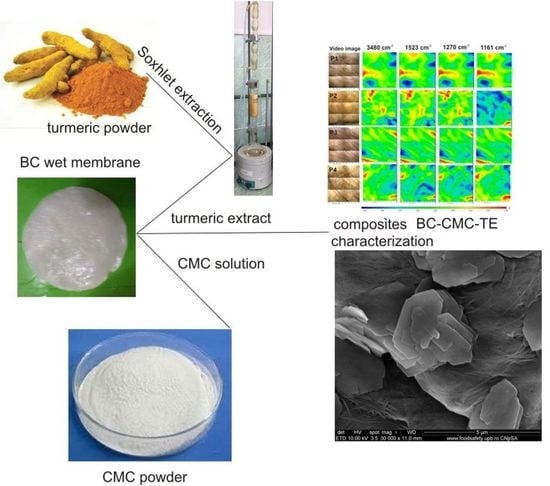
Bacterial Cellulose—Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of BC–CMC Composites
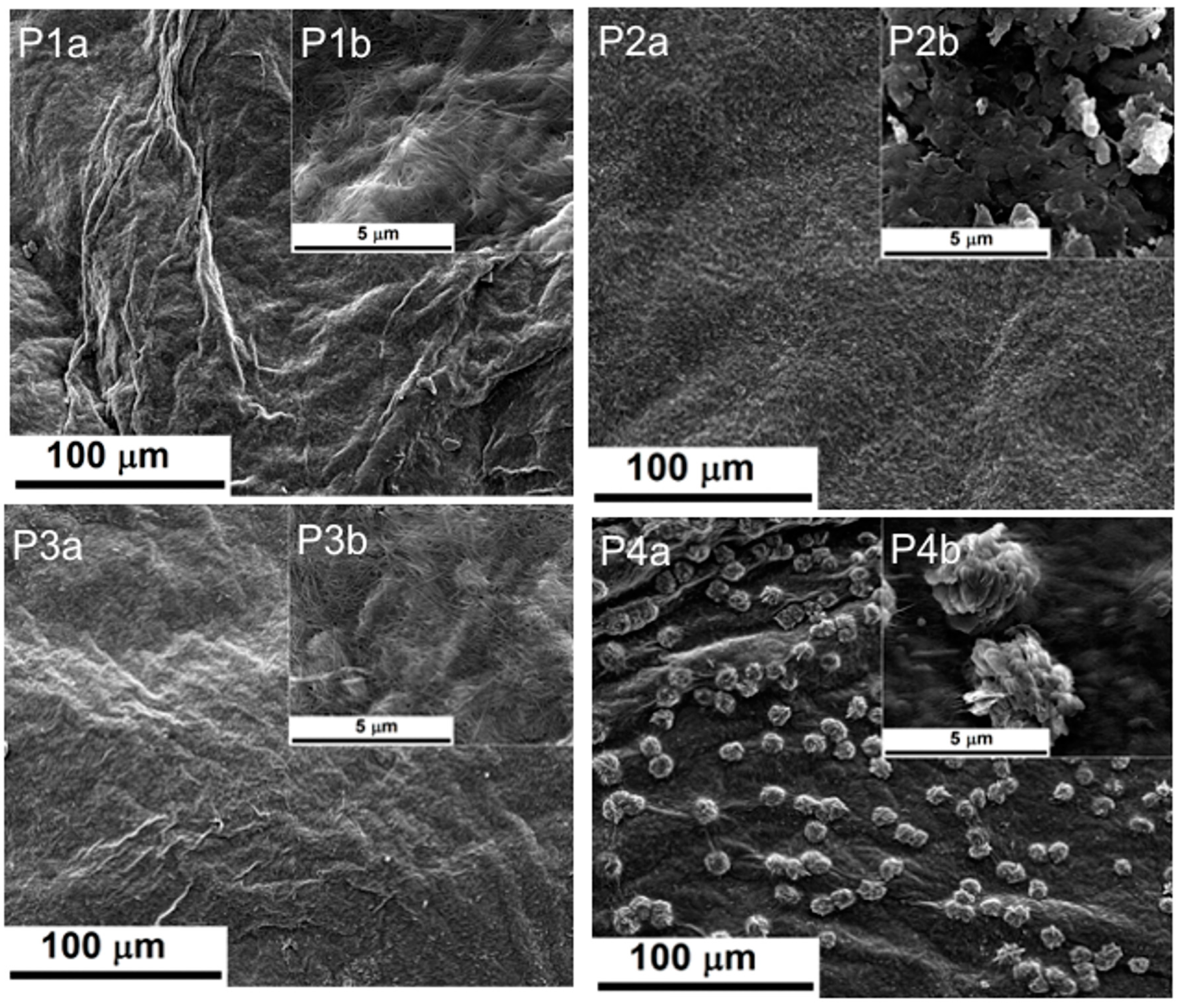
2.1.1. Scanning Electron Microscopy
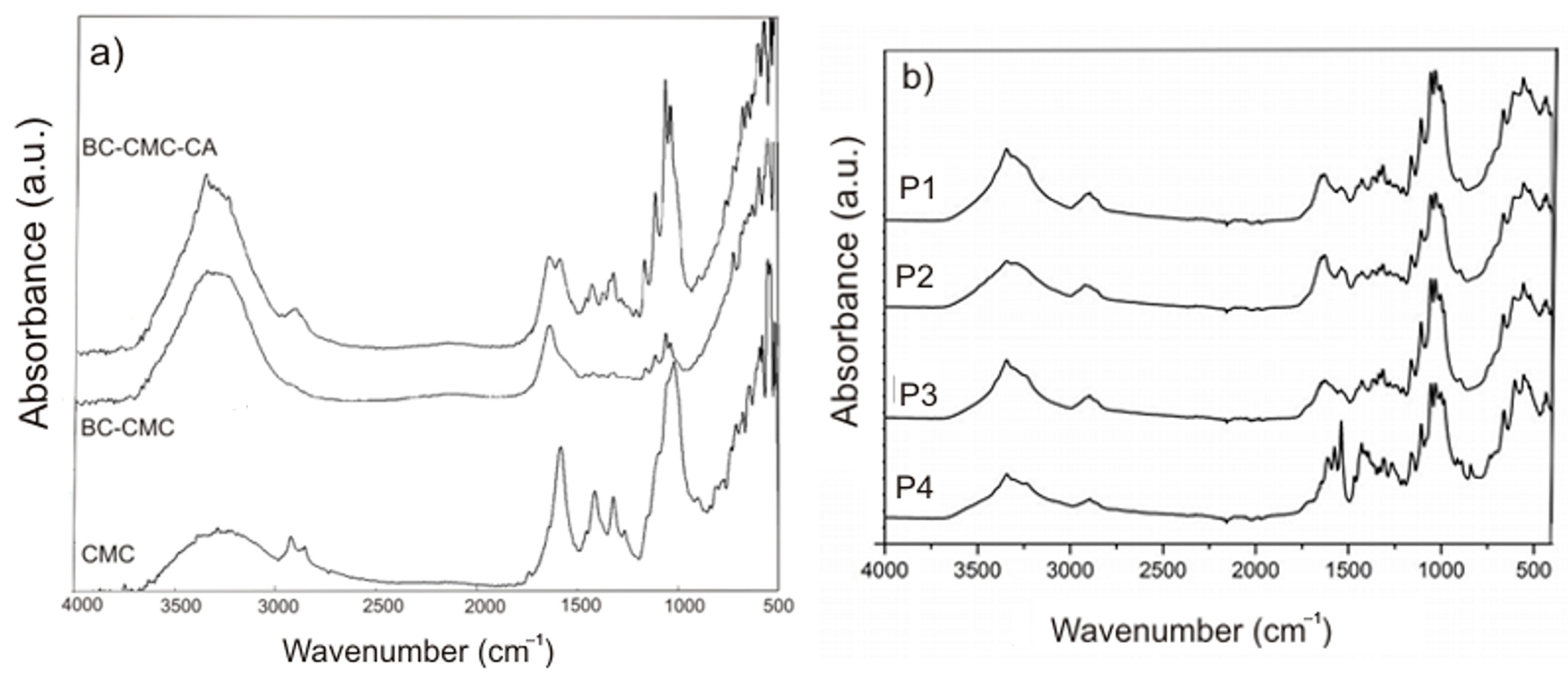
2.1.2. FTIR Analysis
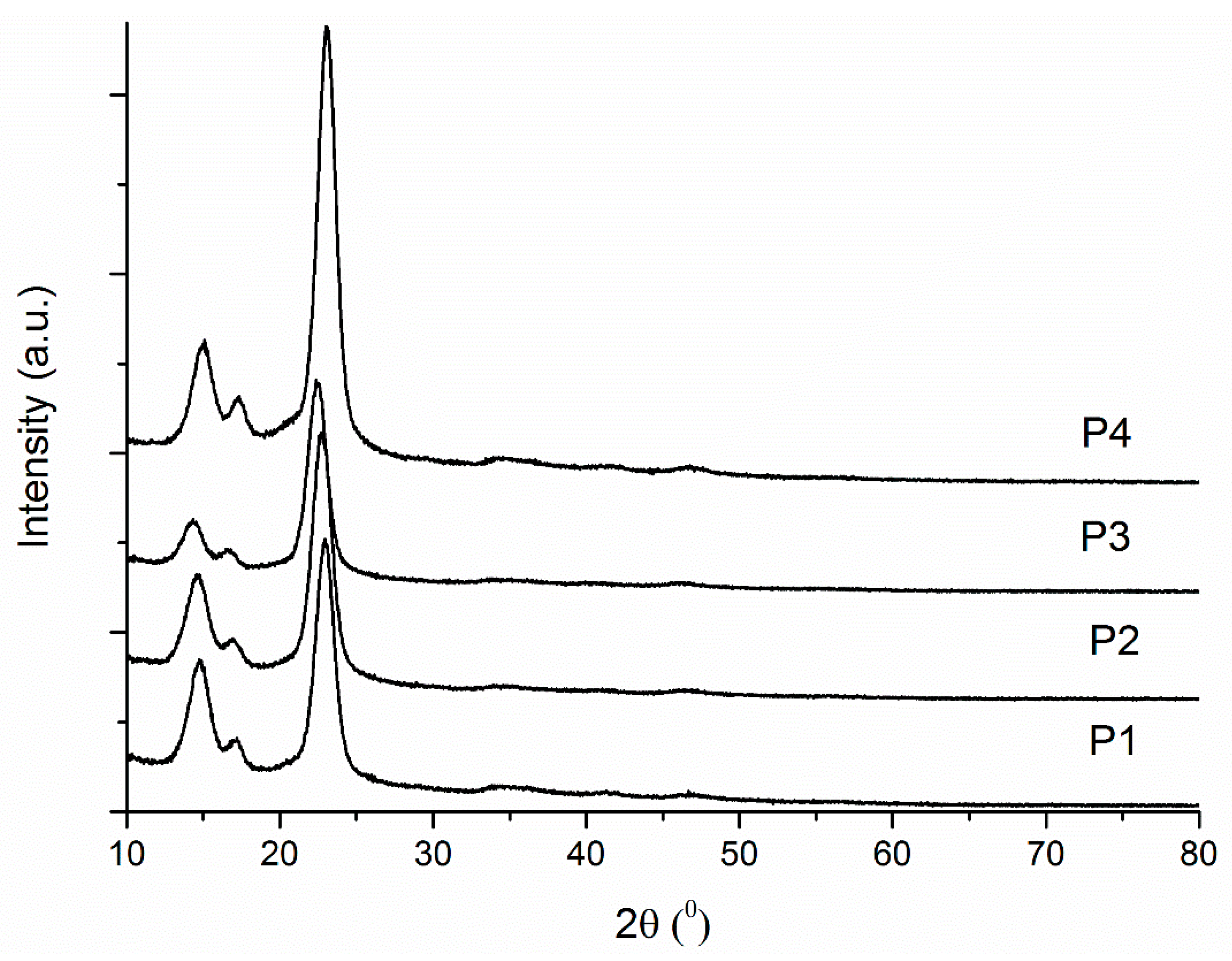
2.1.3. XRD
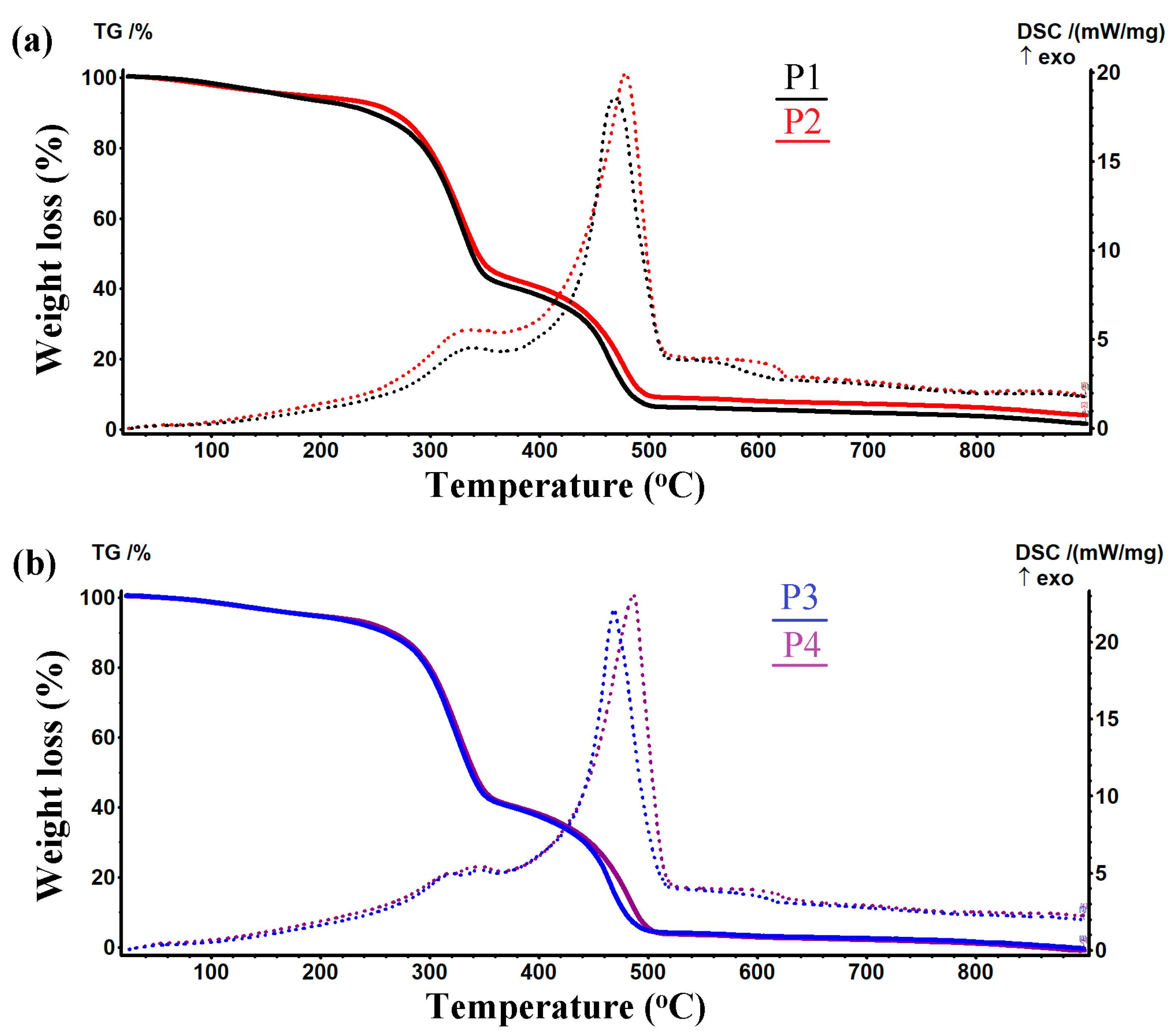
2.1.4. Thermogravimetric Analysis
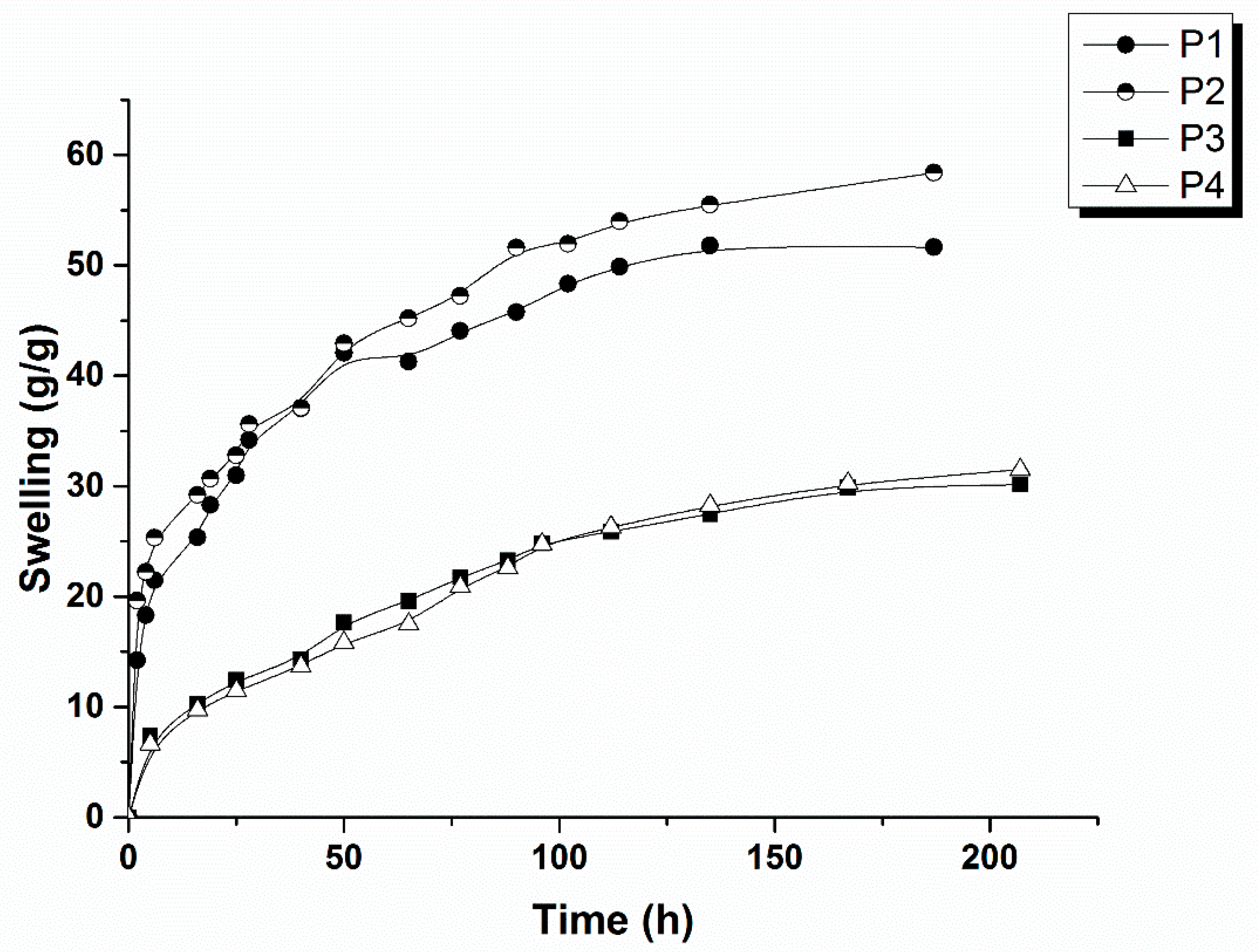
2.1.5. Swelling Studies
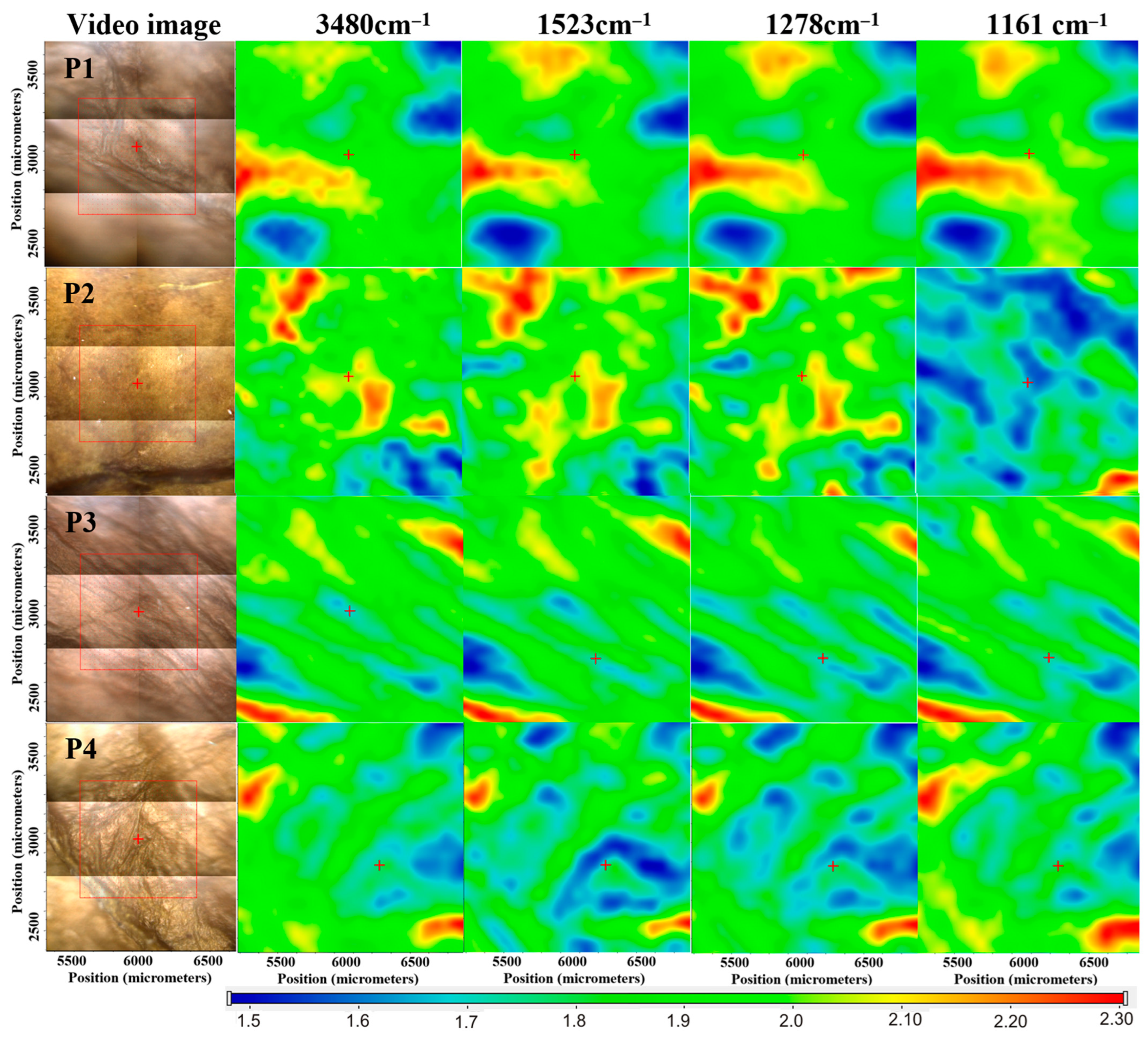
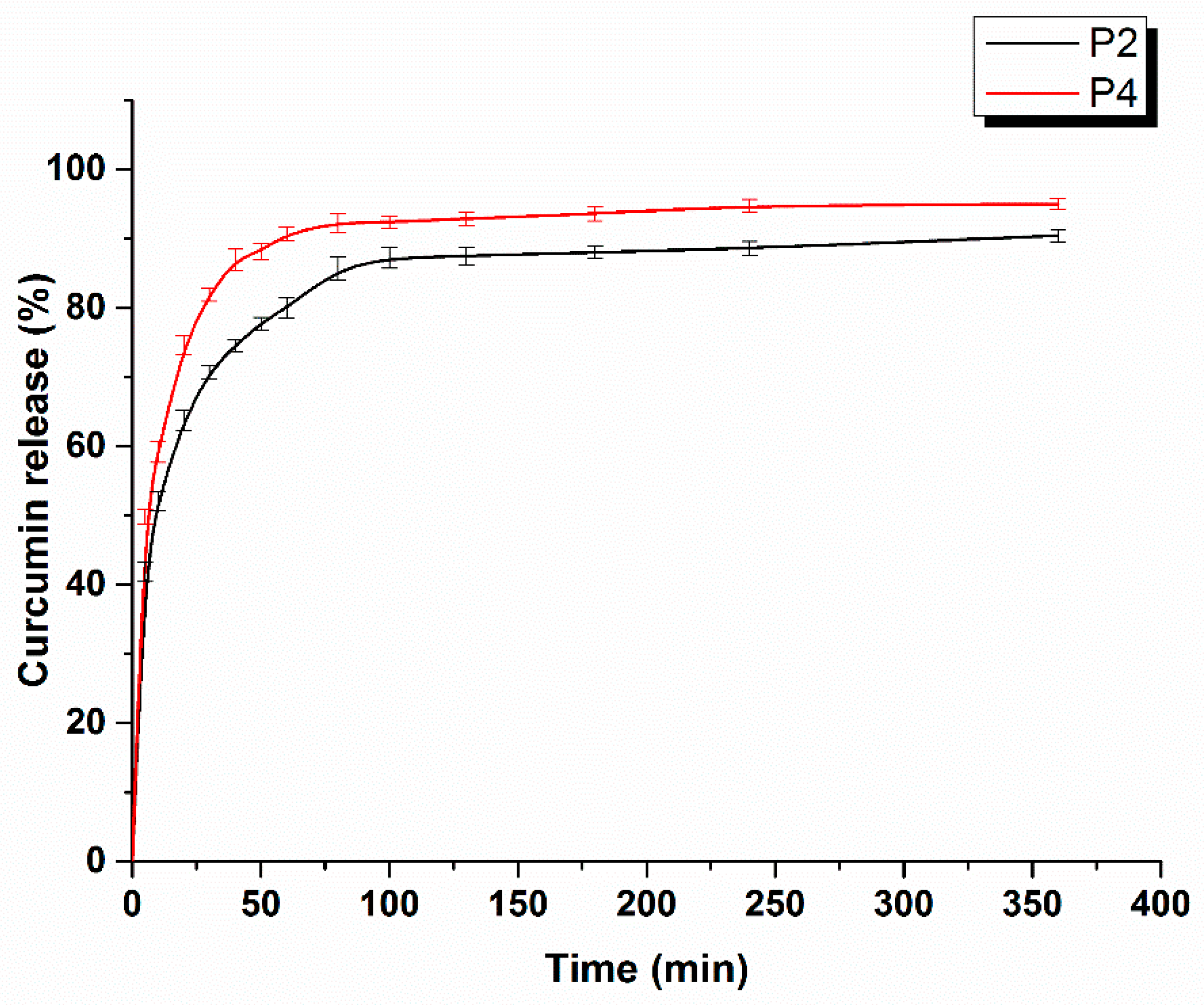
2.1.6. Curcumin Release Studies
2.1.7. Antioxidant Activity
2.1.8. Antimicrobial Activity
2.1.9. In Vitro Cell Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Bacterial Cellulose
3.3. Preparation of BC–CMC–TE Composites
3.4. Ultra-High-Performance Liquid Chromatography Analysis (UHPLC)
3.5. Characterization of Composites P1–P4
3.6. Swelling Studies
3.7. Kinetics of Curcumin Release
3.8. Antioxidant Capacity
3.9. Antimicrobial Activity
3.10. In Vitro Cell Studies
3.10.1. Extract Preparation
3.10.2. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Dai, K.; Wang, Y.; Song, P.; Li, H.; Tang, P.; Zhang, Z.; Li, Z.; Zhou, Y.; et al. Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol. 2022, 208, 400–408. [Google Scholar] [CrossRef]
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.d.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- He, W.; Wu, J.; Mosselhy, D.A.; Zheng, Y.; Yang, S. Bacterial Cellulose: Functional Modification and Wound Healing Applications. Adv. Wound Care 2021, 10, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Lemnaru, G.-M.; Truşcă, R.D.; Ilie, C.-I.; Ţiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Diţu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef] [PubMed]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material forthird degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Meftahi, A.; Nasrolahi, D.; Babaeipour, V.; Alibakhshi, S.; Shahbazi, S. Investigation of Nano Bacterial Cellulose Coated by Sesamum Oil for Wound Dressing Application. Procedia Mat. Sci. 2015, 11, 212–216. [Google Scholar] [CrossRef]
