Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin
Abstract
1. Introduction
2. Results
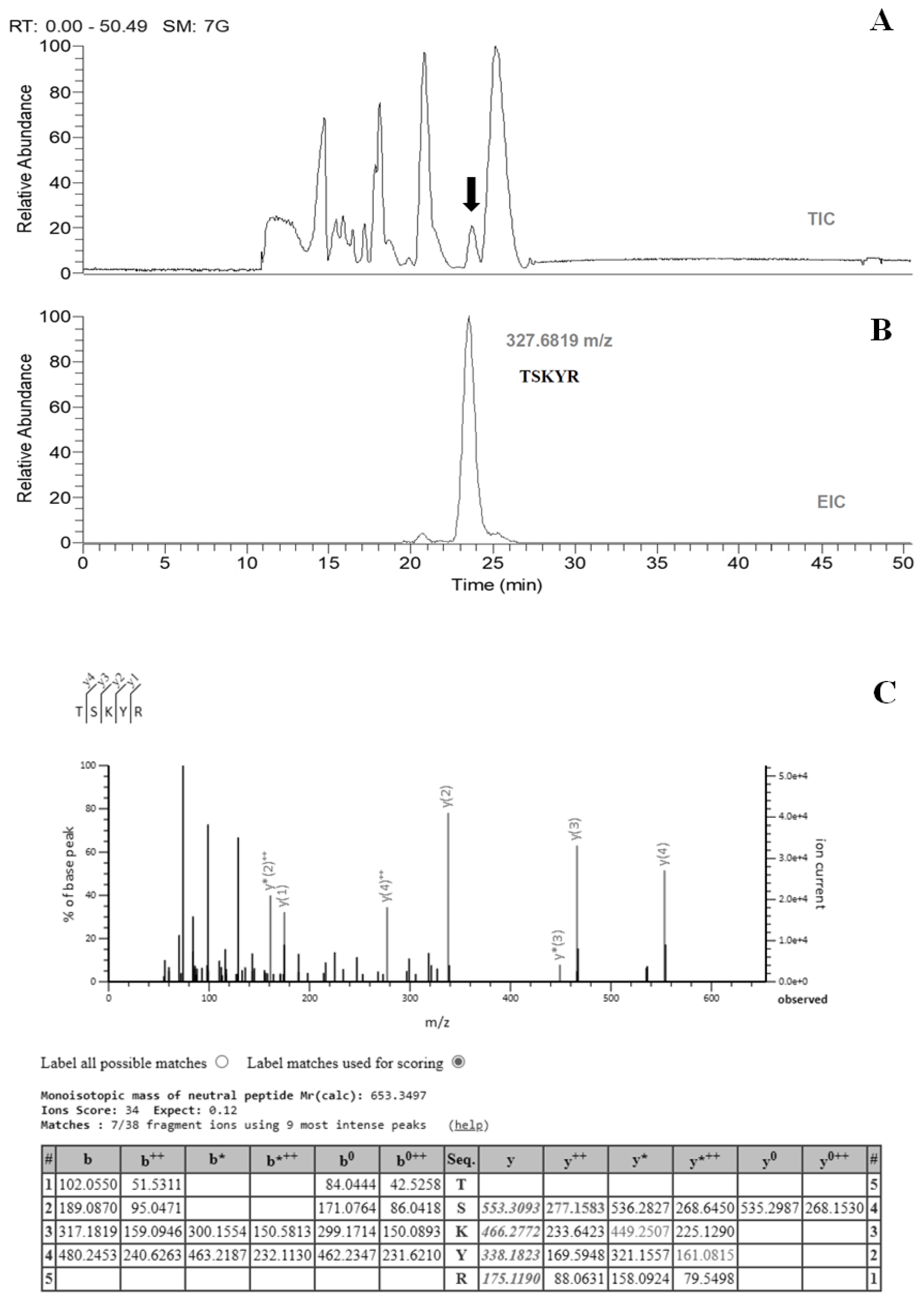
2.1. Sample Preparation, Separation, and Identification
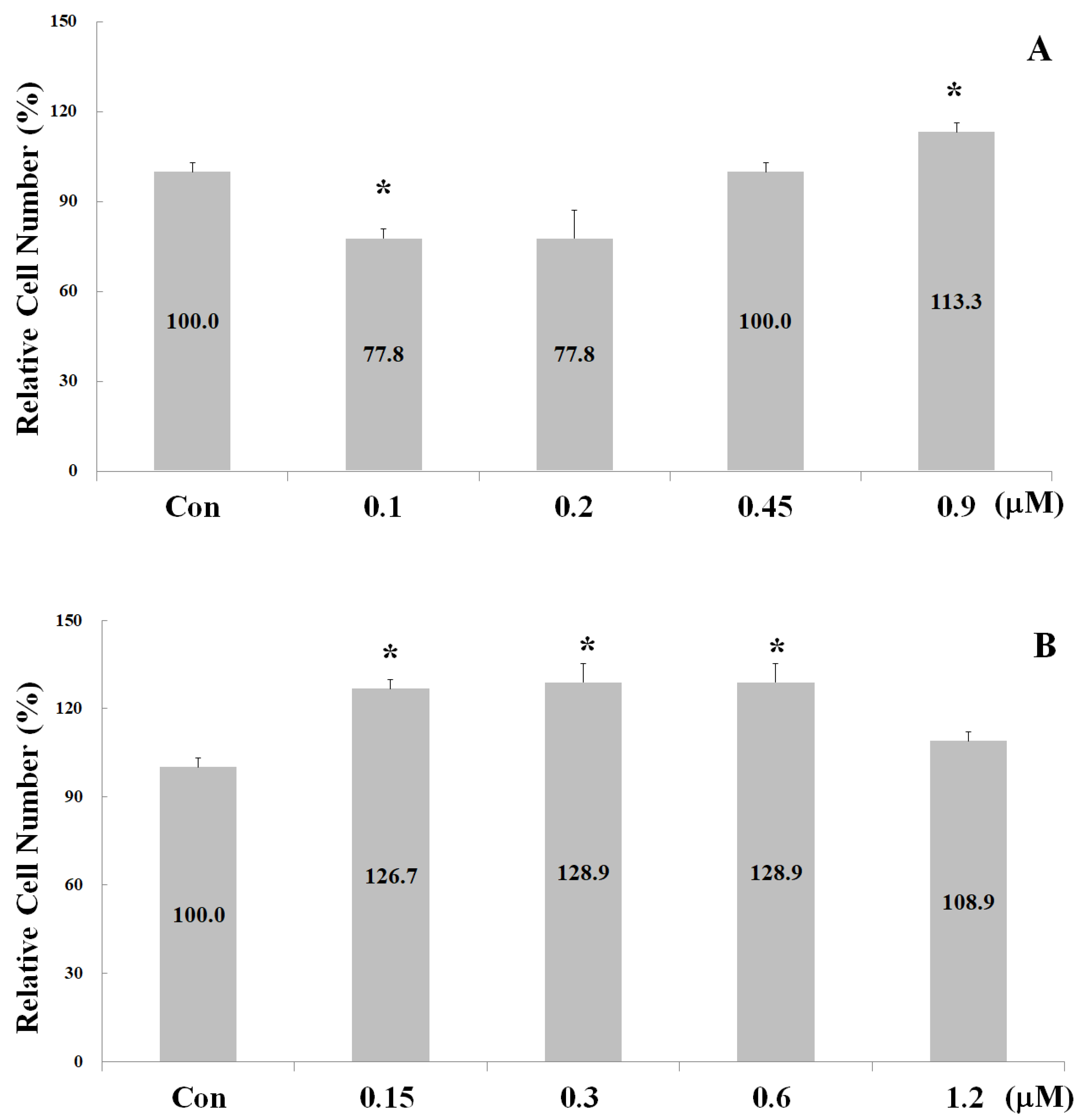
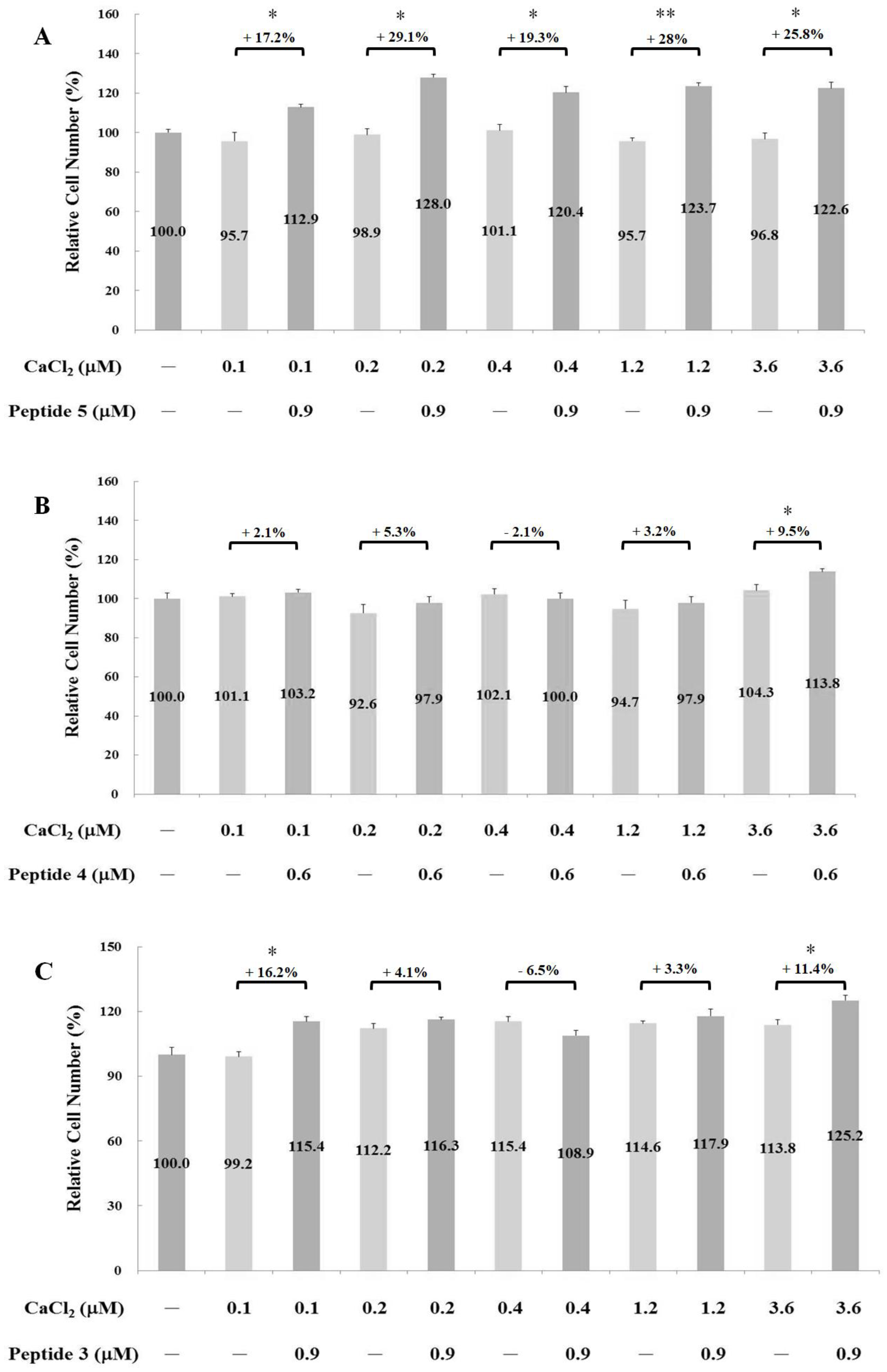
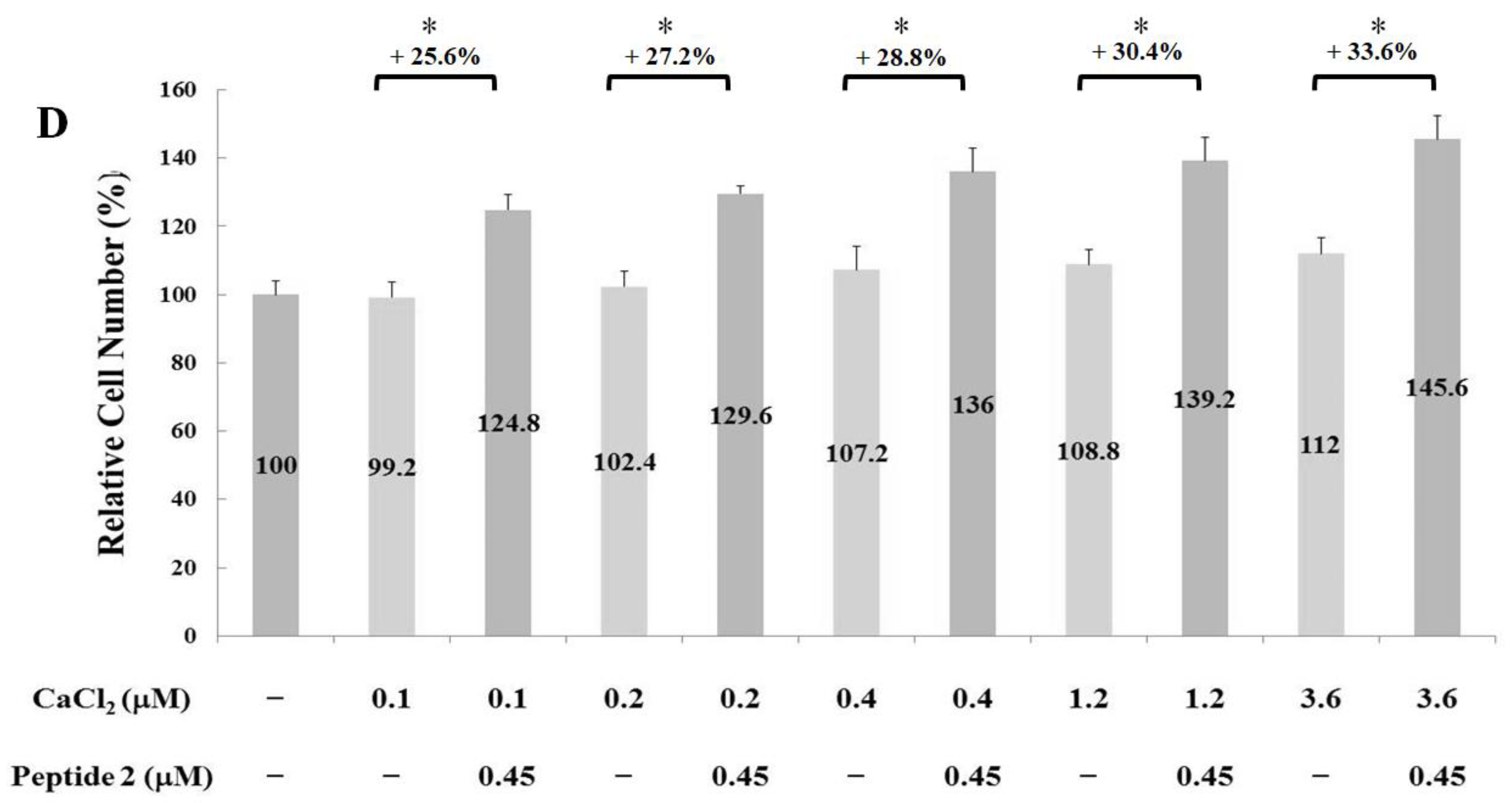
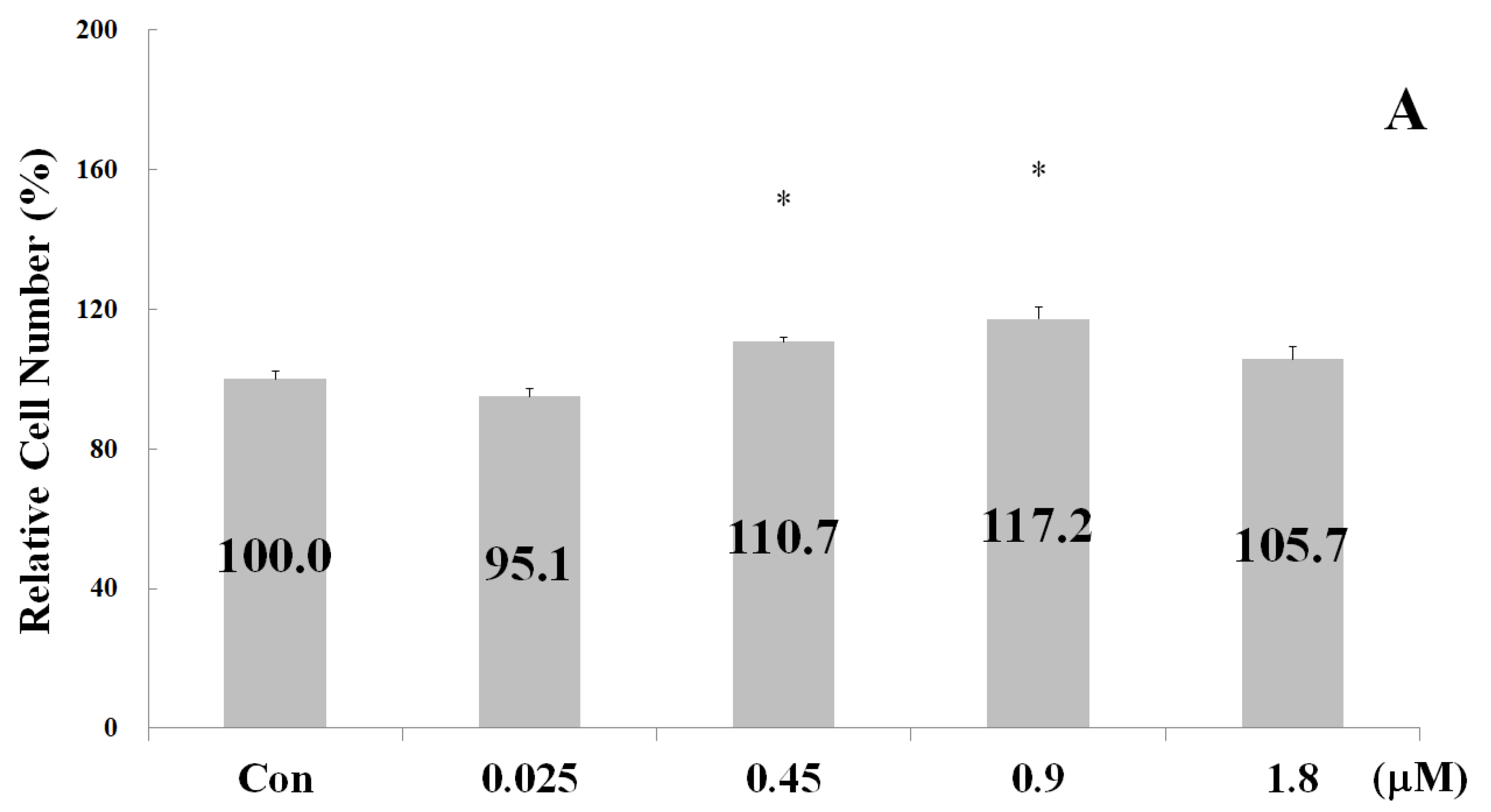
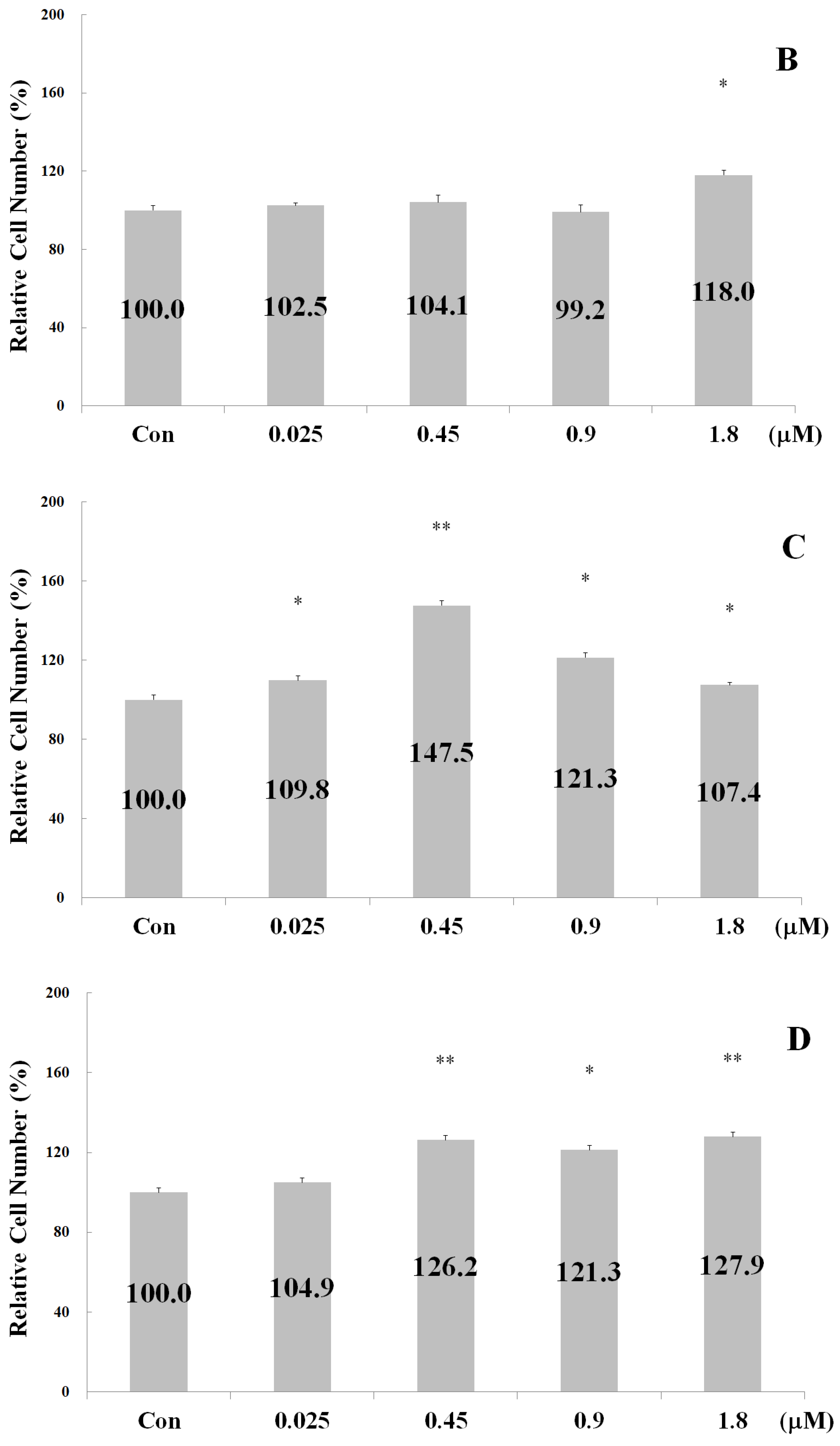
2.2. Measurements of Osteoblast Proliferation-Stimulating Effect
2.3. Measurement of Chondrocyte Proliferation-Stimulating Effect
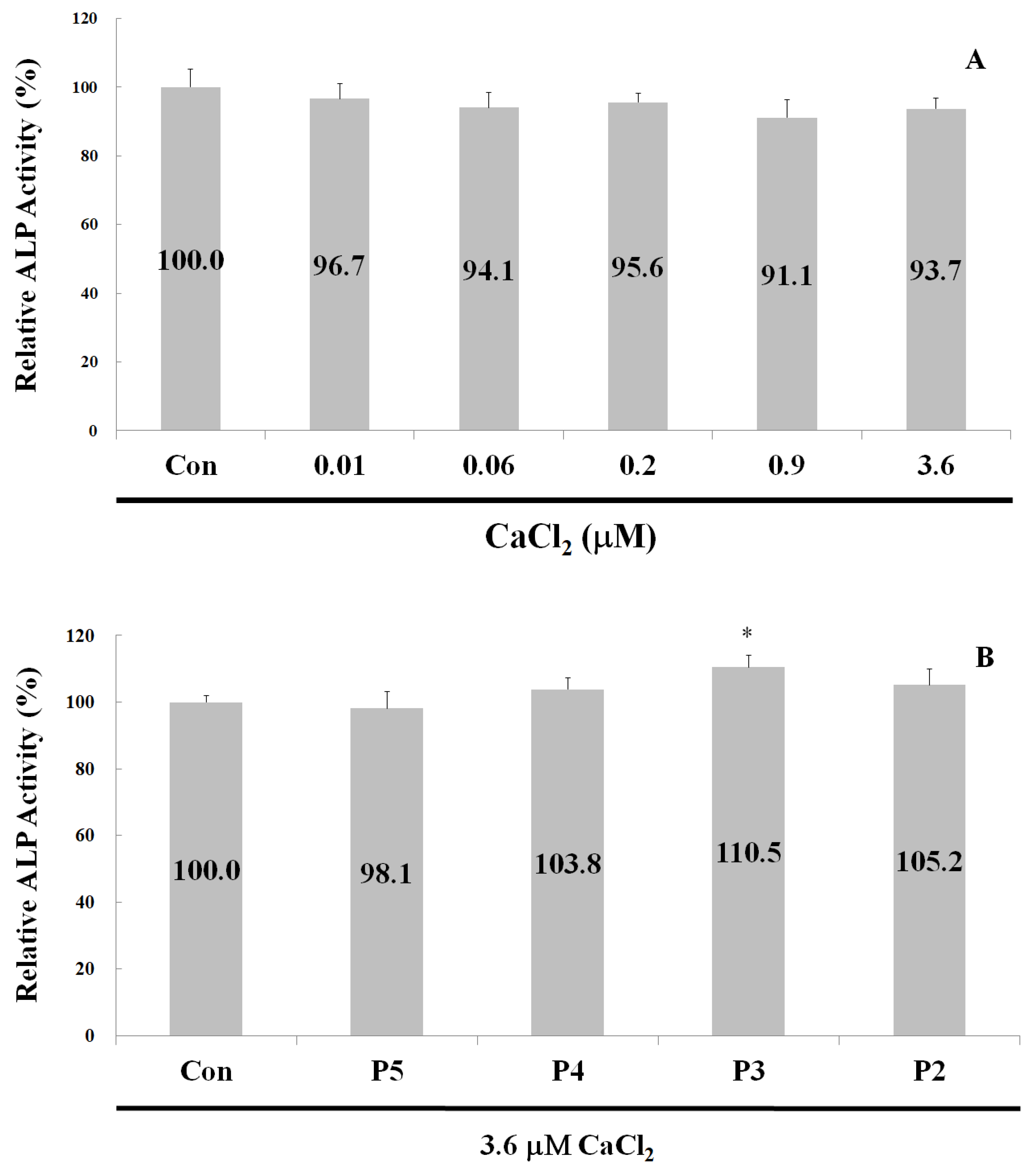
2.4. Alkaline Phosphatase Activity
2.5. Mineralized Nodule Formation
2.6. Gene Expression
3. Discussions
4. Materials and Methods
4.1. Materials
4.2. Separation of Bioactive Peptides by Reverse-Phase High-Performance Liquid Chromatography (HPLC)
4.3. Identification of Bioactive Peptides by Liquid Chromatography–Mass Spectrometry (LC-MS)
4.4. Cell Culture
4.5. Measurement of Cell Proliferation
4.6. Alkaline Phosphatase Activity Assay (ALP)
4.7. Quantification of Bone Nodules
4.8. Reverse Transcription and Quantitative Polymerase Chain Reaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Martín, S.; González-Cantalapiedra, A.; Muñoz, F.; García-González, M.; Permuy, M.; López-Peña, M. Glucosamine and Chondroitin Sulfate: Is There Any Scientific Evidence for Their Effectiveness as Disease-Modifying Drugs in Knee Osteoarthritis Preclinical Studies? A Systematic Review from 