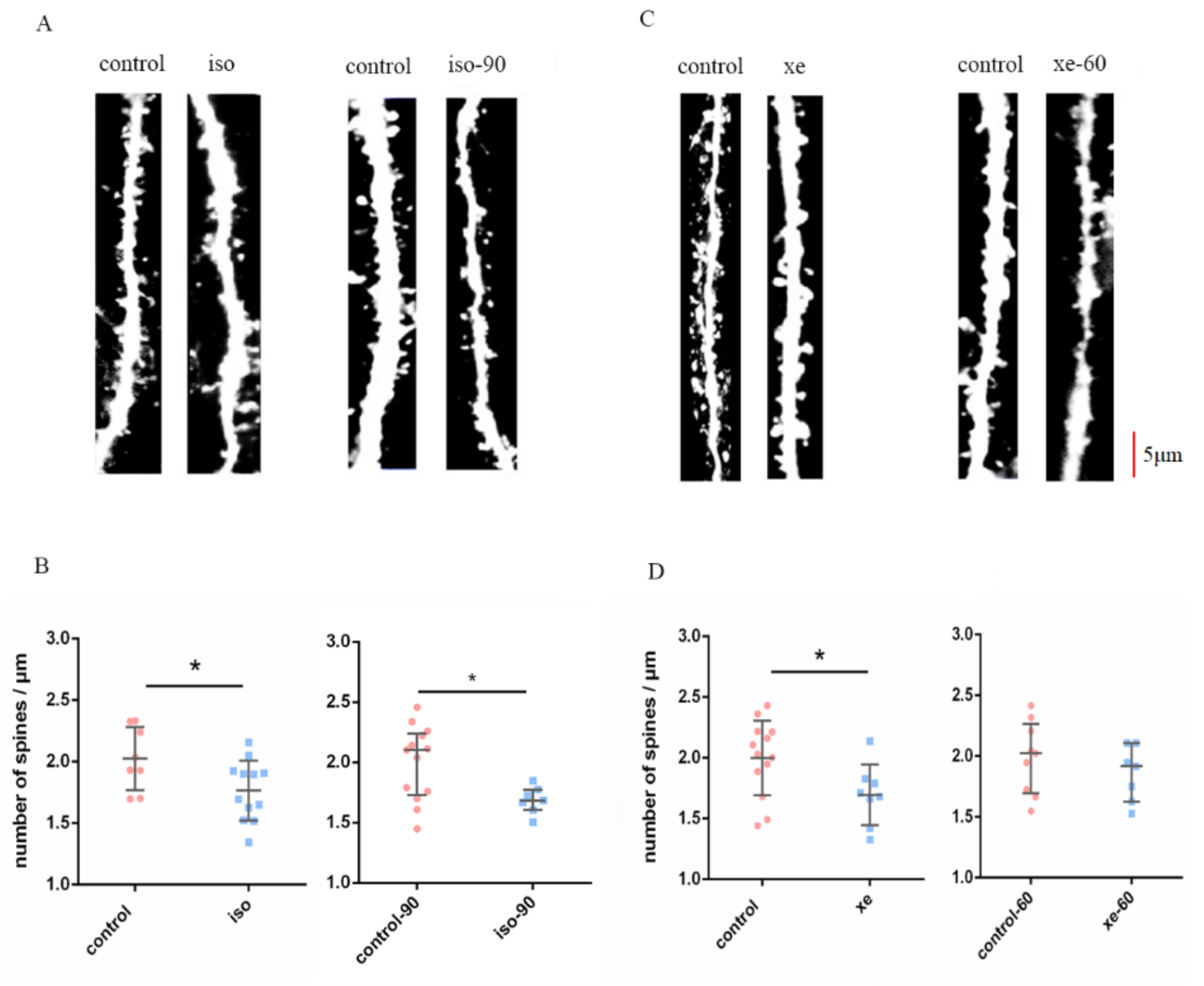
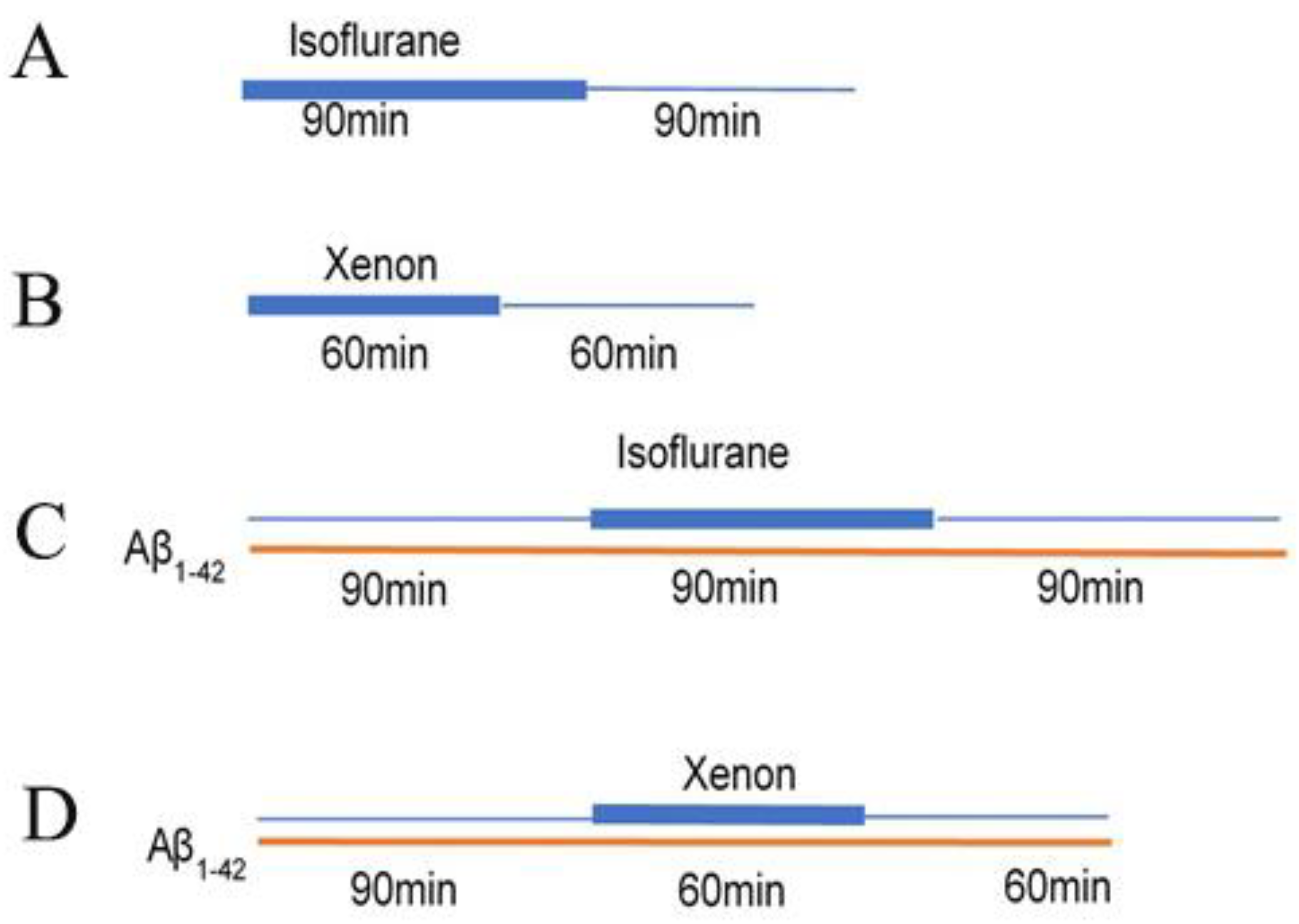
2.1. The Effect of Iso or Xe on DSD
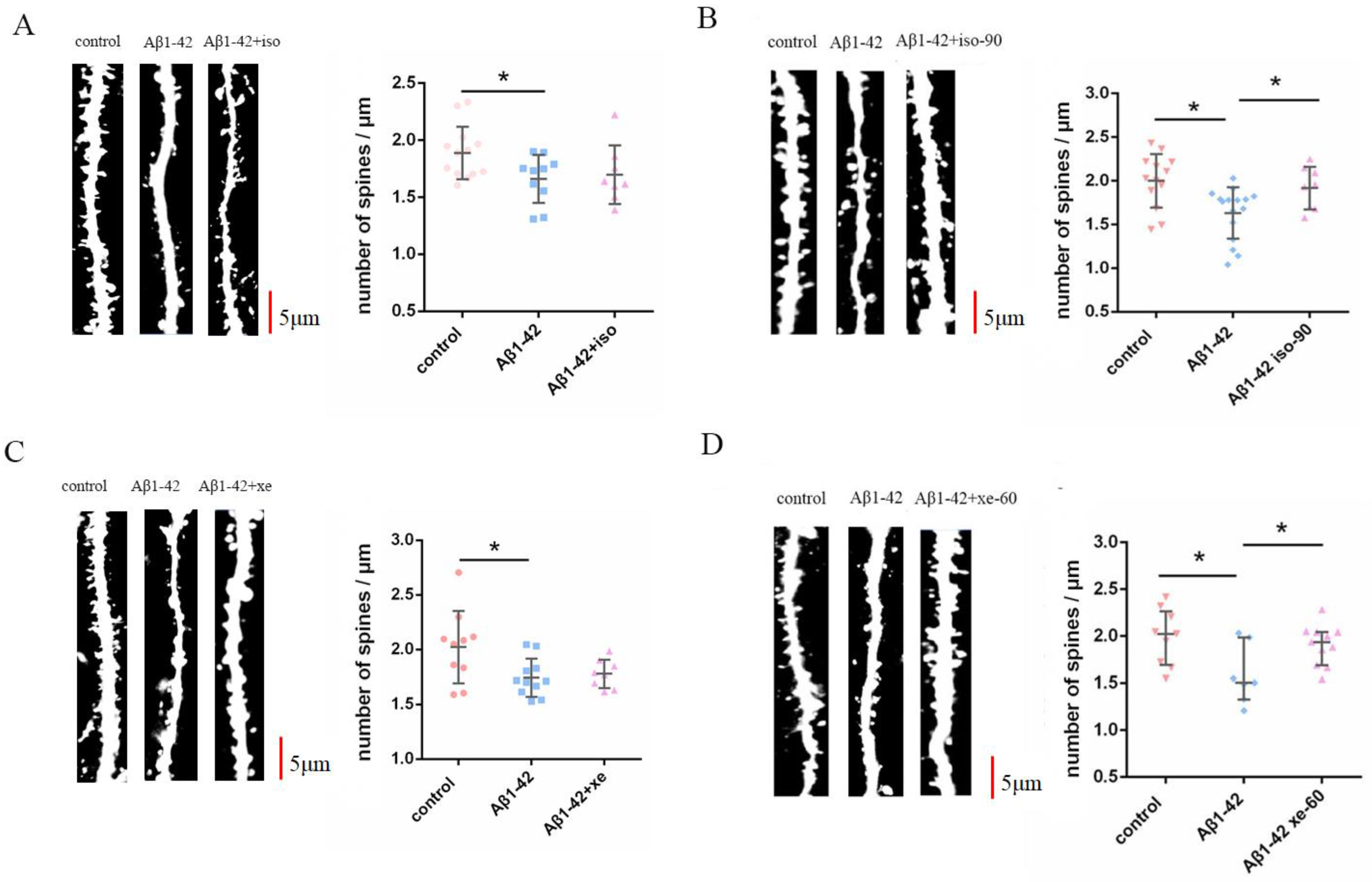
2.2. The Effect of Aβ1–42 Combined with Iso and Xe on DSD
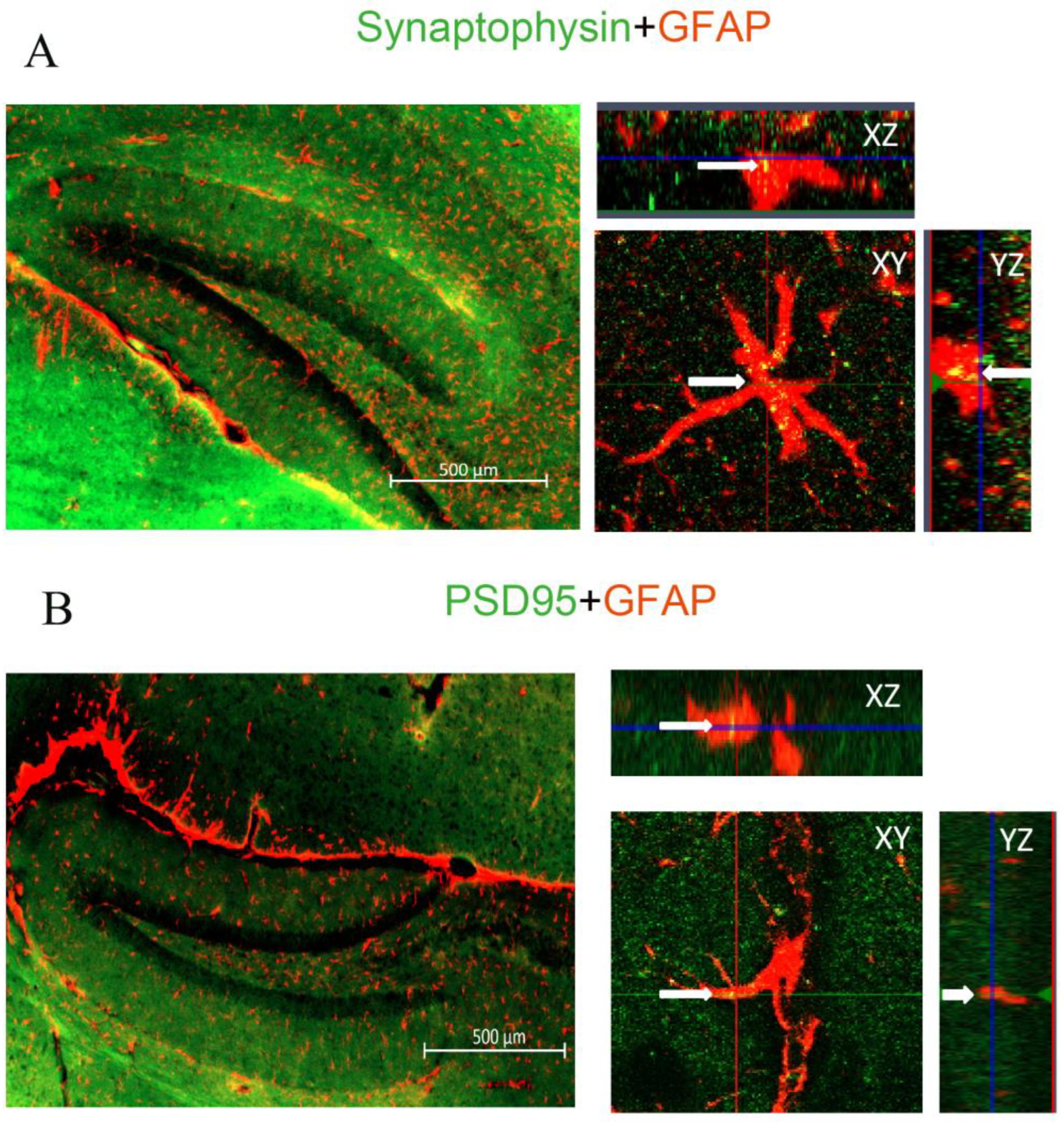
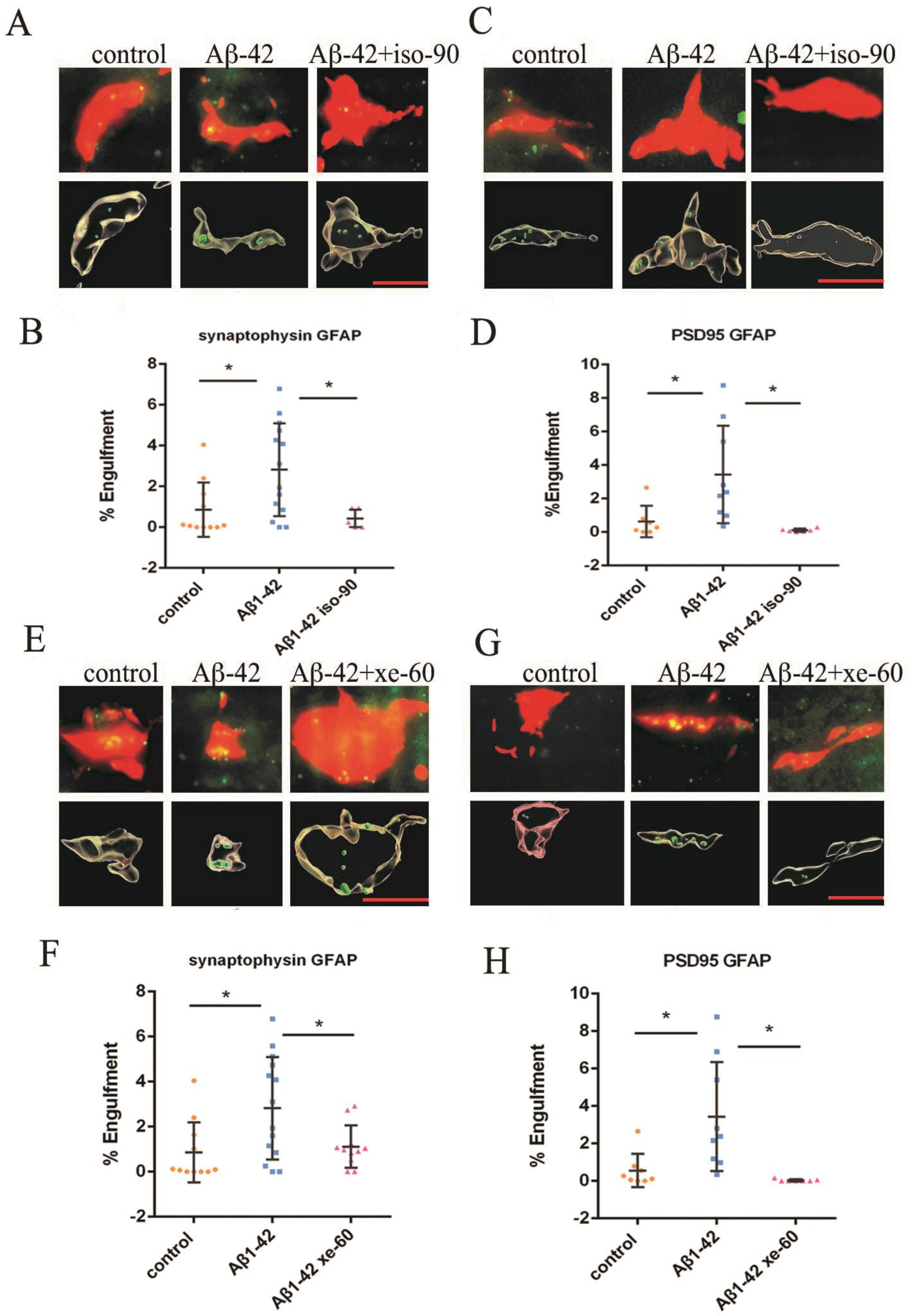
2.3. The Effect of Aβ1–42 on Astrocyte-Dependent Elimination of Synapses Could Be Reversed by Iso or Xe
2.4. Both Iso and Xe Decreased MEGF10 Expression in Astrocytes either in the Presence or in the Absent of Aβ1–42
2.5. AAV-Induced Knock-Down of MEGF10 Reduced Pre- and Postsynaptic Components inside Astrocytes
3. Discussion
4. Materials and Methods
4.1. Animals and Brain Slices Producing
4.2. In Vitro Astrocyte Culture
4.3. Amyloid Beta Application and Exposure to Anaesthetics
4.4. Analysis of Dendritic Spine Density
4.5. AAV Mediated MEGF10 Gen Knock Down
4.6. Western Blot
4.7. Immunofluorescence and Synaptic Engulfment Analysis
4.8. Three Dimensional (3D) Rendering of GFAP-Stained Astrocytes Containing Synaptic Elements
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Marsh, J.; Alifragis, P. Synaptic dysfunction in Alzheimer’s disease: The effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen. Res. 2018, 13, 616–623. [Google Scholar] [PubMed]
- Hamos, J.E.; DeGennaro, L.J.; Drachman, D.A. Synaptic loss in Alzheimer’s disease and other dementias. Neurology 1989, 39, 355–361. [Google Scholar] [CrossRef]
- Merluzzi, A.P.; Carlsson, C.M.; Johnson, S.C.; Schindler, S.E.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B. Neurodegeneration, synaptic dysfunction, and gliosis are phenotypic of Alzheimer dementia. Neurology 2018, 91, e436–e443. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid beta-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Barghorn, S.; Nimmrich, V.; Striebinger, A.; Krantz, C.; Keller, P.; Janson, B.; Bahr, M.; Schmidt, M.; Bitner, R.S.; Harlan, J.; et al. Globular amyloid beta-peptide oligomer—A homogenous and stable neuropathological protein in Alzheimer’s disease. J. Neurochem. 2005, 95, 834–847. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Klein, W.L. The Abeta oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef]