- Asanarong, O.; Quan, V.M.; Boonrungsiman, S.; Sukyai, P. Bioactive wound dressing using bacterial cellulose loaded with papain composite: Morphology, loading/release and antibacterial properties. Eur. Polym. J. 2021, 143, 110224. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Harahap, D.; Niaci, S.; Mardina, V.; Zaura, B.; Qanita, I.; Purnama, A.; Puspita, K.; Rizki, D.R.; Iqhrammullah, M. Antibacterial activities of seven ethnomedicinal plants from family Annonaceae. J. Adv. Pharm. Technol. Res. 2022, 13, 148–153. [Google Scholar]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K.A. Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 12, 186864. [Google Scholar]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yu, Z.; Li, C.; Zhou, J.; Lv, X.; Chen, J.; Wei, M.; Liu, J.; Yu, X.; Wang, C.; et al. Gelatin-based nanofiber membranes loaded with curcumin and borneol as a sustainable wound dressing. Int. J. Biol. Macromol. 2022, 219, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Sahabi, M.; Beigi-Boroujeni, S.; Abdolvand, H.; Makvandi, P.; Isfahani, A.P.; Gharibi, R.; Ebrahimibagha, M. Alginate hydrogel with enhanced curcumin release through HPβCD assisted host-guest interaction. Biomat. Adv. 2022, 141, 213130. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent Developments of Carboxymethyl Cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef]
- Casaburi, A.; Montoya Rojo, Ú.; Cerrutti, P.; Vazquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocol. 2018, 75, 147–156. [Google Scholar] [CrossRef]
- Cheng, H.N.; Takai, W.; Ekong, E.A. Rheology of carboxymethylcellulose made from bacterial cellulose. Macromol. Symp. 1999, 140, 145–153. [Google Scholar] [CrossRef]