2000 to 2021. Animals 2021, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.A.; Yeh, Y.C.; Chang, Z.Y. The efficacy and safety of traditional Chinese medicine Guilu Erxian Jiao in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Complement. Clin. Pr. 2022, 46, 101515. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Yao, Y.; Ma, Y.; Guo, Y.; Yin, H. Effectiveness and safety of Guilu Erxian Glue (a traditional Chinese medicinal product) for the treatment of postmenopausal osteoporosis: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e20773. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chou, Y.Y.; Chen, Y.M.; Tang, Y.J.; Ho, H.C.; Chen, D.Y. Effect of the herbal drug guilu erxian jiao on muscle strength, articular pain, and disability in elderly men with knee osteoarthritis. Evid. Based Complement. Altern. Med. 2014, 2014, 297458. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, N.; Suliburska, J. Nutritional and health factors affecting the bioavailability of calcium: A narrative review. Nutr. Rev. 2021, 79, 1307–1320. [Google Scholar] [CrossRef]
- O’Connor, S.; Solazzo, C.; Collins, M. Advances in identifying archaeological traces of horn and other keratinous hard tissues. Stud. Conserv. 2015, 60, 393–417. [Google Scholar] [CrossRef]
- Woods, J.L.; Harland, D.P.; Vernon, J.A.; Krsinic, G.L.; Walls, R.J. Morphology and ultrastructure of antler velvet hair and body hair from red deer (Cervus elaphus). J. Morphol. 2011, 272, 34–49. [Google Scholar] [CrossRef]