- Xia, W. Brain amyloid beta protein and memory disruption in Alzheimer’s disease. Neuropsychiatr. Dis. Treat 2010, 6, 605–611. [Google Scholar] [CrossRef]
- Masliah, E.; Crews, L.; Hansen, L. Synaptic remodeling during aging and in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9 (Suppl. 3), 91–99. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Dorostkar, M.M.; Zou, C.; Blazquez-Llorca, L.; Herms, J. Analyzing dendritic spine pathology in Alzheimer’s disease: Problems and opportunities. Acta Neuropathol. 2015, 130, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G. Elements of a neurobiological theory of hippocampal function: The role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 2006, 23, 2829–2846. [Google Scholar] [CrossRef]
- Einstein, G.; Buranosky, R.; Crain, B.J. Dendritic pathology of granule cells in Alzheimer’s disease is unrelated to neuritic plaques. J. Neurosci. 1994, 14, 5077–5088. [Google Scholar] [CrossRef]
- Williams, R.S.; Matthysse, S. Age-related changes in Down syndrome brain and the cellular pathology of Alzheimer disease. Prog. Brain Res. 1986, 70, 49–67. [Google Scholar]
- Tatebayashi, Y. The dentate gyrus neurogenesis: A common therapeutic target for Alzheimer disease and senile depression? Seishin Shinkeigaku Zasshi 2003, 105, 398–404. [Google Scholar]
- Ramaiah, R.; Lam, A.M. Postoperative cognitive dysfunction in the elderly. Anesth. Clin 2009, 27, 485–496, table of contents. [Google Scholar] [CrossRef]
- Rundshagen, I. Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 2014, 111, 119–125. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, H.; Zhao, T.; Yan, H.; Zhang, H.; Zhao, X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: The influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015, 15, 154. [Google Scholar] [CrossRef]