- Schlufter, K.; Heinze, T. Carboxymethylation of Bacterial Cellulose. Macromol. Symp. 2010, 294-II, 117–124. [Google Scholar] [CrossRef]
- Cometa, S.; Licini, C.; Bonifacio, M.A.; Mastrorilli, P.; Mattioli-Belmonte, M.; De Giglio, E. Carboxymethyl cellulose-based hydrogel film combined with berberine as an innovative tool for chronic wound management. Carbohydr. Polym. 2022, 283, 119145. [Google Scholar] [CrossRef]
- Naserian, F.; Mesgar, A.S. Development of antibacterial and superabsorbent wound composite sponges containing carboxymethyl cellulose/gelatin/Cu-doped ZnO nanoparticles. Colloids Surf. B Biointerfaces 2022, 218, 112729. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Mansur, L.L.; Ramos, C.P.; Lage, A.P.; Mansur, H.S. Physicochemical properties and antimicrobial activity of biocompatible carboxymethylcellulose-silver nanoparticle hybrids for wound dressing and epidermal repair. J. Appl. Polym. Sci. 2017, 135, 45812. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kazeminava, F.; Javanbakht, S.; Nouri, M.; Adibkia, K.; Ganbarov, K.; Yousefi, M.; Ahmadi, M.; Gholizadeh, P.; Kafil, H.S. Electrospun nanofibers based on carboxymethyl cellulose/polyvinyl alcohol as a potential antimicrobial wound dressing. Int. J. Biol. Macromol. 2022, 214, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, I.A.; Leau, S.-A.; Marin, S.; Holban, A.M.; Vasile, B.-S.; Nicoara, A.-I.; Ene, V.L.; Bleotu, C.; Albu Kaya, M.G.; Ficai, A. Collagen-Carboxymethylcellulose Biocomposite Wound-Dressings with Antimicrobial Activity. Materials 2021, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Saraiva, J.N.; Cavaco, G.; Portela, R.P.; Leal, C.R.; Sobral, R.G.; Almeida, P.L. Crosslinked bacterial cellulose hydrogels for biomedical applications. Eur. Polym. J. 2022, 177, 111438. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Junka, A.; Fijałkowski, K. The cross-linked bacterial cellulose impregnated with octenidine dihydrochloride-based antiseptic as an antibacterial dressing material for highly-exuding, infected wounds. Microbiol. Res. 2022, 263, 127125. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Somchob, B.; Rodthongkum, N.; Hoven, V.P. Bacterial cellulose-based re-swellable hydrogel: Facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr. Polym. 2021, 256, 117506. [Google Scholar] [CrossRef]
- Pavaloiu, R.D.; Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Dobre, T. Composite films of poly(vinyl alcohol)–chitosan–bacterial cellulose for drug controlled release. Int. J. Biol. Macromol. 2014, 68, 117–124. [Google Scholar] [CrossRef]
- Pavaloiu, R.D.; Stoica, A.; Stroescu, M.; Dobre, T. Controlled release of amoxicillin from bacterial cellulose membranes. Cent. Eur. J. Chem. 2014, 12, 962–967. [Google Scholar] [CrossRef]
- Juncu, G.; Stoica-Guzun, A.; Stroescu, M.; Isopencu, G.; Jinga, S.I. Drug release kinetics from carboxymethylcellulose-bacterial cellulose composite films. Int. J. Pharm. 2016, 510, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Tangsatianpan, V.; Torgbo, S.; Sukyai, P. Release Kinetic Model and Antimicrobial Activity of Freeze-Dried Curcumin-loaded Bacterial Nanocellulose. Composite. Polym. Sci. Ser. A 2020, 62, 218–227. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D.; Pirayesh, H. Intelligent pH- and ammonia-sensitive indicator films using neutral red immobilized onto cellulose nanofibrils. Carbohydr. Polym. 2022, 296, 119910. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, S.; Bahmani, M.; Ashori, A.; Pirayesh, H.; Yu, Q.; Dafchahi, M.N. Preparation and characterization of air nanofilters based on cellulose nanofibers. Int. J. Biol. Macrom. 2021, 182, 392–1398. [Google Scholar] [CrossRef]