- Kito, H.; Ohya, S. Role of K(+) and Ca(2+)-Permeable Channels in Osteoblast Functions. Int. J. Mol. Sci. 2021, 22, 10459. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakamura-Takahashi, A.; Kasahara, M.; Yamaguchi, A.; Azuma, T. Tissue-nonspecific alkaline phosphatase promotes the osteogenic differentiation of osteoprogenitor cells. Biochem. Biophys. Res. Commun. 2020, 524, 702–709. [Google Scholar] [CrossRef]
- Kawane, T.; Qin, X.; Jiang, Q.; Miyazaki, T.; Komori, H.; Yoshida, C.A.; Matsuura-Kawata, V.; Sakane, C.; Matsuo, Y.; Nagai, K.; et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci. Rep. 2018, 8, 13551. [Google Scholar] [CrossRef]
- Dambroise, E.; Ktorza, I.; Brombin, A.; Abdessalem, G.; Edouard, J.; Luka, M.; Fiedler, I.; Binder, O.; Pelle, O.; Patton, E.E.; et al. Fgfr3 Is a Positive Regulator of Osteoblast Expansion and Differentiation During Zebrafish Skull Vault Development. J. Bone Min. Res. 2020, 35, 1782–1797. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef]
- Zhou, T.; Yan, Y.; Zhao, C.; Xu, Y.; Wang, Q.; Xu, N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019, 24, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Miraoui, H.; Sévère, N. FGF/FGFR signaling in bone formation: Progress and perspectives. Growth Factors 2012, 30, 117–123. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, H.; Qi, F.; Liu, H.; Gao, L.; Wang, X. Local delivery of insulin/IGF-1 for bone regeneration: Carriers, strategies, and effects. Nanotheranostics 2020, 4, 242–255. [Google Scholar] [CrossRef]
