The Effects of GCSF Primary Prophylaxis on Survival Outcomes and Toxicity in Patients with Advanced Non-Small Cell Lung Cancer on First-Line Chemoimmunotherapy: A Sub-Analysis of the Spinnaker Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| AEs | adverse events |
| NSCLC | non-small-cell lung cancer |
| ECOG | PS Eastern Cooperative Oncology Group performance status |
| ICI | immune checkpoint inhibitors |
| irAEs | immune-related AEs |
| LCPI | Lung Cancer Prognostic Index |
| LIPS | Lung Immuno-oncology Prognostic Score |
| NHS-Lung score | N = number of metastatic sites (cut-off ≥ 3) H = histology (i.e., squamous) S = SII (≥1440) |
| NLR | neutrophils-to-lymphocytes ratio |
| OS | overall survival |
| SII | systemic immune-inflammatory index |
| PD-L1 | programmed death ligand-1 |
| PFS | progression-free survival |
| TPS | tumour proportion score |
References
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef]
- Aapro, M.S.; Bohlius, J.; Cameron, D.A.; Dal Lago, L.; Donnelly, J.P.; Kearney, N.; Lyman, G.H.; Pettengell, R.; Tjan-Heijnen, V.C.; Walewski, J.; et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer 2011, 47, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Addeo, A.; Von Garnier, C.; Blackhall, F.; Planchard, D.; Felip, E.; Dziadziuszko, R.; de Marinis, F.; Reck, M.; Bouchaab, H.; et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: Lung cancer. ESMO Open 2020, 5, e000820. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.; Curioni-Fontecedro, A.; Friedlaender, A.; Addeo, A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: Primum non nocere. ESMO Open 2019, 4, e000765. [Google Scholar] [CrossRef] [PubMed]
- Nawar, T.; Morjaria, S.; Kaltsas, A.; Patel, D.; Perez-Johnston, R.; Daniyan, A.F.; Mailankody, S.; Parameswaran, R. Granulocyte-colony stimulating factor in COVID-19: Is it stimulating more than just the bone marrow? Am. J. Hematol. 2020, 95, E210–E213. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Zuccaro, V.; Ferraris, E.; Rizzo, G.; Tancredi, R.J.; Pedrazzoli, P. How to Use Prophylactic G-CSF in the Time of COVID-19. JCO Oncol. Pract. 2020, 16, 771–772. [Google Scholar] [CrossRef]
