Genome-Wide Identification and Expression Analysis Reveals Roles of the NRAMP Gene Family in Iron/Cadmium Interactions in Peanut
Abstract
1. Introduction
2. Results
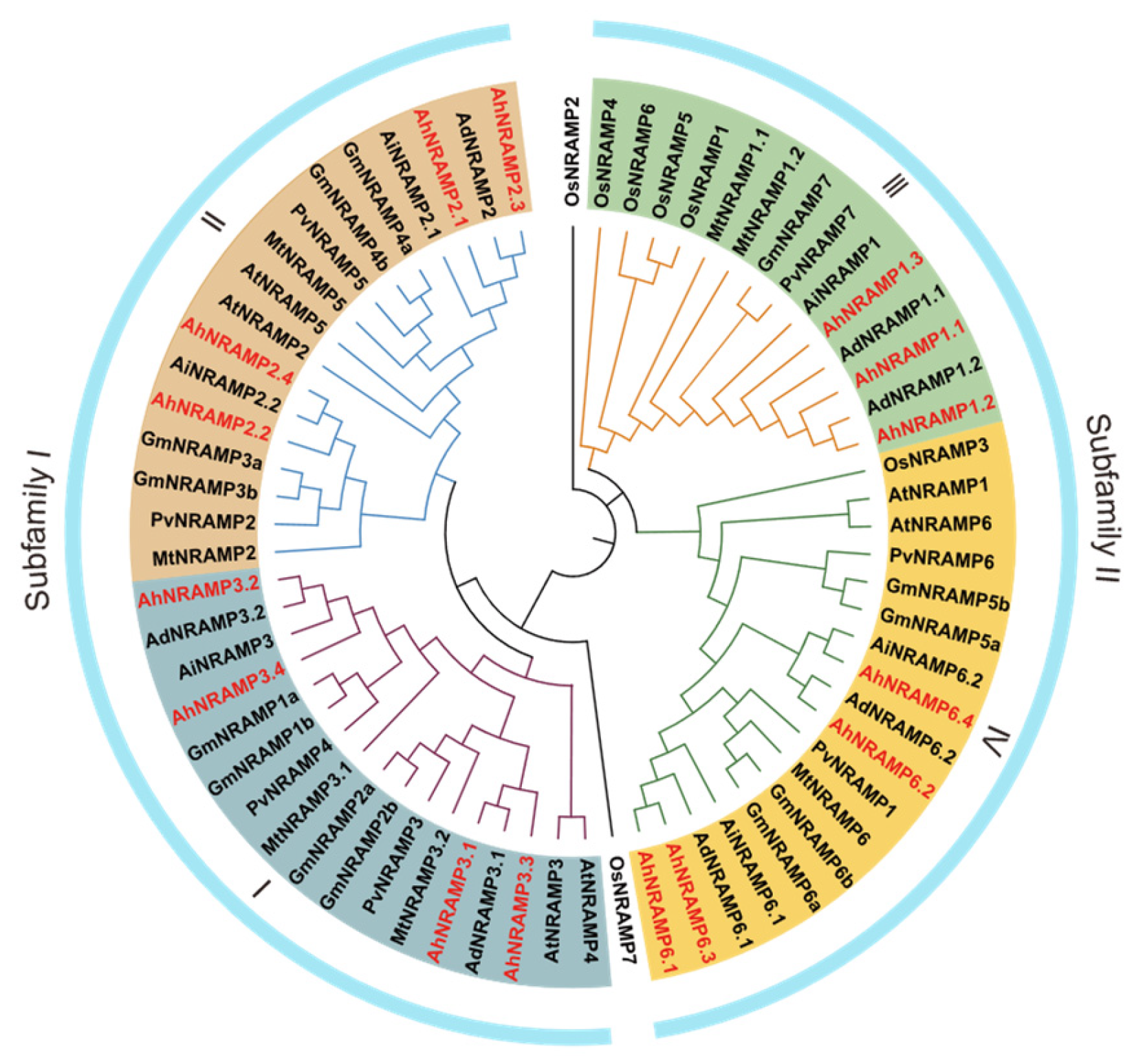
2.1. Identification and Phylogenetic Analysis of the AhNRAMP Family in Peanut
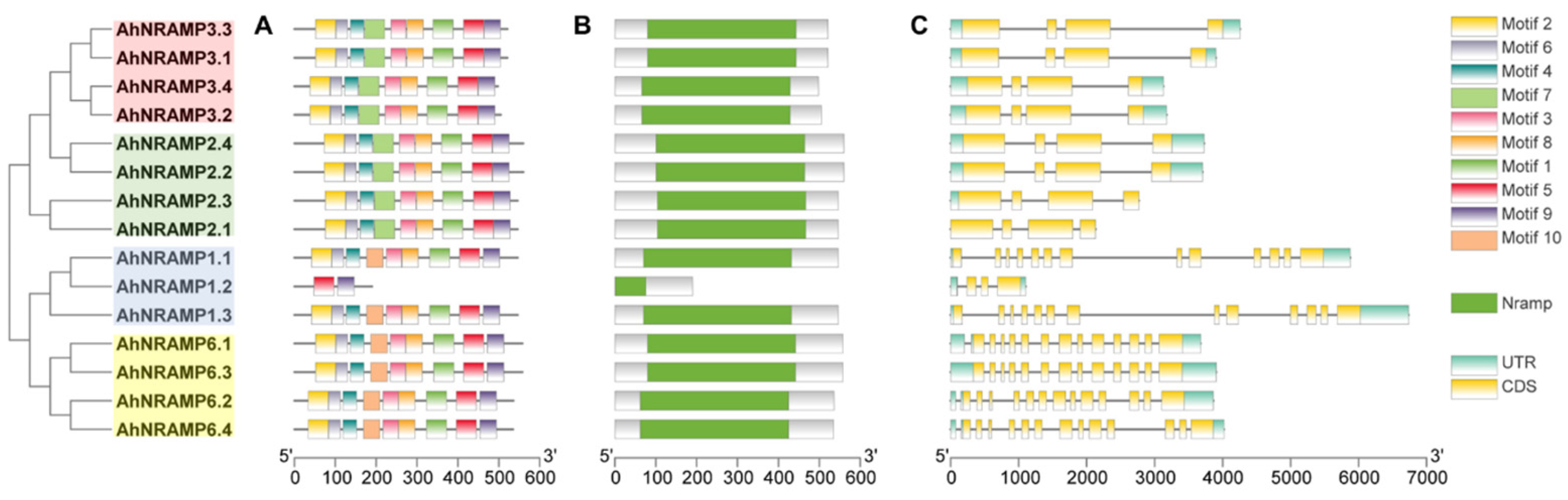
2.2. Conserved Motifs, Domains, and Models of AhNRAMP Proteins
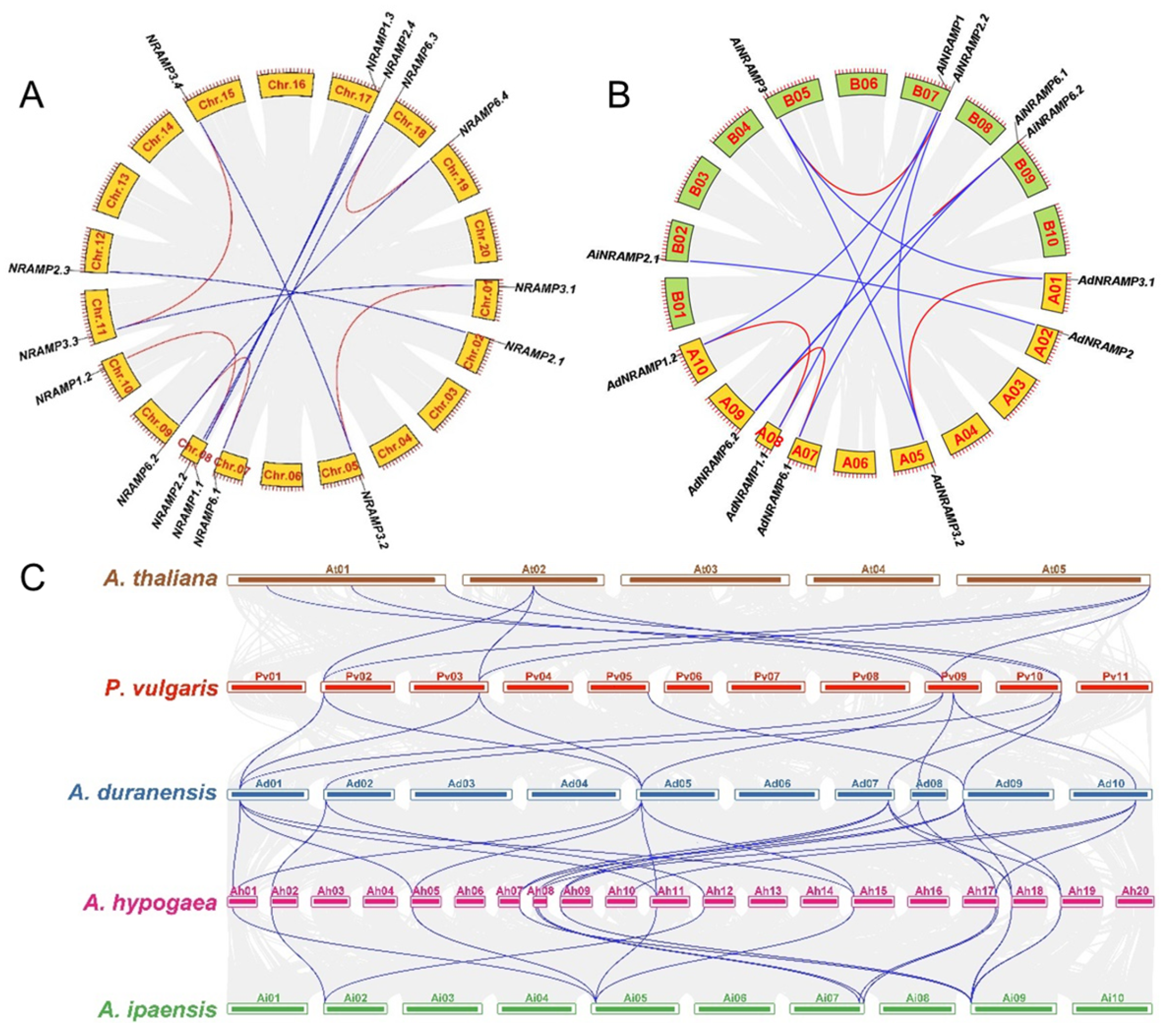
2.3. Exon-Intron Structure, Duplication, and Ka/Ks of the AhNRAMP Family
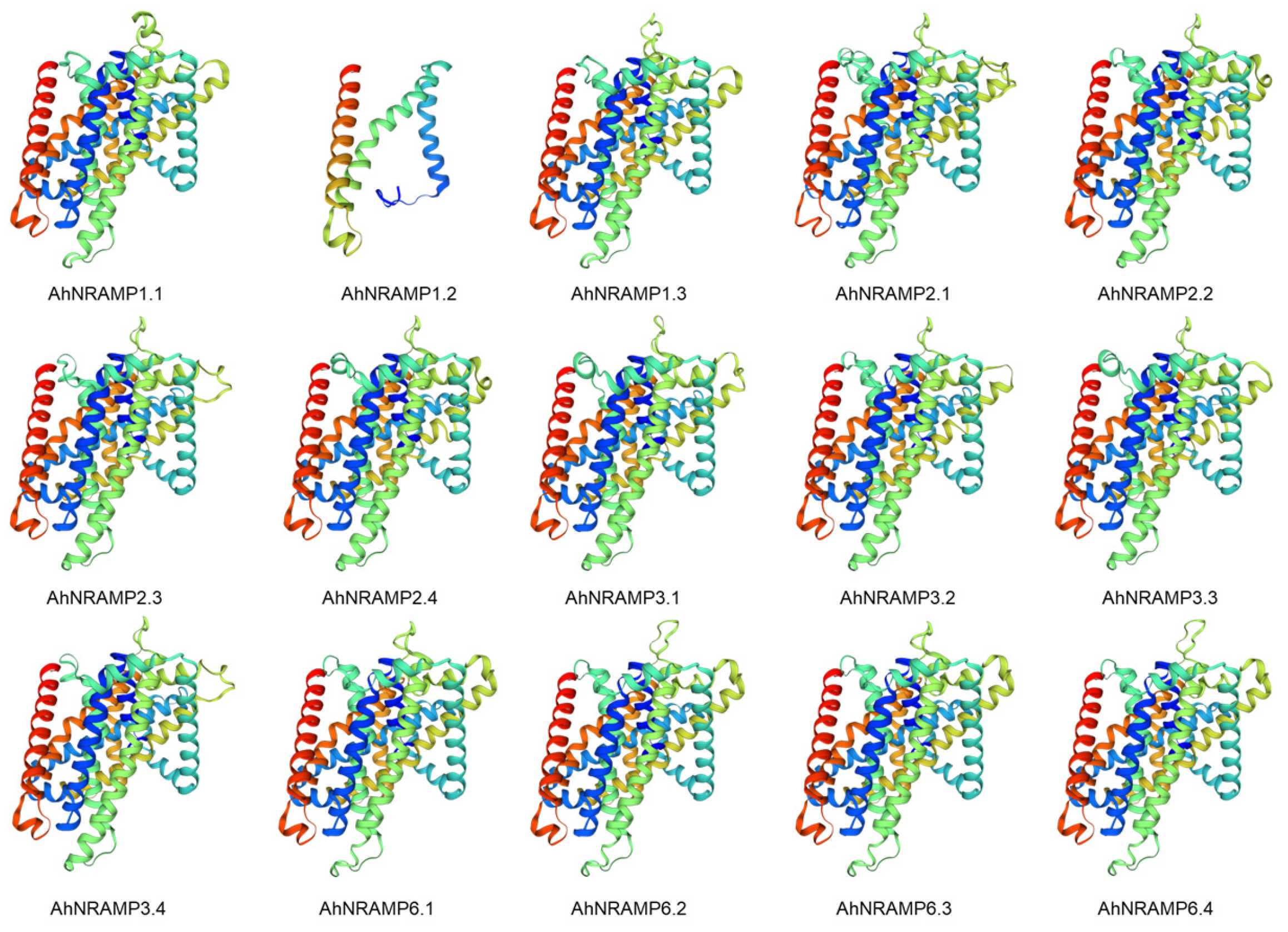
2.4. 3D Model Predictions and Multiple Sequence Alignment of AhNRAMP Proteins
2.5. cis-Acting Elements of AhNRAMP Genes
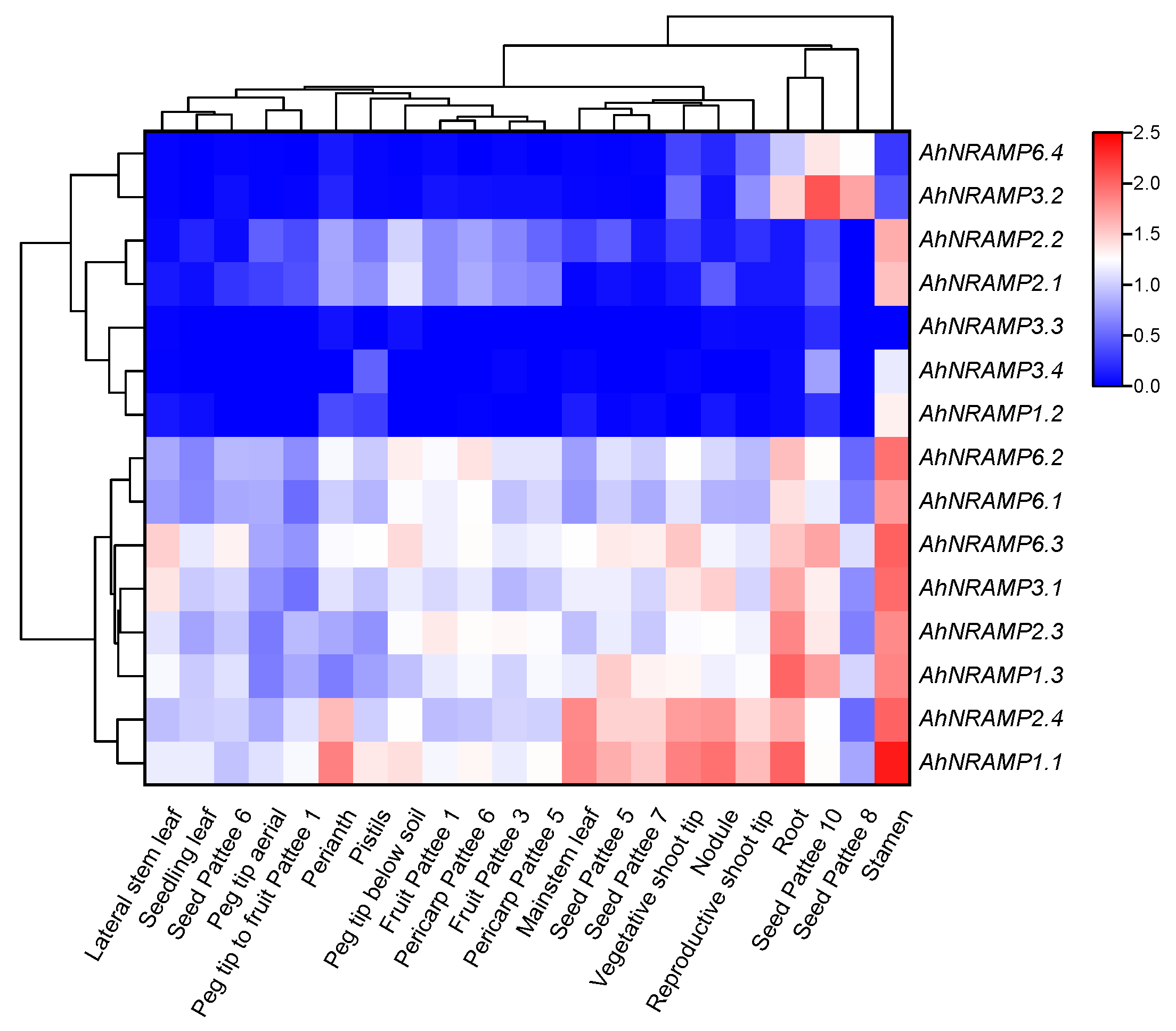
2.6. Tissue-Specific Expression Profiles of AhNRAMP Genes
2.7. Gene Expression of AhNRAMPs in Response to Fe-Deficiency and Cd Exposure
2.8. Fe/Cd Accumulation and Translocation in Two Peanut Cultivars
2.9. Relationship of Gene Expression of AhNRAMPs and Fe/Cd Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Fe and Cd in Peanut Plants
4.3. Identification and Bioinformatics Analyses of NRAMP Family in Peanut
4.4. Gene Expression Analysis Based on RNA-Seq Data
4.5. RNA Extraction and qRT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Boston, MA, USA, 1995. [Google Scholar]
- Liu, F.; Zhang, Y.; Pu, X.; Cai, N.; Sui, X.; Rengel, Z.; Chen, Q.; Song, Z. Physiological and molecular changes in cherry red tobacco in response to iron deficiency stress. Front. Plant Sci. 2022, 13, 861081. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Palumbo, G.; He, J.-Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soil Sediment 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Shao, G.; Chen, M.; Wang, W.; Mou, R.; Zhang, G. Iron nutrition affects cadmium accumulation and toxicity in rice plants. Plant Growth Regul. 2007, 53, 33–42. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Q.; Jiang, Q.; Li, J.; Yu, R.; Shi, G. Comparative transcriptome analysis reveals gene network regulating cadmium uptake and translocation in peanut roots under iron deficiency. BMC Plant Biol. 2019, 19, 35. [Google Scholar] [CrossRef]