- Xu, C.; Xie, X.; Zhao, H.; Wu, Y.; Wang, J.; Feng, J.Q. TGF-Beta Receptor II Is Critical for Osteogenic Progenitor Cell Proliferation and Differentiation During Postnatal Alveolar Bone Formation. Front. Physiol. 2021, 12, 721775. [Google Scholar] [CrossRef]
- Qiu, J.; Huang, G.; Na, N.; Chen, L. MicroRNA-214-5p/TGF-β/Smad2 signaling alters adipogenic differentiation of bone marrow stem cells in postmenopausal osteoporosis. Mol. Med. Rep. 2018, 17, 6301–6310. [Google Scholar] [CrossRef]
- Li, Y.; Tian, A.Y.; Ophene, J.; Tian, M.Y.; Yao, Z.; Chen, S.; Li, H.; Sun, X.; Du, H. TGF-β Stimulates Endochondral Differentiation after Denervation. Int. J. Med. Sci. 2017, 14, 382–389. [Google Scholar] [CrossRef]
- Qin, Y.; Tang, S.; Zhen, G.; Ding, Q.; Ding, S.; Cao, X. Bone-targeted delivery of TGF-β type 1 receptor inhibitor rescues uncoupled bone remodeling in Camurati-Engelmann disease. Ann. N. Y. Acad. Sci. 2018, 1433, 29–40. [Google Scholar] [CrossRef]
- Ueda, H. Review of Kyotorphin Research: A Mysterious Opioid Analgesic Dipeptide and Its Molecular, Physiological, and Pharmacological Characteristics. Front. Med. Technol. 2021, 3, 662697. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Shiomi, H.; Ueda, H.; Amano, H. A novel analgesic dipeptide from bovine brain is a possible Met-enkephalin releaser. Nature 1979, 282, 410–412. [Google Scholar] [CrossRef]
