Synthesis and Antimicrobial Activity of the Pathogenic E. coli Strains of p-Quinols: Additive Effects of Copper-Catalyzed Addition of Aryl Boronic Acid to Benzoquinones
Abstract
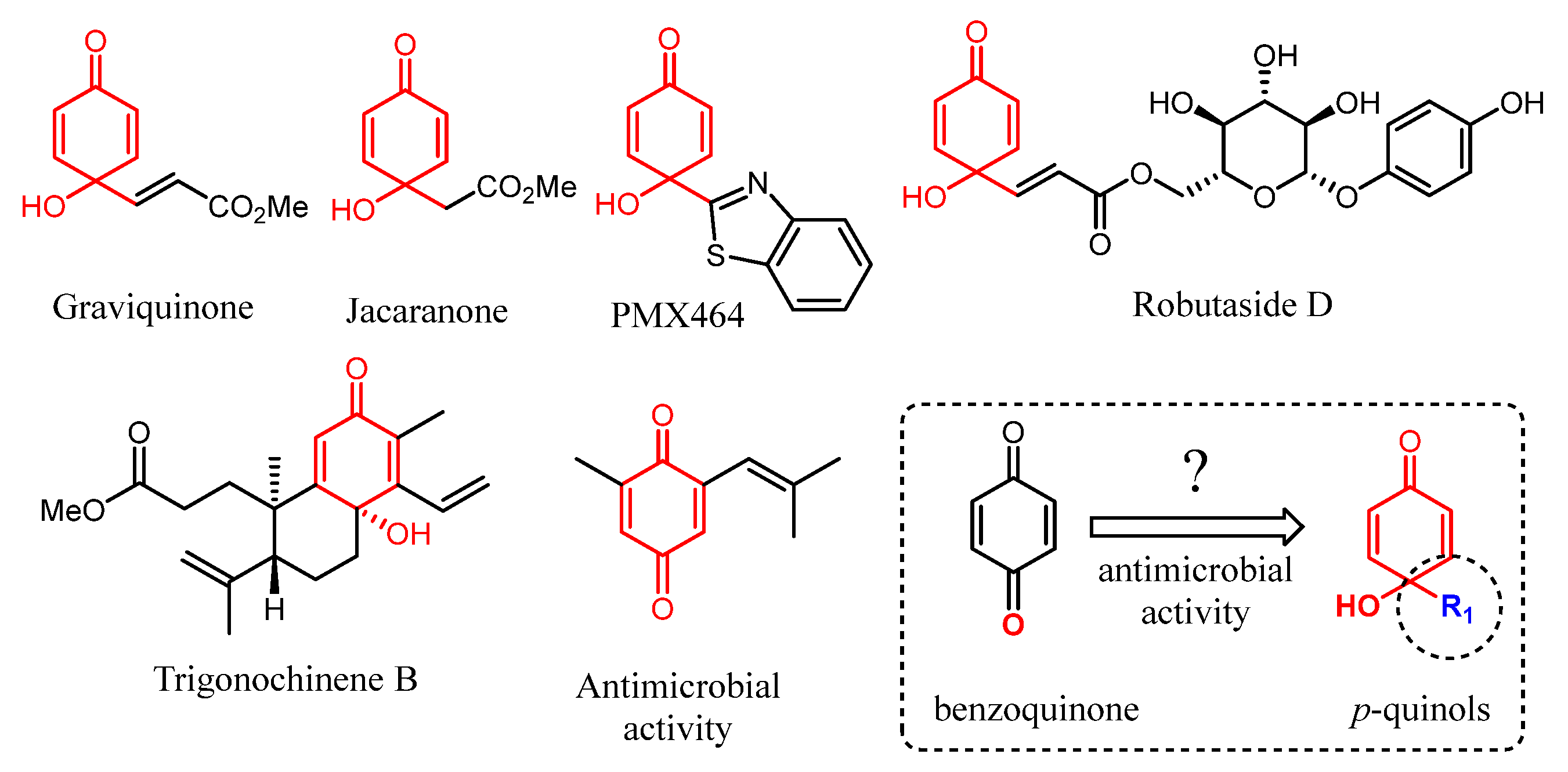
1. Introduction
2. Results and Discussion
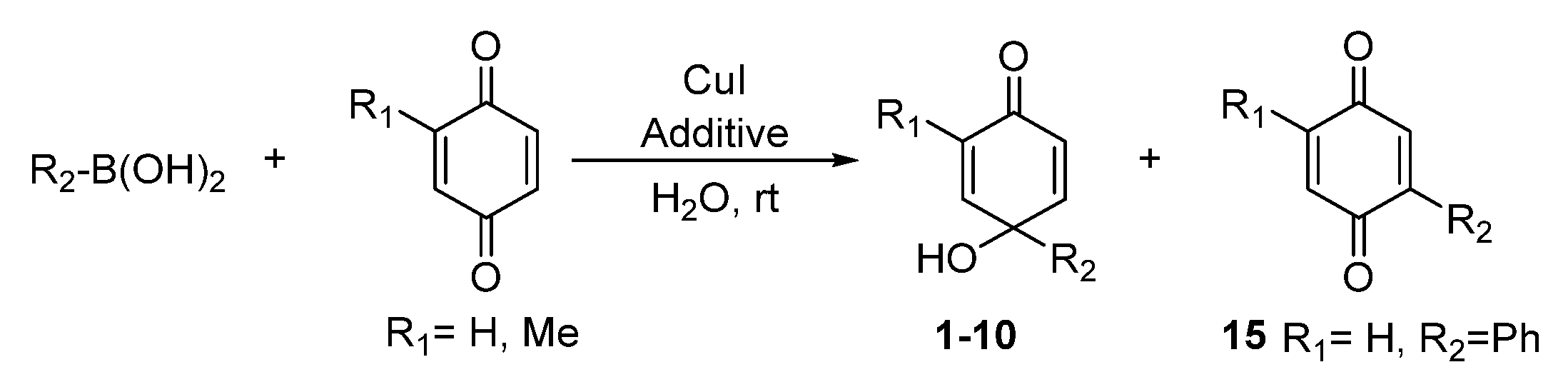
