DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation
Abstract
1. Introduction
2. Results
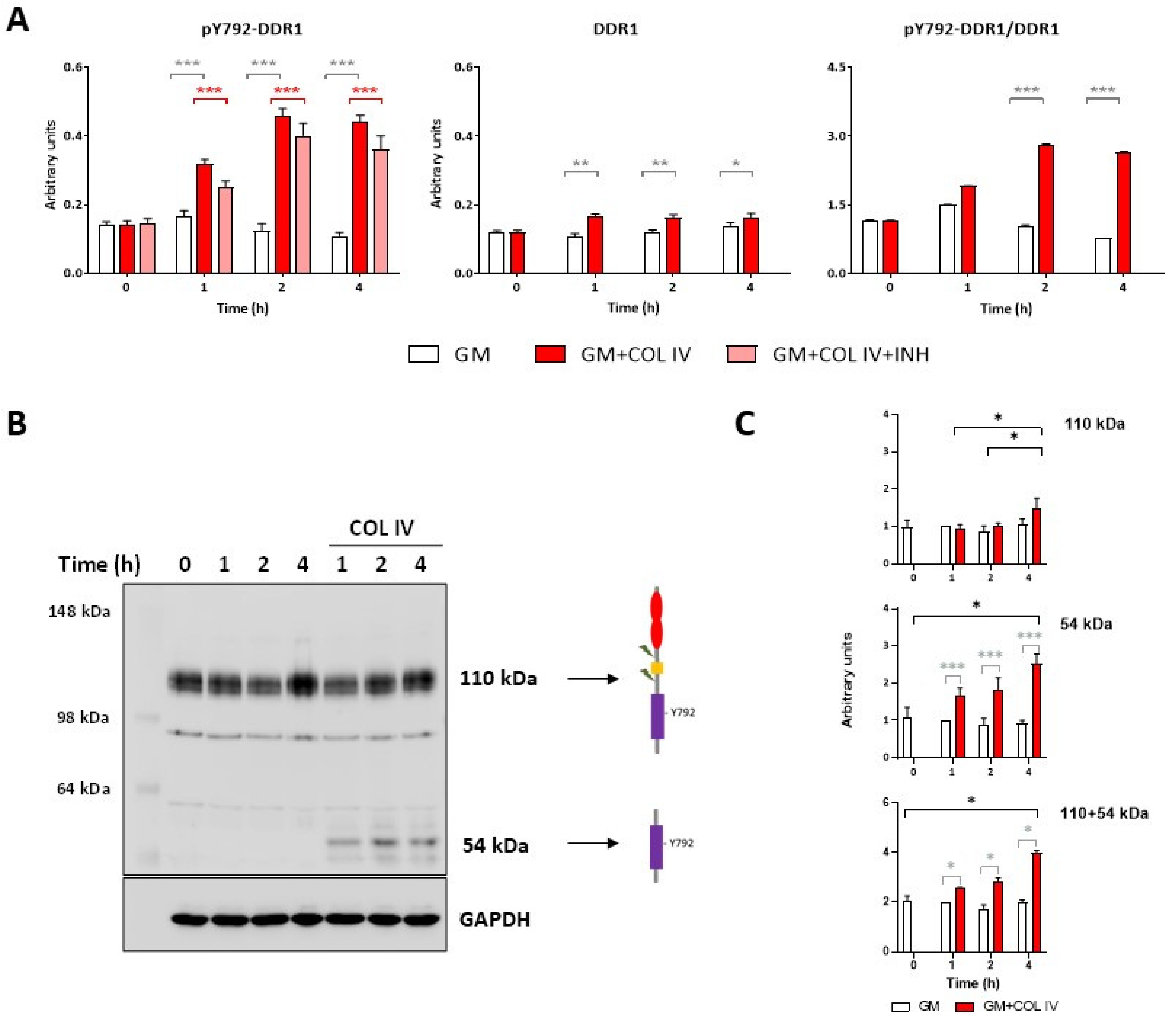
2.1. Collagen IV Induces Y792-DDR1 Phosphorylation in HOG16 Oligodendroglial Cells
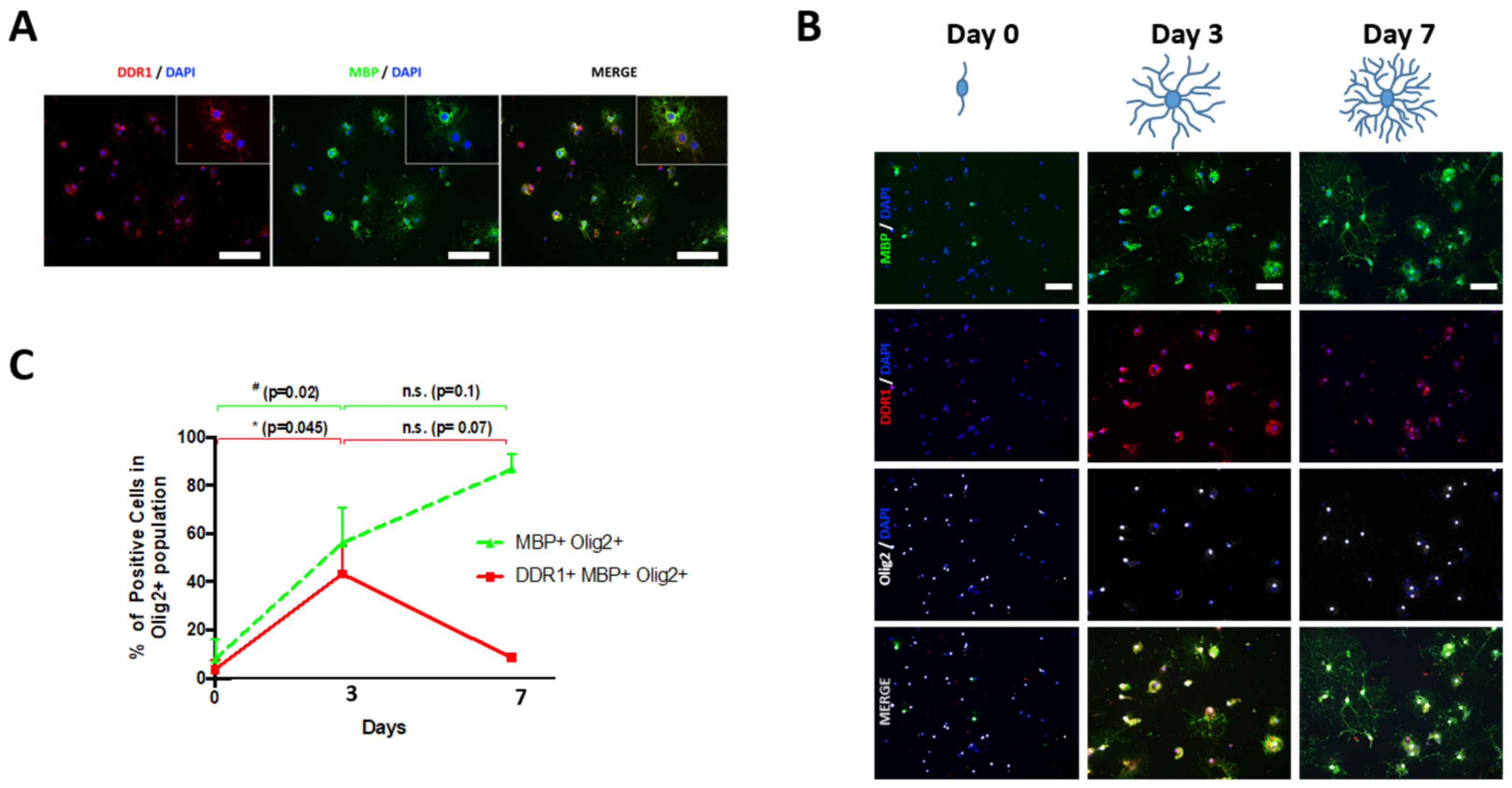
2.2. DDR1 Is Temporally Expressed in Newly Formed OLs Derived from NSCs
2.3. Collagen IV Promotes Branching in Newly Formed OLs Derived from NSCs
3. Discussion
4. Materials and Methods
4.1. HOG16 Cell Line and Culture
4.2. Adult Neural Stem Cell Cultures
4.3. Primary OL Cultures Derived from Adult Rat NSCs
4.4. ELISA
4.5. RT-qPCR
4.6. Western Blot
4.7. Immunocytofluorescence
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nave, K.-A.; Werner, H.B. Myelination of the Nervous System: Mechanisms and Functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Origin and Development of Glial Cells in Peripheral Nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, N.A.; Fuss, B. Extracellular Cues Influencing Oligodendrocyte Differentiation and (Re)Myelination. Exp. Neurol. 2016, 283, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Piao, X. Adhesion G-Protein Coupled Receptors and Extracellular Matrix Proteins: Roles in Myelination and Glial Cell Development. Dev. Dyn. 2017, 246, 275–284. [Google Scholar] [CrossRef]
- Leitinger, B. Transmembrane Collagen Receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Hubert, T.; Grimal, S.; Carroll, P.; Fichard-Carroll, A. Collagens in the Developing and Diseased Nervous System. Cell. Mol. Life Sci. 2009, 66, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Chernousov, M.A.; Yu, W.M.; Chen, Z.L.; Carey, D.J.; Strickland, S. Regulation of Schwann Cell Function by the Extracellular Matrix. Glia 2008, 56, 1498–1507. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Bonaldo, P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol. Neurobiol. 2015, 52, 216–225. [Google Scholar] [CrossRef]