- Su, Y.; Wang, X.; Liu, C.; Shi, G. Variation in cadmium accumulation and translocation among peanut cultivars as affected by iron deficiency. Plant Soil 2013, 363, 201–213. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Shi, G.; Sun, L.; Wang, X.; Liu, C. Leaf responses to iron nutrition and low cadmium in peanut: Anatomical properties in relation to gas exchange. Plant Soil 2014, 375, 99–111. [Google Scholar] [CrossRef]
- Cellier, M.; Privé, G.; Belouchi, A.; Kwan, T.; Rodrigues, V.; Chia, W.; Gros, P. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10089–10093. [Google Scholar] [CrossRef]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Cailliatte, R.; Schikora, A.; Briat, J.F.; Mari, S.; Curie, C. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 2010, 22, 904–917. [Google Scholar] [CrossRef]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high-affinity metal transporters NRAMP1 and IRT1 team up to take up iron under sufficient metal provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef]
- Alejandro, S.; Cailliatte, R.; Alcon, C.; Dirick, L.; Domergue, F.; Correia, D.; Castaings, L.; Briat, J.-F.; Mari, S.; Curie, C. Intracellular distribution of manganese by the trans-golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. Plant Cell 2017, 29, 3068–3084. [Google Scholar] [CrossRef]
- Gao, H.; Xie, W.; Yang, C.; Xu, J.; Li, J.; Wang, H.; Chen, X.; Huang, C.-F. NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol. 2018, 217, 179–193. [Google Scholar] [CrossRef]
- Thomine, S.; Lelièvre, F.; Debarbieux, E.; Schroeder, J.I.; Barbier-Brygoo, H. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J. 2003, 34, 685–695. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelièvre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Krämer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef]
- Cailliatte, R.; Lapeyre, B.; Briat, J.F.; Mari, S.; Curie, C. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem. J. 2009, 422, 217–228. [Google Scholar] [CrossRef]
- Chang, J.D.; Huang, S.; Konishi, N.; Wang, P.; Chen, J.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Overexpression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain. J. Exp. Bot. 2020, 71, 5705–5715. [Google Scholar] [CrossRef]