- Kaisar-Iluz, N.; Arpinati, L.; Shaul, M.E.; Mahroum, S.; Qaisi, M.; Tidhar, E.; Fridlender, Z.G. The Bilateral Interplay between Cancer Immunotherapies and Neutrophils’ Phenotypes and Sub-Populations. Cells 2022, 11, 783. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Wilkins, C.R.; Mones, J.V.; Mauro, M.J. A Novel Paradigm Between Leukocytosis, G-CSF Secretion, Neutrophil-to-Lymphocyte Ratio, Myeloid-Derived Suppressor Cells, and Prognosis in Non-small Cell Lung Cancer. Front. Oncol. 2019, 9, 295. [Google Scholar] [CrossRef]
- Zer, A.; Sung, M.R.; Walia, P.; Khoja, L.; Maganti, M.; Labbe, C.; Shepherd, F.A.; Bradbury, P.A.; Feld, R.; Liu, G.; et al. Correlation of Neutrophil to Lymphocyte Ratio and Absolute Neutrophil Count with Outcomes with PD-1 Axis Inhibitors in Patients with Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, 426–434. [Google Scholar] [CrossRef]
- Takeda, T.; Takeuchi, M.; Saitoh, M.; Takeda, S. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac. Cancer 2018, 9, 1291–1299. [Google Scholar] [CrossRef]
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.-W.; Seog Heo, D.S.; Lee, J.S. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol. Immunother. 2018, 67, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Signori, A.; Stellato, M.; Santini, D.; Maruzzo, M.; De Giorgi, U.; Pedrazzoli, P.; Galli, L.; Zucali, P.A.; Fantinel, E.; et al. The prognostic value of baseline and early variations of peripheral blood inflammatory ratios and their cellular components in patients with metastatic renal cell carcinoma treated with nivolumab: The Δ-Meet-URO analysis. Front. Oncol. 2022, 12, 955501. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, L.; Banna, O.C.; Muthuramalingam, S.; Cave, J.; Comins, C.; Cortellini, A.; Addeo, A.; Signori, A.; McKenzie, H.; Escriu, C.; et al. Efficacy outcomes and prognostic factors from real-world patients with advanced non-small-cell lung cancer treated with first-line chemoimmunotherapy: The Spinnaker retrospective study. Int. Immunopharmacol. 2022, 110, 108985. [Google Scholar]
- Fujita, A.; Ohkubo, T.; Hoshino, H.; Takabatake, H.; Tagaki, S.; Sekine, K.; Abe, S. Phase II study of cisplatin, ifosfamide, and irinotecan with rhG-CSF support in patients with stage IIIb and IV non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Kornek, G.V.; Raderer, M.; Ulrich-Pur, H.; Fiebiger, W.C.; Marosi, L.; Schneeweiss, B.; Greul, R.; Scheithauer, W. Treatment of patients with advanced nonsmall cell lung carcinoma using docetaxel and gemcitabine plus granulocyte-colony stimulating factor. Cancer 2000, 89, 516–522. [Google Scholar] [CrossRef]