- Mogha, A.; D’Rozario, M.; Monk, K.R. G Protein-Coupled Receptors in Myelinating Glia. Trends Pharmacol. Sci. 2016, 37, 977–987. [Google Scholar] [CrossRef]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The Discoidin Domain Receptor Tyrosine Kinases Are Activated by Collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Shrivastava, A.; Radziejewski, C.; Campbell, E.; Kovac, L.; McGlynn, M.; Ryan, T.E.; Davis, S.; Goldfarb, M.P.; Glass, D.J.; Lemke, G.; et al. An Orphan Receptor Tyrosine Kinase Family Whose Members Serve as Nonintegrin Collagen Receptors. Mol. Cell 1997, 1, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Franco-Pons, N.; Virgos, C.; Vogel, W.F.; Ureña, J.M.; Soriano, E.; del Rio, J.A.; Vilella, E. Expression of Discoidin Domain Receptor 1 during Mouse Brain Development Follows the Progress of Myelination. Neuroscience 2006, 140, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Muntané, G.; Chillida, M.; Aranda, S.; Navarro, A.; Vilella, E. Coexpression of the Discoidin Domain Receptor 1 Gene with Oligodendrocyte-Related and Schizophrenia Risk Genes in the Developing and Adult Human Brain. Brain Behav. 2021, 11, e2309. [Google Scholar] [CrossRef] [PubMed]
- Roig, B.; Franco-Pons, N.; Martorell, L.; Tomas, J.; Vogel, W.F.; Vilella, E. Expression of the Tyrosine Kinase Discoidin Domain Receptor 1 (DDR1) in Human Central Nervous System Myelin. Brain Res. 2010, 1336, 22–29. [Google Scholar] [CrossRef]
- Franco-Pons, N.; Tomàs, J.; Roig, B.; Auladell, C.; Martorell, L.; Vilella, E. Discoidin Domain Receptor 1, a Tyrosine Kinase Receptor, Is Upregulated in an Experimental Model of Remyelination and during Oligodendrocyte Differentiation in Vitro. J. Mol. Neurosci. 2009, 38, 2–11. [Google Scholar] [CrossRef]
- Vilella, E.; Gas, C.; Garcia, B.; Rivera, F.J. Expression of DDR1 in the CNS and in Myelinating Oligodendrocytes. Biochim. Biophys. acta. Mol. cell Res. 2019, 1866, 118483. [Google Scholar] [CrossRef]
- Fu, H.-L.; Sohail, A.; Valiathan, R.R.; Wasinski, B.D.; Kumarasiri, M.; Mahasenan, K.V.; Bernardo, M.M.; Tokmina-Roszyk, D.; Fields, G.B.; Mobashery, S.; et al. Shedding of Discoidin Domain Receptor 1 by Membrane-Type Matrix Metalloproteinases. J. Biol. Chem. 2013, 288, 12114–12129. [Google Scholar] [CrossRef]