- Arora, S.S.; Gooch, J.L.; Garcia, P.S. Postoperative cognitive dysfunction, Alzheimer’s disease, and anesthesia. Int. J. Neurosci. 2014, 124, 236–242. [Google Scholar] [CrossRef]
- Lewis, M.C.; Nevo, I.; Paniagua, M.A.; Ben-Ari, A.; Pretto, E.; Eisdorfer, S.; Davidson, E.; Matot, I.; Eisdorfer, C. Uncomplicated general anesthesia in the elderly results in cognitive decline: Does cognitive decline predict morbidity and mortality? Med. Hypotheses 2007, 68, 484–492. [Google Scholar] [CrossRef]
- Lin, D.; Zuo, Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011, 61, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Lavaur, J.; Lemaire, M.; Pype, J.; Le Nogue, D.; Hirsch, E.C.; Michel, P.P. Neuroprotective and neurorestorative potential of xenon. Cell Death Dis. 2016, 7, e2182. [Google Scholar] [CrossRef] [PubMed]
- Bein, B.; Hocker, J.; Scholz, J. Xenon--the ideal anaesthetic agent? Anästhesiol Intensiv. Notf. Schmerzther 2007, 42, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Baskar, N.; Hunter, J.D. Xenon as an anaesthetic gas. Br. J. Hosp. Med. 2006, 67, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, S.; Mattusch, C.; Kochs, E.; Eder, M.; Haseneder, R.; Rammes, G. Xenon attenuates hippocampal long-term potentiation by diminishing synaptic and extrasynaptic N-methyl-D-aspartate receptor currents. Anesthesiology 2012, 116, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Granger, A.J.; Nicoll, R.A. Expression mechanisms underlying long-term potentiation: A postsynaptic view, 10 years on. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130136. [Google Scholar] [CrossRef]
- Chung, W.S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Chung, W.S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Fujita, Y.; Maeda, T.; Sato, C.; Sato, M.; Hatakeyama, H.; Ota, Y.; Iwabuchi, N.; Tatesawa, K.; Nomura, A.; Zou, K.; et al. Engulfment of Toxic Amyloid beta-protein in Neurons and Astrocytes Mediated by MEGF10. Neuroscience 2020, 443, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iram, T.; Ramirez-Ortiz, Z.; Byrne, M.H.; Coleman, U.A.; Kingery, N.D.; Means, T.K.; Frenkel, D.; El Khoury, J. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J. Neurosci. 2016, 36, 5185–5192. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, I.-S. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp. Mol. Med. 2017, 49, e331. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, A.; Brown, G.C. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2018, 285, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Welsh, C.A.; Barres, B.A.; Stevens, B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015, 18, 1539–1545. [Google Scholar] [CrossRef]
- Jung, Y.J.; Chung, W.S. Phagocytic Roles of Glial Cells in Healthy and Diseased Brains. Biomol. Ther. 2018, 26, 350–357. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Noh, S.; Lee, H.; Lee, S.Y.; Mun, J.Y.; Park, H.; Chung, W.S. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 2021, 590, 612–617. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Heller, C.T.; Stevens, B. An engulfment assay: A protocol to assess interactions between CNS phagocytes and neurons. J. Vis. Exp. 2014, 88, 51482. [Google Scholar] [CrossRef]
- Platholi, J.; Herold, K.F.; Hemmings, H.C., Jr.; Halpain, S. Isoflurane reversibly destabilizes hippocampal dendritic spines by an actin-dependent mechanism. PLoS ONE 2014, 9, e102978. [Google Scholar] [CrossRef]
- Haseneder, R.; Kratzer, S.; von Meyer, L.; Eder, M.; Kochs, E.; Rammes, G. Isoflurane and sevoflurane dose-dependently impair hippocampal long-term potentiation. Eur. J. Pharmacol. 2009, 623, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Mattusch, C.; Kratzer, S.; Buerge, M.; Kreuzer, M.; Engel, T.; Kopp, C.; Biel, M.; Hammelmann, V.; Ying, S.W.; Goldstein, P.A.; et al. Impact of Hyperpolarization-activated, Cyclic Nucleotide-gated Cation Channel Type 2 for the Xenon-mediated Anesthetic Effect: Evidence from In Vitro and In Vivo Experiments. Anesthesiology 2015, 122, 1047–1059, Erratum in Anesthesiology 2017, 127, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Suwa, K.; Uezono, S.; Ichinose, F.; Uchiyama, M.; Morita, S. The blood-gas partition coefficient of xenon may be lower than generally accepted. Br. J. Anaesth. 