- Alexander, M.; Wolfe, R.; Ball, D.; Conron, M.; Stirling, R.G.; Solomon, B.; MacManus, M.; Officer, A.; Karnam, S.; Burbury, K.; et al. Lung cancer prognostic index: A risk score to predict overall survival after the diagnosis of non-small-cell lung cancer. Br. J. Cancer 2017, 117, 744–751. [Google Scholar] [CrossRef]
- Banna, G.L.; Cortellini, A.; Cortinovis, D.L.; Tiseo, M.; Aerts, J.G.J.V.; Barbieri, F.; Giusti, R.; Bria, E.; Grossi, F.; Pizzutilo, P.; et al. The lung immuno-oncology prognostic score (LIPS-3): A prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer. ESMO Open 2021, 6, 100078. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, F.; Zhou, Q.; Li, Y.; Wu, J.; Wang, R.; Song, Q. Prognostic significance of PD-L1 in advanced non-small cell lung carcinoma. Medicine 2020, 99, e23172. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Zhan, P.; Song, Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: A meta-analysis. Transl. Lung Cancer Res. 2015, 4, 203–208. [Google Scholar] [CrossRef]
- Jung, J.H.; Shin, D.W.; Kim, J.; Lee, J.C.; Hwang, J.H. Primary Granulocyte Colony-Stimulating Factor Prophylaxis in Metastatic Pancreatic Cancer Patients Treated with FOLFIRINOX as the First-Line Treatment. Cancers 2020, 12, 3137. [Google Scholar] [CrossRef]
- Straus, D.; Collins, G.; Walewski, J.; Zinzani, P.L.; Grigg, A.; Sureda, A.; Illes, A.; Kim, T.M.; Alekseev, S.; Specht, L.; et al. Primary prophylaxis with G-CSF may improve outcomes in patients with newly diagnosed stage III/IV Hodgkin lymphoma treated with brentuximab vedotin plus chemotherapy. Leuk. Lymphoma 2020, 61, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Ruste, V.; Goldschmidt, V.; Laparra, A.; Messayke, S.; Danlos, F.-X.; Romano-Martin, P.; Champiat, S.; Voisin, A.-L.; Baldini, C.; Massard, C.; et al. The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: A prospective study of the French REISAMIC registry. Eur. J. Cancer 2021, 158, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
| Characteristic | G-CSF (No. 59) No. (%) [Range] | No G-CSF Unmatched (No. 249) No. (%) [Range] | χ2 Test (Log-Rank) p-Value | No G-CSF Matched a (No. 114) No. (%) [Range] | χ2 Test (Log-Rank) p-Value |
|---|---|---|---|---|---|
| Age | 0.591 | 0.919 | |||
| ≥70 years | 21 (36) | 77 (31) | 40 (35) | ||
| <70 years | 38 (64) | 172 (69) | 74 (65) | ||
| Gender | 0.714 | 0.463 | |||