- Leitinger, B. Discoidin Domain Receptor Functions in Physiological and Pathological Conditions. Int. Rev. Cell Mol. Biol. 2014, 310, 39–87. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Tomoda, T.; Fang, Y.; Hatten, M.E. Discoidin Domain Receptor 1 Functions in Axon Extension of Cerebellar Granule Neurons. Genes Dev. 2000, 14, 2216–2228. [Google Scholar] [CrossRef]
- Steffenhagen, C.; Kraus, S.; Dechant, F.X.; Kandasamy, M.; Lehner, B.; Poehler, A.M.; Furtner, T.; Siebzehnrubl, F.A.; Couillard-Despres, S.; Strauss, O.; et al. Identity, Fate and Potential of Cells Grown as Neurospheres: Species Matters. Stem Cell Rev. Rep. 2011, 7, 815–835. [Google Scholar] [CrossRef]
- Kim, H.-G.; Tan, L.; Weisberg, E.L.; Liu, F.; Canning, P.; Choi, H.G.; Ezell, S.A.; Wu, H.; Zhao, Z.; Wang, J.; et al. Discovery of a Potent and Selective DDR1 Receptor Tyrosine Kinase Inhibitor. ACS Chem. Biol. 2013, 8, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.; Lange, S.; Hinrichsen, B.; Philp, A.R.; Reyes, C.R.; Halabi, D.; Mansilla, J.B.; Rotheneichner, P.; Guzman de la Fuente, A.; Couillard-Despres, S.; et al. Pericytes Favor Oligodendrocyte Fate Choice in Adult Neural Stem Cells. Front. Cell. Neurosci. 2019, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, A.G.; Lange, S.; Silva, M.E.; Gonzalez, G.A.; Tempfer, H.; van Wijngaarden, P.; Zhao, C.; Di Canio, L.; Trost, A.; Bieler, L.; et al. Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination. Cell Rep. 2017, 20, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W. Discoidin Domain Receptors: Structural Relations and Functional Implications. FASEB J. 1999, 13, S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Miaczynska, M. Effects of Membrane Trafficking on Signaling by Receptor Tyrosine Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a009035. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shackel, N.A.; Wang, X.M.; Ajami, K.; McCaughan, G.W.; Gorrell, M.D. Discoidin Domain Receptor 1: Isoform Expression and Potential Functions in Cirrhotic Human Liver. Am. J. Pathol. 2011, 178, 1134–1144. [Google Scholar] [CrossRef]
- Slack, B.E.; Siniaia, M.S.; Blusztajn, J.K. Collagen Type I Selectively Activates Ectodomain Shedding of the Discoidin Domain Receptor 1: Involvement of Src Tyrosine Kinase. J. Cell. Biochem. 2006, 98, 672–684. [Google Scholar] [CrossRef]