- Kiso, Y.; Kitagawa, K.; Kawai, N.; Akita, T.; Takagi, H.; Amano, H.; Fukui, K. Neo-kyotorphin (Thr-Ser-Lys-Tyr-Arg), a new analgesic peptide. FEBS Lett. 1983, 155, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yoshihara, Y.; Fukushima, N.; Shiomi, H.; Nakamura, A.; Takagi, H. Kyotorphin (tyrosine-arginine) synthetase in rat brain synaptosomes. J. Biol. Chem. 1987, 262, 8165–8173. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Tamura, S.; Fukushima, N.; Katada, T.; Ui, M.; Satoh, M. Inositol 1,4,5-trisphosphate-gated calcium transport through plasma membranes in nerve terminals. J. Neurosci. 1996, 16, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhang, Y.; Yan, W.; Zheng, X. [Effects of serum containing oral liquid of Guilu-Erxian on the therapy of osteoporosis at the cellular level]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2008, 25, 897–902. [Google Scholar] [PubMed]
- Yin, H. Experiment Based on Regulation of TGF-b/SMADS Signal Transduction Pathway by Gui Lu Er Xian Jiao which Instumulate Osteoblast Synthesis of Type I Collagen to Improve Steoporosis. Ph.D. Dissertation, Nanjing University of Chinese Medicine, Nanjing, China, 2016; pp. 79–84. (In Chinese). [Google Scholar]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.Y.; Wen, J. Improved RP-HPLC method for determination of bovine lactoferrin and its proteolytic degradation in simulated gastrointestinal fluids. Biomed. Chromatogr. 2013, 27, 197–202. [Google Scholar] [CrossRef]