- Tang, L.; Dong, J.; Qu, M.; Lv, Q.; Zhang, L.; Peng, C.; Hu, Y.; Li, Y.; Ji, Z.; Mao, B.; et al. Knockout of OsNRAMP5 enhances rice tolerance to cadmium toxicity in response to varying external cadmium concentrations via distinct mechanisms. Sci. Total Environ. 2022, 832, 155006. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, L.; Hu, J.; Zhang, X.; Lu, K.; Dong, H.; Wang, D.; Zhao, F.J.; Huang, C.F.; et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Bashir, K.; Nakanishi, H.; Nishizawa, N.K. OsNRAMP5, a major player for constitutive iron and manganese uptake in rice. Plant Signal. Behav. 2012, 7, 763–766. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-D.; Gao, W.; Wang, P.; Zhao, F.-J. OsNRAMP5 is a major transporter for lead uptake in rice. Environ. Sci. Technol. 2022, 56, 17481–17490. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.D.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F.J. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal. Behav. 2011, 6, 1813–1816. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yu, Y.; Dai, X.; Gong, C.; Gu, D.; Xu, E.; Liu, Y.; Zou, Y.; Zhang, P.; et al. The tonoplast-localized transporter OsNRAMP2 is involved in iron homeostasis and affects seed germination in rice. J. Exp. Bot. 2021, 72, 4839–4852. [Google Scholar] [CrossRef]
- Yamaji, N.; Sasaki, A.; Xia, J.X.; Yokosho, K.; Ma, J.F. A node-based switch for preferential distribution of manganese in rice. Nat. Commun. 2013, 4, 2442. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Dong, H.; Zhang, Y.; Lv, K.; Wang, D.; Lian, X. OsNRAMP3 is a vascular bundles-specific manganese transporter that is responsible for manganese distribution in rice. PLoS ONE 2013, 8, e83990. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.; Dong, D.; Jia, X.; McCouch, S.R.; Kochian, L.V. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 6503–6508. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 18381–18385. [Google Scholar] [CrossRef]