- Underkoffler, L.A.; Carr, E.; Nelson, A.; Ryan, M.J.; Schulz, R.; Loomes, K.M. Microarray Data Reveal Relationship between Jag1 and Ddr1 in Mouse Liver. PLoS ONE 2013, 8, e84383. [Google Scholar] [CrossRef]
- Buraschi, S.; Morcavallo, A.; Neill, T.; Stefanello, M.; Palladino, C.; Xu, S.-Q.; Belfiore, A.; Iozzo, R.V.; Morrione, A. Discoidin Domain Receptor 1 Functionally Interacts with the IGF-I System in Bladder Cancer. Matrix Biol. Plus 2020, 6–7, 100022. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, W.; Ko, Y.; Lai, T.; Cheng, A.; Shiah, S.; Hsiao, J.; Chang, J.; Lin, S. Angiolymphatic Invasion in Oral Cancer. Cancers 2020, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Canning, P.; Tan, L.; Chu, K.; Lee, S.W.; Gray, N.S.; Bullock, A.N. Structural Mechanisms Determining Inhibition of the Collagen Receptor DDR1 by Selective and Multi-Targeted Type II Kinase Inhibitors. J. Mol. Biol. 2014, 426, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Lemeer, S.; Bluwstein, A.; Wu, Z.; Leberfinger, J.; Müller, K.; Kramer, K.; Kuster, B. Phosphotyrosine Mediated Protein Interactions of the Discoidin Domain Receptor 1. J. Proteom. 2012, 75, 3465–3477. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cescon, M.; Megighian, A.; Bonaldo, P. Collagen VI Regulates Peripheral Nerve Myelination and Function. FASEB J. 2014, 28, 1145–1156. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The Integrin-Collagen Connection—A Glue for Tissue Repair? J. Cell Sci. 2016, 129, 1284. [Google Scholar] [CrossRef]
- Meyer Zum Gottesberge, A.M.; Hansen, S. The Collagen Receptor DDR1 Co-Localizes with the Non-Muscle Myosin IIA in Mice Inner Ear and Contributes to the Cytoarchitecture and Stability of Motile Cells. Cell Tissue Res. 2014, 358, 729–736. [Google Scholar] [CrossRef]
- Huang, Y.; Arora, P.; Mcculloch, C.A.; Vogel, W.F. The Collagen Receptor DDR1 Regulates Cell Spreading and Motility by Associating with Myosin IIA. J. Cell Sci. 2009, 122, 1637–1646. [Google Scholar] [CrossRef]
- Coelho, N.M.; Arora, P.D.; van Putten, S.; Boo, S.; Petrovic, P.; Lin, A.X.; Hinz, B.; McCulloch, C.A. Discoidin Domain Receptor 1 Mediates Myosin-Dependent Collagen Contraction. Cell Rep. 2017, 18, 1774–1790. [Google Scholar] [CrossRef]