- Hashem, M.; Sharaf, S.; Abd El-Hady, M.M.; Hebeish, A. Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulolse–hydrogel–ZnO-nanocomposites. Carbohydr. Polym. 2013, 95, 421–427. [Google Scholar] [CrossRef]
- Pandey, V.K.; Ajmal, G.; Upadhyay, S.N.; Mishra, P.K. Nano-fibrous scaffold with curcumin for anti-scar wound healing. Int. J. Pharm. 2020, 589, 119858. [Google Scholar] [CrossRef]
- Elakkiya, T.; Malarvizhi, G.; Rajiv, S.; Natarajan, T.S. Curcumin loaded electrospun Bombyx mori silk nanofibers for drug delivery. Polym. Int. 2014, 63, 100–105. [Google Scholar] [CrossRef]
- Rahmi, R.; Lelifajri, L.; Iqbal, M.; Fathurrahmi, F.; Jalaluddin, J.; Sembiring, R.; Iqhrammullah, M. Preparation, characterization PEDGE-cross-linked magnetic chitosan (PEDGE-MCh) microspheres for Cd2+ removal. Arabian J. Sci. Eng. 2020, 1–9. [Google Scholar] [CrossRef]
- Ojagh, S.M.A.; Vahabzadeh, F.; Karimi, A. Synthesis and characterization of bacterial cellulose-based composites for drug delivery. Carbohydr. Polym. 2021, 273, 118587. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Rakoczy, R.; Nowak, A.; Konopacki, M.; Klebeko, J.; Świątek, E.; Janus, E.; Duchnik, W.; Wenelska, K.; Kucharski, Ł.; et al. Transdermal Delivery Systems for Ibuprofen and Ibuprofen Modified with Amino Acids Alkyl Esters Based on Bacterial Cellulose. Int. J. Mol. Sci. 2021, 22, 6252. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Composite wound dressings of pectin and gelatin with aloe vera and curcumin as bioactive agents. Int. J. Biol. Macromol. 2016, 82, 104–113. [Google Scholar] [CrossRef]
- Akter, J.; Hossain, A.; Takara, K.; Islam, Z.; Hou, D.-X. Antioxidant activity of different species and varieties of turmeric (Curcuma spp): Isolation of active compounds. Comp. Biochem. Physiol. C 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Roşu, M.