1998, 80, 255–256. [Google Scholar] [CrossRef]
- Esper, T.; Wehner, M.; Meinecke, C.D.; Rueffert, H. Blood/Gas partition coefficients for isoflurane, sevoflurane, and desflurane in a clinically relevant patient population. Obstet. Anesthesia Dig. 2015, 120, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Franks, N.P.; Lieb, W.R. Effects of temperature and volatile anesthetics on GABA(A) receptors. Anesthesiology 1999, 90, 484–491. [Google Scholar] [CrossRef]
- Jones, M.V.; Brooks, P.A.; Harrison, N.L. Enhancement of gamma-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J. Physiol. 1992, 449, 279–293. [Google Scholar] [CrossRef]
- Haseneder, R.; Kratzer, S.; Kochs, E.; Hofelmann, D.; Auberson, Y.; Eder, M.; Rammes, G. The xenon-mediated antagonism against the NMDA receptor is non-selective for receptors containing either NR2A or NR2B subunits in the mouse amygdala. Eur. J. Pharmacol. 2009, 619, 33–37. [Google Scholar] [CrossRef]
- Weigt, H.U.; Fohr, K.J.; Georgieff, M.; Georgieff, E.M.; Senftleben, U.; Adolph, O. Xenon blocks AMPA and NMDA receptor channels by different mechanisms. Acta NeuroBiol. Exp. 2009, 69, 429–440. [Google Scholar]
- Rammes, G.; Seeser, F.; Mattusch, K.; Zhu, K.; Haas, L.; Kummer, M.; Heneka, M.; Herms, J.; Parsons, C.G. The NMDA receptor antagonist Radiprodil reverses the synaptotoxic effects of different amyloid-beta (Abeta) species on long-term potentiation (LTP). Neuropharmacology 2018, 140, 184–192. [Google Scholar] [CrossRef]