- Kohler, W.; Curiel, J.; Vanderver, A. Adulthood Leukodystrophies. Nat. Rev. Neurol. 2018, 14, 94–105. [Google Scholar] [CrossRef]
- Lemus, H.N.; Warrington, A.E.; Rodriguez, M. Multiple Sclerosis: Mechanisms of Disease and Strategies for Myelin and Axonal Repair. Neurol. Clin. 2018, 36, 1–11. [Google Scholar] [CrossRef]
- Haroutunian, V.; Katsel, P.; Roussos, P.; Davis, K.L.; Altshuler, L.L.; Bartzokis, G. Myelination, Oligodendrocytes, and Serious Mental Illness. Glia 2014, 62, 1856–1877. [Google Scholar] [CrossRef] [PubMed]
- Gas, C.; Canales-Rodríguez, E.J.; Radua, J.; Abasolo, N.; Cortés, M.J.; Salvadó, E.; Muntané, G.; Alemán-Gómez, Y.; Julià, T.; Marsal, S.; et al. Discoidin Domain Receptor 1 Gene Variants Are Associated with Decreased White Matter Fractional Anisotropy and Decreased Processing Speed in Schizophrenia. J. Psychiatr. Res. 2019, 110, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Roig, B.; Virgos, C.; Franco, N.; Martorell, L.; Valero, J.; Costas, J.; Carracedo, A.; Labad, A.; Vilella, E. The Discoidin Domain Receptor 1 as a Novel Susceptibility Gene for Schizophrenia. Mol. Psychiatry 2007, 12, 833–841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roig, B.; Abasolo, N.; Moyano, S.; Martorell, L.; Vilella, E. Increased Expression of the Spliced DDR1c Isoform in Brain Tissues from Schizophrenia Patients. J. Psychiatr. Res. 2012, 46, 825–827. [Google Scholar] [CrossRef]
- Agnihotri, S.; Jalali, S.; Wilson, M.R.; Danesh, A.; Li, M.; Klironomos, G.; Krieger, J.R.; Mansouri, A.; Khan, O.; Mamatjan, Y.; et al. The Genomic Landscape of Schwannoma. Nat. Genet. 2016, 48, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.R.; Marco, M.; Leitinger, B.; Kleer, C.G.; Fridman, R. Discoidin Domain Receptor Tyrosine Kinases: New Players in Cancer Progression. Cancer Metastasis Rev. 2012, 31, 295–321. [Google Scholar] [CrossRef]
- De Kleijn, K.M.A.; Zuure, W.A.; Peijnenborg, J.; Heuvelmans, J.M.; Martens, G.J.M. Reappraisal of Human HOG and MO3.13 Cell Lines as a Model to Study Oligodendrocyte Functioning. Cells 2019, 8, 1096. [Google Scholar] [CrossRef]