| Male | 31 (53) | 140 (56) | 68 (60) | ||
| Female | 28 (47) | 109 (44) | 46 (40) | ||
| Smoking history | 0.494 | 0.951 | |||
| Never | 3 (5) | 22 (9) | 7 (6) | ||
| Former/Current | 56 (95) | 227 (91) | 107 (94) | ||
| Histology | 0.550 | 0.643 | |||
| Squamous | 12 (21) | 39 (16) | 27 (25) | ||
| Adenocarcinoma | 46 (79) | 200 (84) | 80 (75) | ||
| Other | 1 (2) | 10 (4) | 7 (6) | ||
| ECOG PS | 0.351 | 0.161 | |||
| 0 | 28 (47) | 99 (40) | 39 (35) | ||
| 1 | 31 (53) | 150 (60) | 72 (65) | ||
| Stage | 0.275 | 0.654 | |||
| IIIB/IVA | 22 (37) | 115 (46) | 48 (42) | ||
| IVB | 37 (63) | 134 (54) | 66 (58) | ||
| BMI | 0.306 | 0.713 | |||
| Underweight/Normal | 27 (46) | 135 (54) | 57 (50) | ||
| Overweight/Obese | 32 (54) | 114 (46) | 57 (50) | ||
| Number of metastatic sites | 0.706 | 0.935 | |||
| <3 | 41 (69) | 164 (66) | 80 (70) | ||
| ≥3 | 18 (31) | 85 (34) | 34 (30) | ||
| Brain metastases | 6 (19) | 25 (10) | 0.833 | 13 (11) | 0.992 |
| Liver metastases | 4 (7) | 33 (13) | 0.249 | 14 (12) | 0.389 |
| PD-L1 IHC Ab b | 0.495 | 0.416 | |||
| Negative | 34 (61) | 131 (55) | 58 (53) | ||
| Positive | 22 (39) | 109 (45) | 52 (47) | ||
| NA | 3 (5) | 9 (4) | 4 (4) | ||
| Pre-treatment steroids | 6 (10) | 27 (11) | 0.933 | 13 (11) | 0.992 |
| Pre-treatment NLR ≥ 4 | 34 (58) | 130 (52) | 0.545 | 59 (52) | 0.566 |
| Pre-treatment SII ≥ 1440 | 30 (51) | 124 (50) | 1.000 | 58 (51) | 0.875 |
| Type of chemotherapy | 0.007 | 0.867 | |||
| Cisplatin–Pemetrexed | 3 (5) | 21 (8) | 4 (4) | ||
| Carboplatin–Pemetrexed | 45 (76) | 195 (78) | 87 (76) | ||
| Carboplatin–Paclitaxel | 11 (19) | 33 (13) | 23 (20) | ||
| Best response c | 0.744 | 0.706 | |||
| CR/PR | 37 (65) | 161 (68) | 68 (64) | ||
| SD | 12 (21) | 40 (17) | 19 (18) | ||
| PD | 8 (14) | 37 (16) | 20 (19) | ||
| NA | 1 (2) | 11 (4) | |||
| G1/2 AE–G1/2 irAE | 33 (56)–24 (41) | 86 (35)–76 (31) | 0.004–0.199 | 29 (25)–26 (23) | <0.001–0.023 |
| G3/4 AE–G3/4 irAE | 14 (24)–9 (15) | 48 (19)–40 (16) | 0.537–0.993 | 18 (16)–17 (15) | 0.285–0.869 |
| Treatment discontinuation | 14 (24) | 58 (23) | 0.920 | 19 ()17) | 0.359 |
| Follow-up, median, mo. [95%] | 12.8 [12.3–13.3] | 20.7 [19.0–22.5] | (<0.001) | 13.9 [9.9–17.8] | 0.533 |
| Deaths | 29 (49) | 151 (61) | 0.143 | 56 (49) | 0.875 |
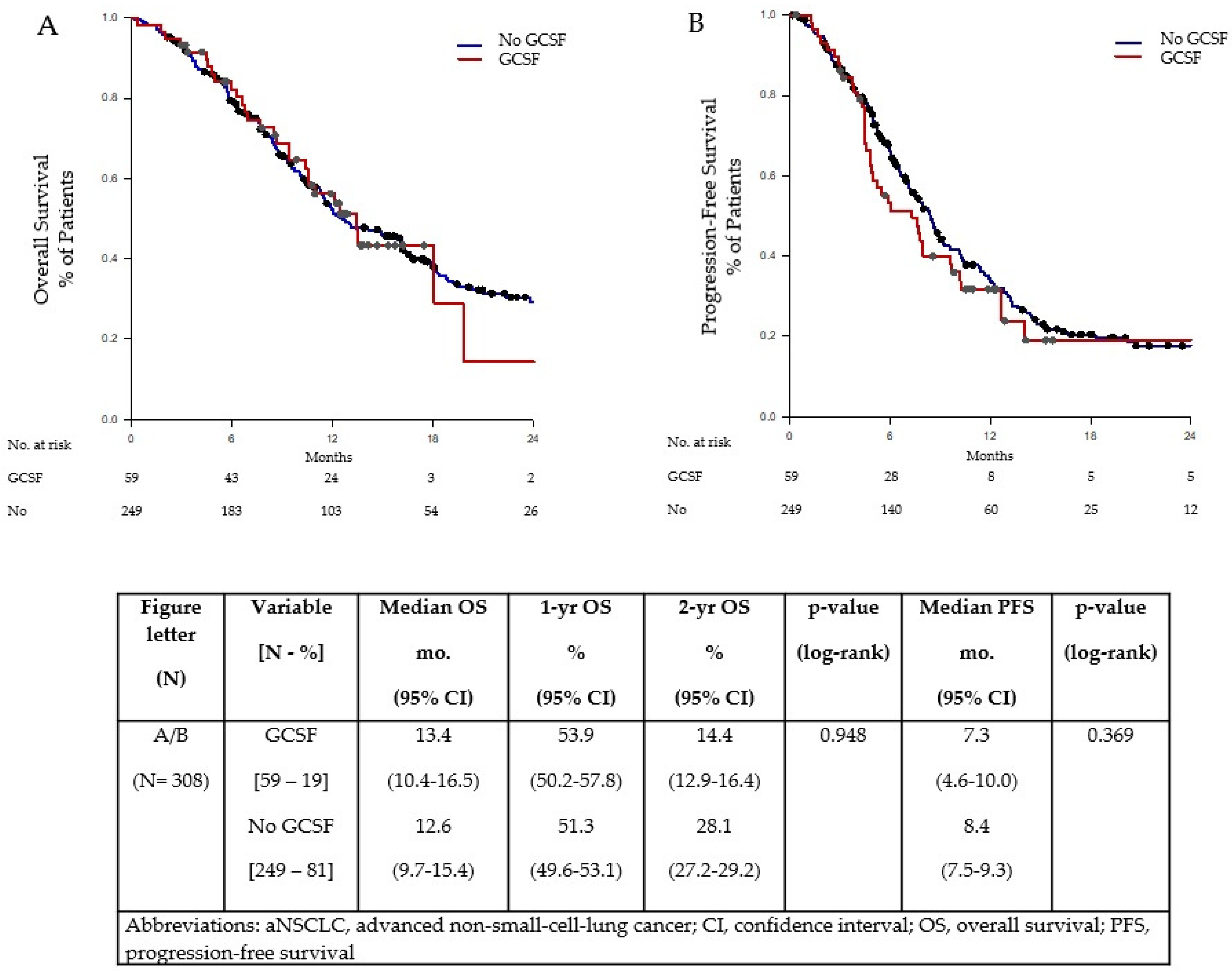
| OS, median, mo. [95% CI] | 13.4 [10.4–16.5] | 12.6 [9.7–15.4] | (0.948) | 11.3 [9.0–13.5] | 0.357 |