-C.; Páll, E.; Socaci, C.; Măgeruşan, L.; Pogăcean, F.; Coroş, M.; Turza, A.; Pruneanu, S. Cytotoxicity of methylcellulose-based films containing graphenes and curcumin on human lung fibroblasts. Process Biochem. 2017, 52, 243–249. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macrom. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Abel, M.; Ruth, P.; Elsner, P.; Hipler, U.-C. In vitro assessment of the antimicrobial activity of wound dressings: Influence of the test method selected and impact of the pH. J. Mater. Sci. Mater. Med. 2015, 26, 18. [Google Scholar] [CrossRef]
- Thorn, R.M.S.; Greenman, J.; Austin, A.J. In vitro method to assess the antimicrobial activity and potential efficacy of novel types of wound dressings. J. Appl. Microbiol. 2005, 99, 895–901. [Google Scholar] [CrossRef]
- Schumacher, A.; Vranken, T.; Malhotra, A.; Arts, J.J.C.; Habibovic, P. In vitro antimicrobial susceptibility testingmethods: Agar dilution to 3D tissue-engineered models. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 187–208. [Google Scholar] [CrossRef]
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.-M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol. 2016, 68, 195–207. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Suwanmajo, T.; Sanchavanakit, N.; Phisalaphong, M. Curcumin-Loaded Bacterial Cellulose/Alginate/Gelatin as A Multifunctional Biopolymer Composite Film. Molecules 2020, 25, 3800. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica-Guzun, A.; Stroescu, M. Controlled release of sorbic acid from bacterial cellulose based mono and multilayer antimicrobial films. LWT-Food Sci. Technol. 2012, 47, 400–406. [Google Scholar] [CrossRef]
- Stroescu, M.; Stoica-Guzun, A.; Jipa, I.M. Vanillin release from poly(vinyl alcohol)-bacterial cellulose mono and multilayer films. J. Food Eng. 2013, 114, 153–157. [Google Scholar] [CrossRef]