- Buntinx, M.; Vanderlocht, J.; Hellings, N.; Vandenabeele, F.; Lambrichts, I.; Raus, J.; Ameloot, M.; Stinissen, P.; Steels, P. Characterization of Three Human Oligodendroglial Cell Lines as a Model to Study Oligodendrocyte Injury: Morphology and Oligodendrocyte-Specific Gene Expression. J. Neurocytol. 2003, 32, 25–38. [Google Scholar] [CrossRef]
- Roig, B.; Moyano, S.; Martorell, L.; Costas, J.; Vilella, E. The Discoidin Domain Receptor 1 Gene Has a Functional A2RE Sequence. J. Neurochem. 2012, 120, 408–418. [Google Scholar] [CrossRef]
- Wachs, F.-P.; Couillard-Despres, S.; Engelhardt, M.; Wilhelm, D.; Ploetz, S.; Vroemen, M.; Kaesbauer, J.; Uyanik, G.; Klucken, J.; Karl, C.; et al. High Efficacy of Clonal Growth and Expansion of Adult Neural Stem Cells. Lab. Investig. 2003, 83, 949–962. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.E.; Hernández-Andrade, M.; Abasolo, N.; Espinoza-Cruells, C.; Mansilla, J.B.; Reyes, C.R.; Aranda, S.; Esteban, Y.; Rodriguez-Calvo, R.; Martorell, L.; et al. DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation. Int. J. Mol. Sci. 2023, 24, 1742. https://doi.org/10.3390/ijms24021742
Silva ME, Hernández-Andrade M, Abasolo N, Espinoza-Cruells C, Mansilla JB, Reyes CR, Aranda S, Esteban Y, Rodriguez-Calvo R, Martorell L, et al. DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation. International Journal of Molecular Sciences. 2023; 24(2):1742. https://doi.org/10.3390/ijms24021742
Chicago/Turabian StyleSilva, Maria Elena, Matías Hernández-Andrade, Nerea Abasolo, Cristóbal Espinoza-Cruells, Josselyne B. Mansilla, Carolina R. Reyes, Selena Aranda, Yaiza Esteban, Ricardo Rodriguez-Calvo, Lourdes Martorell, and et al. 2023. "DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation" International Journal of Molecular Sciences 24, no. 2: 1742. https://doi.org/10.3390/ijms24021742
APA StyleSilva, M. E., Hernández-Andrade, M., Abasolo, N., Espinoza-Cruells, C., Mansilla, J. B., Reyes, C. R., Aranda, S., Esteban, Y., Rodriguez-Calvo, R., Martorell, L., Muntané, G., Rivera, F. J., & Vilella, E. (2023). DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation. International Journal of Molecular Sciences, 24(2), 1742. https://doi.org/10.3390/ijms24021742