- Kang, L.; Weng, N.; Jian, W. LC-MS bioanalysis of intact proteins and peptides. Biomed. Chromatogr. 2020, 34, e4633. [Google Scholar] [CrossRef]
- Tong, S.; Xue, L.; Xu, D.P.; Liu, Z.M.; Du, Y.; Wang, X.K. In vitro culture of hFOB1.19 osteoblast cells on TGF-β1-SF-CS three-dimensional scaffolds. Mol. Med. Rep. 2016, 13, 181–187. [Google Scholar] [CrossRef]
- Finger, F.; Schörle, C.; Soder, S.; Zien, A.; Goldring, M.B.; Aigner, T. Phenotypic characterization of human chondrocyte cell line C-20/A4: A comparison between monolayer and alginate suspension culture. Cells Tissues Organs 2004, 178, 65–77. [Google Scholar] [CrossRef]
- Tsai, R.K.; Chang, C.H.; Hseu, C.M.; Chang, S.M.; Wu, J.R.; Wang, H.Z.; Wu, W.C.; Wu, W.S. Ethambutol induces PKC-dependent cytotoxic and antiproliferative effects on human retinal pigment cells. Exp. Eye Res. 2008, 87, 594–603. [Google Scholar] [CrossRef]
- Wehner, C.; Lettner, S.; Moritz, A.; Andrukhov, O.; Rausch-Fan, X. Effect of bisphosphonate treatment of titanium surfaces on alkaline phosphatase activity in osteoblasts: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 125. [Google Scholar] [CrossRef]
- Chaves Neto, A.H.; Machado, D.; Yano, C.L.; Ferreira, C.V. Antioxidant defense and apoptotic effectors in ascorbic acid and β-glycerophosphate-induced osteoblastic differentiation. Dev. Growth Differ. 2011, 53, 88–96. [Google Scholar] [CrossRef]