- Uyguna, B.E.; Bou-Akla, T.; Albannaa, M.; Matthewa, H.W.T. Membrane thickness is an important variable in membrane scaffolds: Influence of chitosan membrane structure on the behavior of cells. Acta Biomater. 2010, 6, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Cryst. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Caglioti, G.; Paoletti, A.; Ricci, F.P. Choice of collimators for a crystal spectrometer for neutron diffraction. Nucl. Inst. 1958, 3, 223–228. [Google Scholar] [CrossRef]
| Sample Abbreviation | Description of the Sample |
|---|---|
| P1 | BC-CMC-CA-1 (1:0.4:0.12 ratio) * |
| P2 | BC-CMC-CA-1 loaded with TE |
| P3 | BC-CMC-CA-2 (1:0.4:0.24 ratio) * |
| P4 | BC-CMC-CA-2 loaded with TE |
| BC is reported as dry material. |
| Sample | Mass Loss (%) | DSC Effects | ||||
|---|---|---|---|---|---|---|
| RT-200 (°C) | 200–375 (°C) | 375–520 (°C) | * Endo I (°C) | * Exo I (°C) | * Exo II (°C) | |
| P1 | 6.70 | 52.83 | 34.26 | 60.1 | 340.1 | 469.0 |
| P2 | 5.58 | 51.66 | 33.87 | 60.1 | 334.9 | 478.5 |
| P3 | 5.45 | 54.50 | 35.94 | 60.8 | 322.5/345.8 | 469.0 |
| P4 | 5.32 | 54.23 | 36.79 | 60.0 | 315.5/345.7 | 486.6 |
| Sample Code | TEAC (μg/mL) | AE (%) |
|---|---|---|
| P2 | 15.49 ± 1.011 | 22.17 ± 0.014 |
| P4 | 11.65 ± 0.93 | 16.81 ± 0.011 |
| Curcumin | 59.14 ± 1.025 | 84.65 ± 0.025 |
| Turmeric | 43.08 ± 1.02 | 61.65 ± 0.03 |
| Sample Code | IZ, mm ± SD Mean | ||
|---|---|---|---|
| E. coli | S. aureus | C. albicans | |
| P1 | 0.67 ± 0.31 | 1.00 ± 0.41 | 2.04 ± 0.63 |
| P2 | 1.33 ± 0.47 | 2.02 ± 0.33 | 3.67 ± 1.70 |
| P3 | 0.17 ± 0.24 | 1.00 ± 0.27 | 1.94 ± 0.67 |
| P4 | 1.33 ± 0.47 | 1.00 ± 0.29 | 2.44 ± 0.42 |
| Control polymeric matrix | nd | nd | nd |
| Control TE extract | >20 mm | 2.01 ± 0.37 | nd |
| Sample Code | Time | Cell Viability (%) | p | |
|---|---|---|---|---|
| Control | 1 day | 24 h | 100 ± 0.01 | - |
| 48 h | 100 ± 0.01 | - | ||
| 6 day | 24 h | 100 ± 0.01 | - | |
| 48 h | 100 ± 0.01 | - | ||
| P1 | 1 day | 24 h | 99.018 ± 8.068 | 0.9995 |