- Shrestha, B.R.; Vitolo, O.V.; Joshi, P.; Lordkipanidze, T.; Shelanski, M.; Dunaevsky, A. Amyloid beta peptide adversely affects spine number and motility in hippocampal neurons. Mol. Cell. Neurosci. 2006, 33, 274–282. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.O.; Ip, N.Y. Structural plasticity of dendritic spines: The underlying mechanisms and its dysregulation in brain disorders. Biochim. Biophys. Acta 2013, 1832, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Toni, N.; Buchs, P.A.; Nikonenko, I.; Bron, C.R.; Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 1999, 402, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Engert, F.; Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 1999, 399, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Rammes, G.; Gravius, A.; Ruitenberg, M.; Wegener, N.; Chambon, C.; Sroka-Saidi, K.; Jeggo, R.; Staniaszek, L.; Spanswick, D.; O’Hare, E.; et al. MRZ-99030—A novel modulator of Abeta aggregation: II—Reversal of Abeta oligomer-induced deficits in long-term potentiation (LTP) and cognitive performance in rats and mice. Neuropharmacology 2015, 92, 170–182. [Google Scholar] [CrossRef]
- Birnbaum, J.H.; Bali, J.; Rajendran, L.; Nitsch, R.M.; Tackenberg, C. Calcium flux-independent NMDA receptor activity is required for Abeta oligomer-induced synaptic loss. Cell Death Dis. 2015, 6, e1791. [Google Scholar] [CrossRef]
- Whitcomb, D.J.; Hogg, E.L.; Regan, P.; Piers, T.; Narayan, P.; Whitehead, G.; Winters, B.L.; Kim, D.H.; Kim, E.; St George-Hyslop, P.; et al. Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus. Sci. Rep. 2015, 5, 10934. [Google Scholar] [CrossRef]
- Ulrich, D. Amyloid-beta Impairs Synaptic Inhibition via GABA(A) Receptor Endocytosis. J. Neurosci. 2015, 35, 9205–9210. [Google Scholar] [CrossRef]
- Lin, L.H.; Chen, L.L.; Zirrolli, J.A.; Harris, R.A. General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acidA receptors expressed by Xenopus oocytes: Lack of involvement of intracellular calcium. J. Pharmacol. Exp. Ther. 1992, 263, 569–578. [Google Scholar]