- Blick, C.; Ramachandran, A.; Wigfield, S.; McCormick, R.; Jubb, A.; Buffa, F.M.; Turley, H.; Knowles, M.A.; Cranston, D.; Catto, J.; et al. Hypoxia regulates FGFR3 expression via HIF-1α and miR-100 and contributes to cell survival in non-muscle invasive bladder cancer. Br. J. Cancer 2013, 109, 50–59. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Deberg, M.A.; Piccardi, N.; Msika, P.; Reginster, J.Y.L.; Henrotin, Y.E. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2005, 13, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, G.; Yu, L.; Feng, Y.; Li, X.; Zhang, Z.; Wang, Y.; Sun, D.; Pradhan, S. High-efficiency generation of induced pluripotent mesenchymal stem cells from human dermal fibroblasts using recombinant proteins. Stem Cell Res. Ther. 2016, 7, 99. [Google Scholar] [CrossRef] [PubMed]





| First Separation | Second Separation for LC-MS Analysis | |||
|---|---|---|---|---|
| Time (min) | Eluent (B%) | Time (min) | Eluent (B%) | Eluent (C%) |
| 0–15 | 0 | 0–10 | 0 | 0 |
| 15–20 | 0–15 | 10–25 | 0–100 | 0 |
| 20–40 | 15 | 25–30 | 100 | 80–100 |
| 40–45 | 15–20 | 30–40 | 100–0 | 0–100 |
| 45–75 | 20 | 40–45 | 100 | |
| 75–80 | 20–100 | 45–60 | 100–0 | |
| 80–90 | 100 | |||
| 90–100 | 100–0 | |||
| Gene | Primer Sequence | Reference |
|---|---|---|
| Runx2 | FORWARD: TACTTCGTCAGCATCCTATCA REVERSE: TTCCGTCAGCGTCAACAC | [13] |