- Peris-Peris, C.; Serra-Cardona, A.; Sánchez-Sanuy, F.; Campo, S.; Ariño, J.; San Segundo, B. Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in rice. Mol. Plant. Microbe Interact. 2017, 30, 385–398. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Su, G.; Liu, J.; Liu, C.; Shi, G. Genotypic differences in spectral and photosynthetic response of peanut to iron deficiency. J. Plant Nutr. 2015, 38, 145–160. [Google Scholar] [CrossRef]
- Shi, G.; Su, G.; Lu, Z.; Liu, C.; Wang, X. Relationship between biomass, seed components and seed Cd concentration in various peanut (Arachis hypogaea L.) cultivars grown on Cd-contaminated soils. Ecotoxicol. Environ. Saf. 2014, 110, 174–181. [Google Scholar] [CrossRef]
- Liu, C.; Yu, R.; Shi, G. Effects of drought on the accumulation and redistribution of cadmium in peanuts at different developmental stages. Arch. Agron. Soil Sci. 2017, 63, 1049–1057. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Zhang, Z.; Shi, G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Xiong, H.; Kobayashi, T.; Kakei, Y.; Senoura, T.; Nakazono, M.; Takahashi, H.; Nakanishi, H.; Shen, H.; Duan, P.; Guo, X.; et al. AhNRAMP1 iron transporter is involved in iron acquisition in peanut. J. Exp. Bot. 2012, 63, 4437–4446. [Google Scholar] [CrossRef]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. Biophys. Acta 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Mani, A.; Sankaranarayanan, K. In silico analysis of natural resistance-associated macrophage protein (NRAMP) family of transporters in rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Han, P.; Chen, L.; Walk, T.C.; Li, Y.; Hu, X.; Xie, L.; Liao, H.; Liao, X. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.K.; Caldas, D.G.G.; Oliveira, L.R.; Frederici, G.C.; Leite, L.M.P.; Mui, T.S. Genome-wide characterization of the NRAMP gene family in Phaseolus vulgaris provides insights into functional implications during common bean development. Genet. Mol. Biol. 2018, 41, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; He, G.; Qin, L.; Li, D.; Meng, L.; Huang, Y.; He, T. Genome-wide analysis of the NRAMP gene family in potato (Solanum tuberosum): Identification, expression analysis and response to five heavy metals stress. Ecotoxicol. Environ. Saf. 2021, 208, 111661. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duan, Y.; Han, Z.; Shang, X.; Zhang, K.; Zou, Z.; Ma, Y.; Li, F.; Fang, W.; Zhu, X. Genome-wide identification and expression analysis of the NRAMP family genes in tea plant (Camellia sinensis). Plants 2021, 10, 1055. [Google Scholar] [CrossRef]
- Ullah, I.; Wang, Y.; Eide, D.J.; Dunwell, J.M. Evolution, and functional analysis of Natural Resistance-Associated Macrophage Proteins (NRAMPs) from Theobroma cacao and their role in cadmium accumulation. Sci. Rep. 2018, 8, 14412. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Li, G.; Kumar, S.; Sun, Z.; Li, Y.; Guo, W.; Yang, J.; Hou, H. Genome-wide identification of the Nramp gene family in Spirodela polyrhiza and expression analysis under cadmium stress. Int. J. Mol. Sci. 2021, 22, 6414. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Z.; Shi, G. Genome-wide identification of metal tolerance protein genes in peanut: Differential expression in the root of two contrasting cultivars under metal stresses. Front. Plant Sci. 2022, 13, 791200. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, N.; Zhang, Z.; Shi, G. Genome-wide identification and expression profile reveal potential roles of peanut ZIP family genes in zinc/iron-deficiency tolerance. Plants 2022, 11, 786. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, J.; Guan, J.; Tan, Z.; Zhang, Z.; Shi, G. Genome-wide identification and transcript analysis reveal potential roles of oligopeptide transporter genes in iron deficiency induced cadmium accumulation in peanut. Front. Plant Sci. 2022, 13, 894848. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal-Bertioli, S.C.M.; Ren, L.; Farmer, A.D.; Pandey, M.K.; et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef]
- Soltis, D.E.; Visger, C.J.; Soltis, P.S. The polyploidy revolution then…and now: Stebbins revisited. Am. J. Bot. 2014, 101, 1057–1078. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Q.; Liu, H.; Zhang, J.; Hong, Y.; Lan, H.; Li, H.; Wang, J.; Liu, H.; Li, S.; et al. Sequencing of cultivated peanut, Arachis hypogaea, yields insights into genome evolution and oil improvement. Mol. Plant 2019, 12, 920–934. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Qian, W.; Liao, B.-Y.; Chang, A.Y.F.; Zhang, J. Maintenance of duplicate genes and their functional redundancy by reduced expression. Trends Genet. 2010, 26, 425–430. [Google Scholar] [CrossRef]
- Meng, J.G.; Zhang, X.D.; Tan, S.K.; Zhao, K.X.; Yang, Z.M. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. Biometals 2017, 30, 917–931. [Google Scholar] [CrossRef]
- Ehrnstorfer, I.A.; Geertsma, E.R.; Pardon, E.; Steyaert, J.; Dutzler, R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat. Struct. Mol. Biol. 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Thomine, S.; Wang, R.; Ward, J.M.; Crawford, N.M.; Schroeder, J.I. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4991–4996. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Tsirigos, K.D.; Peters, C.; Shu, N.; Käll, L.; Elofsson, A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015, 43, W401–W407. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Clevenger, J.; Chu, Y.; Scheffler, B.; Ozias-Akins, P. A developmental transcriptome map for allotetraploid Arachis hypogaea. Front. Plant Sci. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Gene Length (bp) | CDS (bp) | Aa a | MW b (kDa) | Instability | Aliphatic Index | GRAVY c | pI d | No. of TMD e | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AhNRAMP1.1 | 112706038 | 2062 | 1638 | 546 | 58.78 | 36.06 | 120.26 | 0.564 | 8.93 | 12 | PM f |
| AhNRAMP1.2 | 112717764 | 742 | 570 | 190 | 20.83 | 31.17 | 123.76 | 0.584 | 8.74 | 4 | PM |
| AhNRAMP1.3 | 112766867 | 2393 | 1638 | 546 | 58.77 | 37.32 | 120.79 | 0.566 | 8.93 | 12 | PM |
| AhNRAMP2.1 | 112717946 | 1638 | 1638 | 546 | 60.18 | 41.85 | 111.45 | 0.494 | 5.21 | 12 | VM g |
| AhNRAMP2.2 | 112706457 | 2334 | 1680 | 560 | 61.35 | 35.46 | 108.53 | 0.355 | 5.35 | 12 | VM |
| AhNRAMP2.3 | 112729510 | 1756 | 1638 | 546 | 60.24 | 42.40 | 110.55 | 0.486 | 5.29 | 12 | VM |
| AhNRAMP2.4 | 112764447 | 2341 | 1680 | 560 | 61.39 | 34.44 | 108.87 | 0.363 | 5.27 | 12 | VM |