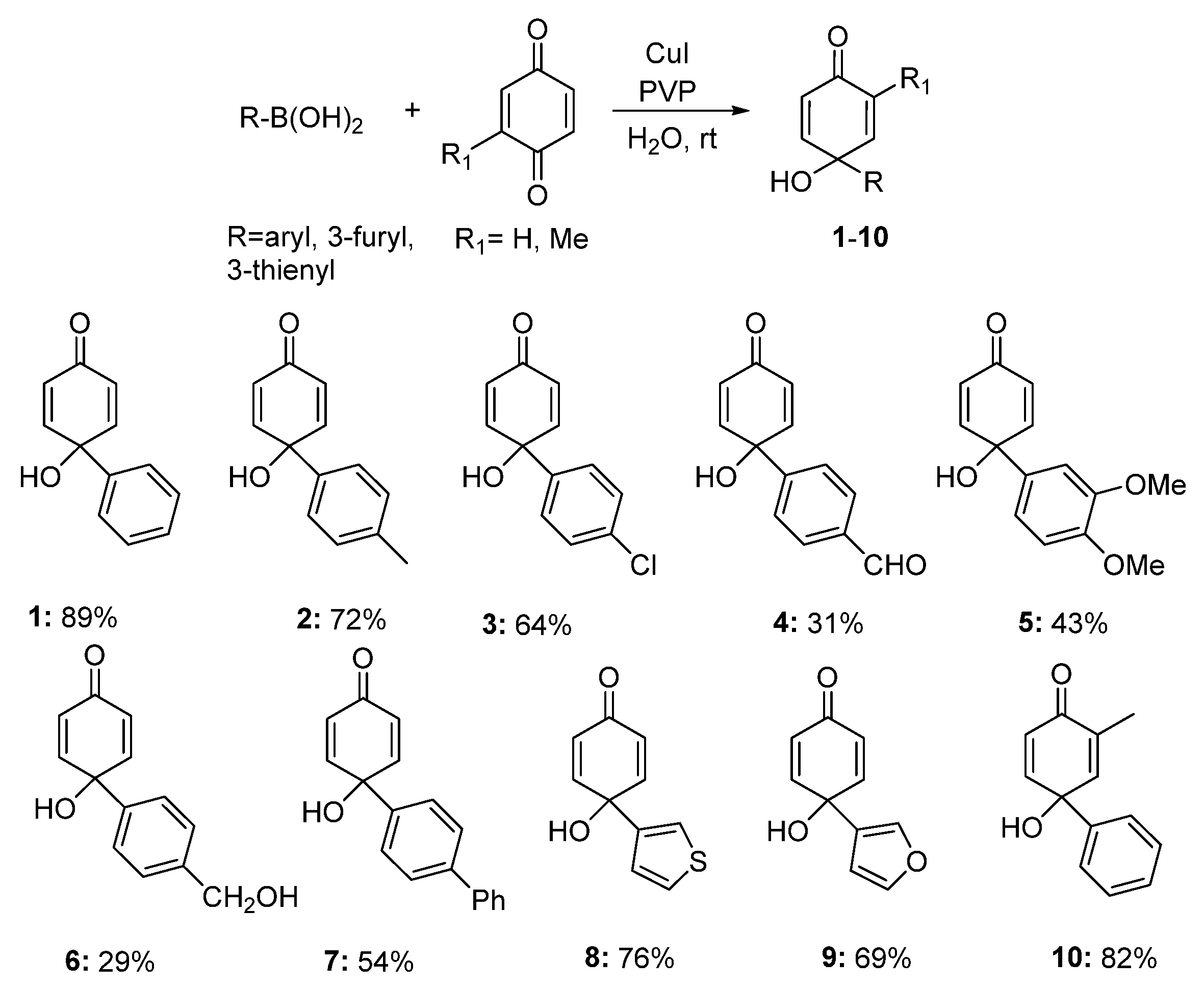
2.1. Chemistry
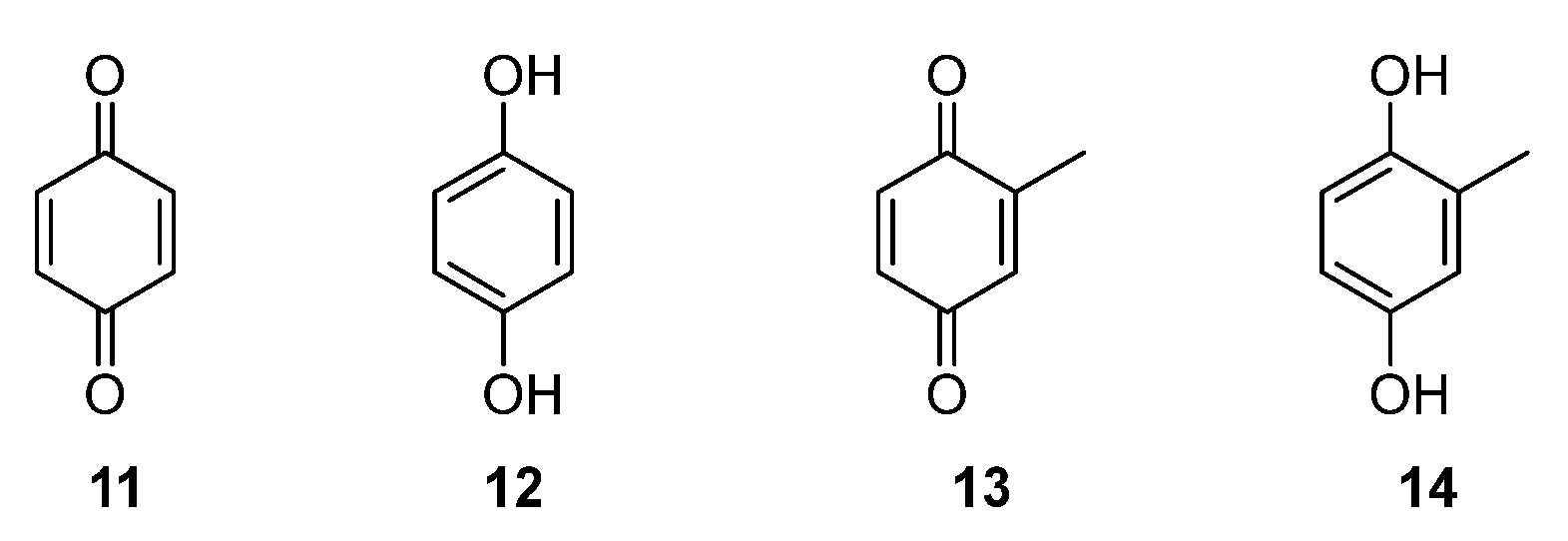
2.2. Cytotoxic Studies of the Library of p-Quinols 1–10, and Parent