- Drakew, A.; Muller, M.; Gahwiler, B.H.; Thompson, S.M.; Frotscher, M. Spine loss in experimental epilepsy: Quantitative light and electron microscopic analysis of intracellularly stained CA3 pyramidal cells in hippocampal slice cultures. Neuroscience 1996, 70, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Galofre, M.; Sunol, C. Reduction of glutamatergic neurotransmission by prolonged exposure to dieldrin involves NMDA receptor internalization and metabotropic glutamate receptor 5 downregulation. Toxicol. Sci. 2010, 113, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Gerace, E.; Zianni, E.; Landucci, E.; Scartabelli, T.; Berlinguer Palmini, R.; Iezzi, D.; Moroni, F.; Di Luca, M.; Mannaioni, G.; Gardoni, F.; et al. Differential mechanisms of tolerance induced by NMDA and 3,5-dihydroxyphenylglycine (DHPG) preconditioning. J. Neurochem. 2020, 155, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.H.; Zhang, S.; Gan, W.B. Dendritic spine dynamics. Annu. Rev. Physiol. 2009, 71, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Losi, G.; Mariotti, L.; Carmignoto, G. GABAergic interneuron to astrocyte signalling: A neglected form of cell communication in the brain. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130609. [Google Scholar] [CrossRef]
- Kirchhoff, F. Analysis of Functional NMDA Receptors in Astrocytes. Methods Mol. Biol. 2017, 1677, 241–251. [Google Scholar][Green Version]
- Fan, D.; Grooms, S.Y.; Araneda, R.C.; Johnson, A.B.; Dobrenis, K.; Kessler, J.A.; Zukin, R.S. AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J. Neurosci. Res. 1999, 57, 557–571. [Google Scholar] [CrossRef]
- Hoft, S.; Griemsmann, S.; Seifert, G.; Steinhauser, C. Heterogeneity in expression of functional ionotropic glutamate and GABA receptors in astrocytes across brain regions: Insights from the thalamus. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130602. [Google Scholar] [CrossRef]
- Seifert, G.; Steinhauser, C. Ionotropic glutamate receptors in astrocytes. Prog. Brain Res. 2001, 132, 287–299. [Google Scholar]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef]