| AhNRAMP3.1 | 112799830 | 1868 | 1563 | 521 | 57.34 | 38.79 | 119.81 | 0.530 | 5.23 | 12 | VM |
| AhNRAMP3.2 | 112800530 | 2085 | 1515 | 505 | 55.15 | 39.60 | 122.66 | 0.667 | 4.94 | 12 | VM |
| AhNRAMP3.3 | 112720549 | 1984 | 1563 | 521 | 57.37 | 37.77 | 119.25 | 0.532 | 5.42 | 12 | VM |
| AhNRAMP3.4 | 112748442 | 2056 | 1494 | 498 | 54.41 | 41.17 | 124.19 | 0.693 | 4.92 | 12 | VM |
| AhNRAMP6.1 | 112703806 | 2277 | 1674 | 558 | 59.78 | 31.48 | 118.01 | 0.580 | 8.62 | 12 | PM |
| AhNRAMP6.2 | 112709742 | 2141 | 1608 | 536 | 58.14 | 38.23 | 118.52 | 0.598 | 8.69 | 12 | PM |
| AhNRAMP6.3 | 112773194 | 2505 | 1674 | 558 | 59.78 | 31.48 | 118.01 | 0.580 | 8.62 | 12 | PM |
| AhNRAMP6.4 | 112776188 | 1869 | 1605 | 535 | 58.04 | 38.65 | 118.56 | 0.598 | 8.57 | 12 | PM |
| Gene Pairs | Duplicate Type | Ka a | Ks b | Ka/Ks c | Positive Selection | Divergence Time (Mya) |
|---|---|---|---|---|---|---|
| AhNRAMP1.1/1.2 | Segmental | 0.0069 | 0.0077 | 0.9017 | No | 0.472 |
| AhNRAMP3.1/3.2 | Segmental | 0.0966 | 0.9455 | 0.1022 | No | 58.221 |
| AhNRAMP3.3/3.4 | Segmental | 0.0865 | 0.8961 | 0.0966 | No | 55.178 |
| AhNRAMP6.1/6.2 | Segmental | 0.1076 | 0.8321 | 0.1294 | No | 51.236 |
| AhNRAMP6.3/6.4 | Segmental | 0.1074 | 0.8417 | 0.1276 | No | 51.826 |
| AhNRAMP1.1/1.3 | Whole-genome | 0.0065 | 0.0201 | 0.3237 | No | 1.240 |
| AhNRAMP2.1/2.3 | Whole-genome | 0.0040 | 0.0481 | 0.0834 | No | 2.963 |
| AhNRAMP2.2/2.4 | Whole-genome | 0.0047 | 0.0329 | 0.1440 | No | 2.023 |
| AhNRAMP3.1/3.3 | Whole-genome | 0.0051 | 0.0368 | 0.1399 | No | 2.265 |
| AhNRAMP3.2/3.4 | Whole-genome | 0.0009 | 0.0219 | 0.0407 | No | 1.351 |
| AhNRAMP6.1/6.3 | Whole-genome | 0.0000 | 0.0000 | - | No | 0.000 |
| AhNRAMP6.2/6.4 | Whole-genome | 0.0008 | 0.0600 | 0.0136 | No | 3.697 |
| Gene Expression | Fe in Roots | Fe in Shoots | Total Fe in Plants | % of Fe in Shoots | Cd in Roots | Cd in Shoots | Total Cd in Plants | % of Cd in Shoots |
|---|---|---|---|---|---|---|---|---|
| AHNRAMP1.1 | −0.646 ** | −0.820 ** | −0.681 ** | −0.294 | 0.699 * | 0.785 ** | 0.700 * | −0.432 |
| AHNRAMP1.2 | −0.541 ** | −0.719 ** | −0.588 ** | −0.278 | 0.699 * | 0.876 ** | 0.738 ** | −0.380 |