| OCN | FORWARD: GAGGGCAATAAGGTAGTGAA REVERSE: CATAGATGCGTTTGTAGGC | [13] |
| FGFR2 | FORWARD: GAGAAGGAGATCACGGCTTC REVERSE: AAGTCTGGCTTCTTGGTCGT | [17,18,19] |
| FGFR3 | FORWARD: GCCTCCTCGGAGTCCTTG REVERSE: GCCTCCTCGGAGTCCTTG | [35] |
| ACAN | FORWARD: CACCTCCCCAACAGATGCTT REVERSE: GGTACTTGTTCCAGCCCTCC | [36] |
| COL2A1 | FORWARD: TGCTGCCCAGATGGCTGGAGGA REVERSE: TGCCTTGAAATCCTTGAGGCCC | [37] |
| β-actin | FORWARD: AGAGCTACGAGCTGCCTGAC REVERSE: AGCACTGTGTTGGCGTACA | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, T.-J.; Lin, J.-H.; Lin, S.Z.; Tsai, W.-T.; Wu, J.-R.; Chen, H.-P. Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin. Int. J. Mol. Sci. 2023, 24, 1759. https://doi.org/10.3390/ijms24021759
Ho T-J, Lin J-H, Lin SZ, Tsai W-T, Wu J-R, Chen H-P. Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin. International Journal of Molecular Sciences. 2023; 24(2):1759. https://doi.org/10.3390/ijms24021759
Chicago/Turabian StyleHo, Tsung-Jung, Jung-Hsing Lin, Shinn Zong Lin, Wan-Ting Tsai, Jia-Ru Wu, and Hao-Ping Chen. 2023. "Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin" International Journal of Molecular Sciences 24, no. 2: 1759. https://doi.org/10.3390/ijms24021759
APA StyleHo, T.-J., Lin, J.-H., Lin, S. Z., Tsai, W.-T., Wu, J.-R., & Chen, H.-P. (2023). Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin. International Journal of Molecular Sciences, 24(2), 1759. https://doi.org/10.3390/ijms24021759