| 48 h | 105.381 ± 2.409 | 0.7744 | ||
| 6 day | 24 h | 103.631 ± 4.569 | 0.9926 | |
| 48 h | 107.906 ± 2.973 | 0.7120 | ||
| P2 | 1 day | 24 h | 79.426 ± 5.676 | 0.0046 * |
| 48 h | 82.368 ± 3.332 | 0.0130 * | ||
| 6 day | 24 h | 100.294 ± 14.233 | 0.9999 | |
| 48 h | 98.089 ± 12.595 | 0.9978 | ||
| P3 | 1 day | 24 h | 102.383 ± 11.305 | 0.9859 |
| 48 h | 106.241 ± 8.770 | 0.6798 | ||
| 6 day | 24 h | 106.975 ± 18.176 | 0.9224 | |
| 48 h | 103.738 ± 4.004 | 0.9728 | ||
| P4 | 1 day | 24 h | 95.755 ± 0.531 | 0.8947 |
| 48 h | 95.805 ± 11.062 | 0.8917 | ||
| 6 day | 24 h | 102.232 ± 13.466 | 0.9989 | |
| 48 h | 105.923 ± 14.269 | 0.8719 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isopencu, G.; Deleanu, I.; Busuioc, C.; Oprea, O.; Surdu, V.-A.; Bacalum, M.; Stoica, R.; Stoica-Guzun, A. Bacterial Cellulose—Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications. Int. J. Mol. Sci. 2023, 24, 1719. https://doi.org/10.3390/ijms24021719
Isopencu G, Deleanu I, Busuioc C, Oprea O, Surdu V-A, Bacalum M, Stoica R, Stoica-Guzun A. Bacterial Cellulose—Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications. International Journal of Molecular Sciences. 2023; 24(2):1719. https://doi.org/10.3390/ijms24021719
Chicago/Turabian StyleIsopencu, Gabriela, Iuliana Deleanu, Cristina Busuioc, Ovidiu Oprea, Vasile-Adrian Surdu, Mihaela Bacalum, Roberta Stoica, and Anicuţa Stoica-Guzun. 2023. "Bacterial Cellulose—Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications" International Journal of Molecular Sciences 24, no. 2: 1719. https://doi.org/10.3390/ijms24021719
APA StyleIsopencu, G., Deleanu, I., Busuioc, C., Oprea, O., Surdu, V.-A., Bacalum, M., Stoica, R., & Stoica-Guzun, A. (2023). Bacterial Cellulose—Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications. International Journal of Molecular Sciences, 24(2), 1719. https://doi.org/10.3390/ijms24021719