| AHNRAMP1.3 | −0.610 ** | −0.828 ** | −0.673 ** | −0.352 | 0.650 * | 0.754 ** | 0.616 * | −0.367 |
| AHNRAMP2.2 | −0.142 | 0.223 | −0.015 | 0.529 ** | 0.657 * | 0.830 ** | 0.656 * | −0.326 |
| AHNRAMP2.4 | −0.349 | −0.544 ** | −0.230 | −0.498 * | −0.183 | −0.261 | −0.001 | −0.026 |
| AHNRAMP3.1 | −0.570 ** | −0.555 ** | −0.501 * | −0.123 | 0.888 ** | 0.802 ** | 0.871 ** | −0.721 ** |
| AHNRAMP3.2 | −0.192 | −0.192 | −0.099 | 0.069 | 0.423 | 0.718 ** | 0.505 | −0.093 |
| AHNRAMP3.3 | −0.594 ** | −0.400 | −0.438 * | 0.010 | 0.847 ** | 0.752 ** | 0.817 ** | −0.705 * |
| AHNRAMP3.4 | −0.561 ** | −0.568 ** | −0.546 ** | 0.051 | 0.670 * | 0.809 ** | 0.678 * | −0.364 |
| AHNRAMP6.2 | −0.596 ** | −0.740 ** | −0.610 ** | −0.235 | 0.603 * | 0.748 ** | 0.622 * | −0.285 |
| AHNRAMP6.3 | −0.261 | 0.246 | 0.055 | 0.464 * | 0.808 ** | 0.908 ** | 0.872 ** | −0.550 |
| AHNRAMP6.4 | −0.178 | −0.331 | −0.445 * | 0.158 | 0.640 * | 0.761 ** | 0.596 * | −0.367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Li, J.; Guan, J.; Wang, C.; Zhang, Z.; Shi, G. Genome-Wide Identification and Expression Analysis Reveals Roles of the NRAMP Gene Family in Iron/Cadmium Interactions in Peanut. Int. J. Mol. Sci. 2023, 24, 1713. https://doi.org/10.3390/ijms24021713
Tan Z, Li J, Guan J, Wang C, Zhang Z, Shi G. Genome-Wide Identification and Expression Analysis Reveals Roles of the NRAMP Gene Family in Iron/Cadmium Interactions in Peanut. International Journal of Molecular Sciences. 2023; 24(2):1713. https://doi.org/10.3390/ijms24021713
Chicago/Turabian StyleTan, Zengjing, Jinxiu Li, Junhua Guan, Chaohui Wang, Zheng Zhang, and Gangrong Shi. 2023. "Genome-Wide Identification and Expression Analysis Reveals Roles of the NRAMP Gene Family in Iron/Cadmium Interactions in Peanut" International Journal of Molecular Sciences 24, no. 2: 1713. https://doi.org/10.3390/ijms24021713
APA StyleTan, Z., Li, J., Guan, J., Wang, C., Zhang, Z., & Shi, G. (2023). Genome-Wide Identification and Expression Analysis Reveals Roles of the NRAMP Gene Family in Iron/Cadmium Interactions in Peanut. International Journal of Molecular Sciences, 24(2), 1713. https://doi.org/10.3390/ijms24021713