| 1 yr OS [95% CI] | 53.9 [50.2–57.8] | 51.3 [49.6–53.1] | 44.7 [42.3–47.3] | ||
| 2 yr OS [95% CI] | 14.4 [12.9–16.4] | 28.1 [27.2–29.2] | 18.2 [16.1–20.9] | ||
| PFS, median, mo. [95% CI] | 7.3 [4.6–10.0] | 8.4 [7.5–9.3] | (0.369) | 7.5 [5.8–9.2] | 0.832 |
| COVID-19-positive | 2 (3) | 8 (3) | 0.734 | 3 (3) | 0.844 |
| COVID-19 deaths | |||||
| Death rate | 1 (3) | 7 (5) | 0.836 | 2 (4) | 0.555 |
| Positive rate | 1 (50) | 7 (88) | 0.843 | 1 (33) | 0.576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anpalakhan, S.; Huddar, P.; Behrouzi, R.; Signori, A.; Cave, J.; Comins, C.; Cortellini, A.; Addeo, A.; Escriu, C.; McKenzie, H.; et al. The Effects of GCSF Primary Prophylaxis on Survival Outcomes and Toxicity in Patients with Advanced Non-Small Cell Lung Cancer on First-Line Chemoimmunotherapy: A Sub-Analysis of the Spinnaker Study. Int. J. Mol. Sci. 2023, 24, 1746. https://doi.org/10.3390/ijms24021746
Anpalakhan S, Huddar P, Behrouzi R, Signori A, Cave J, Comins C, Cortellini A, Addeo A, Escriu C, McKenzie H, et al. The Effects of GCSF Primary Prophylaxis on Survival Outcomes and Toxicity in Patients with Advanced Non-Small Cell Lung Cancer on First-Line Chemoimmunotherapy: A Sub-Analysis of the Spinnaker Study. International Journal of Molecular Sciences. 2023; 24(2):1746. https://doi.org/10.3390/ijms24021746
Chicago/Turabian StyleAnpalakhan, Shobana, Prerana Huddar, Roya Behrouzi, Alessio Signori, Judith Cave, Charles Comins, Alessio Cortellini, Alfredo Addeo, Carles Escriu, Hayley McKenzie, and et al. 2023. "The Effects of GCSF Primary Prophylaxis on Survival Outcomes and Toxicity in Patients with Advanced Non-Small Cell Lung Cancer on First-Line Chemoimmunotherapy: A Sub-Analysis of the Spinnaker Study" International Journal of Molecular Sciences 24, no. 2: 1746. https://doi.org/10.3390/ijms24021746
APA StyleAnpalakhan, S., Huddar, P., Behrouzi, R., Signori, A., Cave, J., Comins, C., Cortellini, A., Addeo, A., Escriu, C., McKenzie, H., Barone, G., Murray, L., Bhatnagar, G., Pinato, D. J., Ottensmeier, C., Gomes, F., & Banna, G. L. (2023). The Effects of GCSF Primary Prophylaxis on Survival Outcomes and Toxicity in Patients with Advanced Non-Small Cell Lung Cancer on First-Line Chemoimmunotherapy: A Sub-Analysis of the Spinnaker Study. International Journal of Molecular Sciences, 24(2), 1746. https://doi.org/10.3390/ijms24021746