- Bramham, C.R. Local protein synthesis, actin dynamics, and LTP consolidation. Curr. Opin. NeuroBiol. 2008, 18, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Haseneder, R.; Kratzer, S.; Kochs, E.; Eckle, V.S.; Zieglgansberger, W.; Rammes, G. Xenon reduces N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology 2008, 109, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.C.; Choi, V.W.; Samulski, R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006, 1, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H.; Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2006, 1, 3166–3173. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Wong, J.K.Y.; Zhu, K.; Noakes, P.G.; Rammes, G. The Anaesthetics Isoflurane and Xenon Reverse the Synaptotoxic Effects of Aβ1–42 on Megf10-Dependent Astrocytic Synapse Elimination and Spine Density in Ex Vivo Hippocampal Brain Slices. Int. J. Mol. Sci. 2023, 24, 912. https://doi.org/10.3390/ijms24020912
Shi D, Wong JKY, Zhu K, Noakes PG, Rammes G. The Anaesthetics Isoflurane and Xenon Reverse the Synaptotoxic Effects of Aβ1–42 on Megf10-Dependent Astrocytic Synapse Elimination and Spine Density in Ex Vivo Hippocampal Brain Slices. International Journal of Molecular Sciences. 2023; 24(2):912. https://doi.org/10.3390/ijms24020912
Chicago/Turabian StyleShi, Dai, Jaime K. Y. Wong, Kaichuan Zhu, Peter G. Noakes, and Gerhard Rammes. 2023. "The Anaesthetics Isoflurane and Xenon Reverse the Synaptotoxic Effects of Aβ1–42 on Megf10-Dependent Astrocytic Synapse Elimination and Spine Density in Ex Vivo Hippocampal Brain Slices" International Journal of Molecular Sciences 24, no. 2: 912. https://doi.org/10.3390/ijms24020912
APA StyleShi, D., Wong, J. K. Y., Zhu, K., Noakes, P. G., & Rammes, G. (2023). The Anaesthetics Isoflurane and Xenon Reverse the Synaptotoxic Effects of Aβ1–42 on Megf10-Dependent Astrocytic Synapse Elimination and Spine Density in Ex Vivo Hippocampal Brain Slices. International Journal of Molecular Sciences, 24(2), 912. https://doi.org/10.3390/ijms24020912