Benzo- and Hydroquinones 11–14
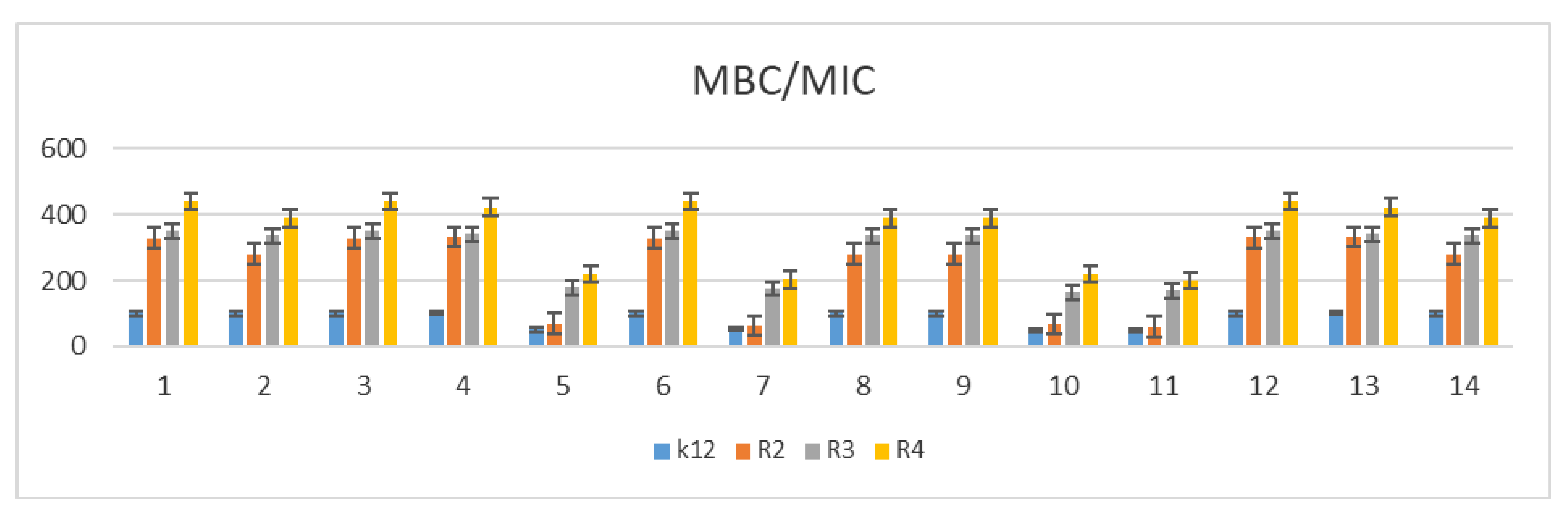
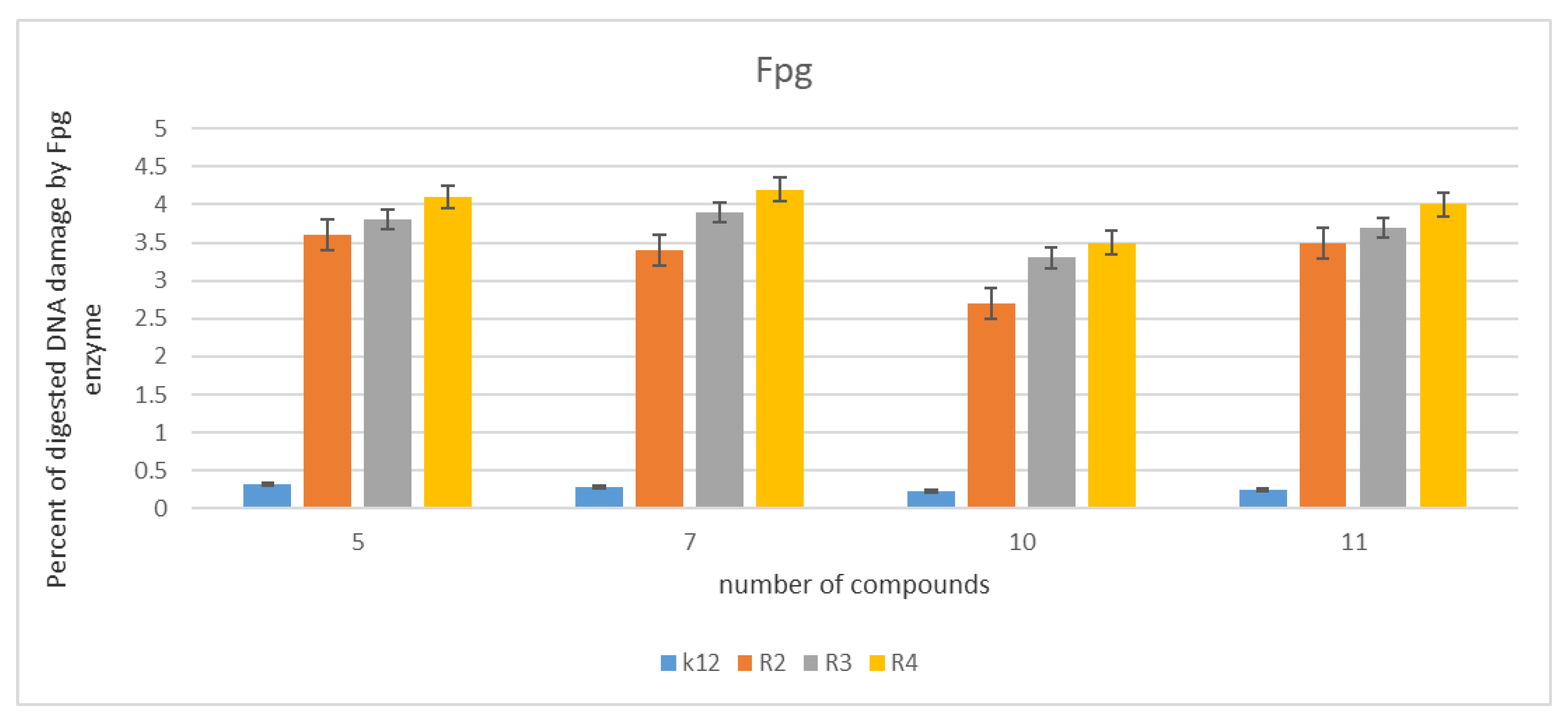
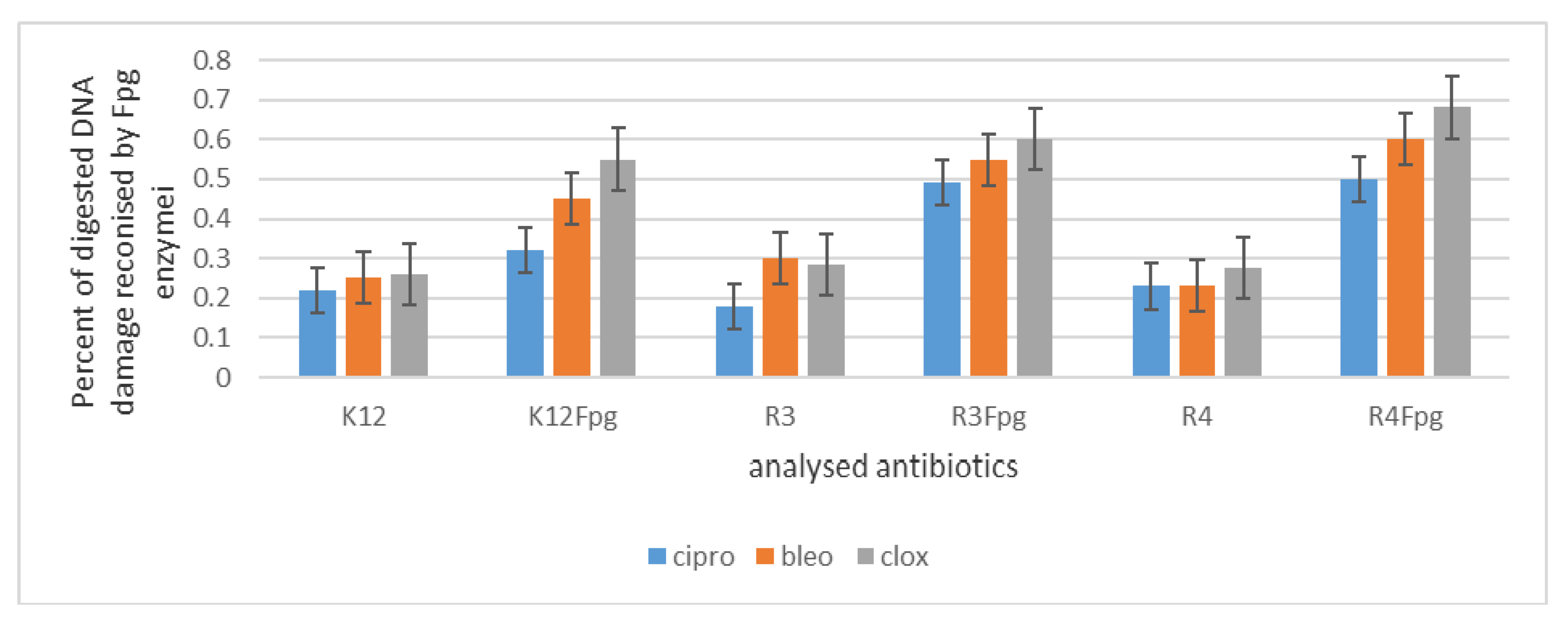
2.3. Analysis of Bacterial DNA Isolated from E. coli R2–R4 Strains Modified with Tested p-Quinols
3. Materials and Methods
3.1. Microorganisms and Media
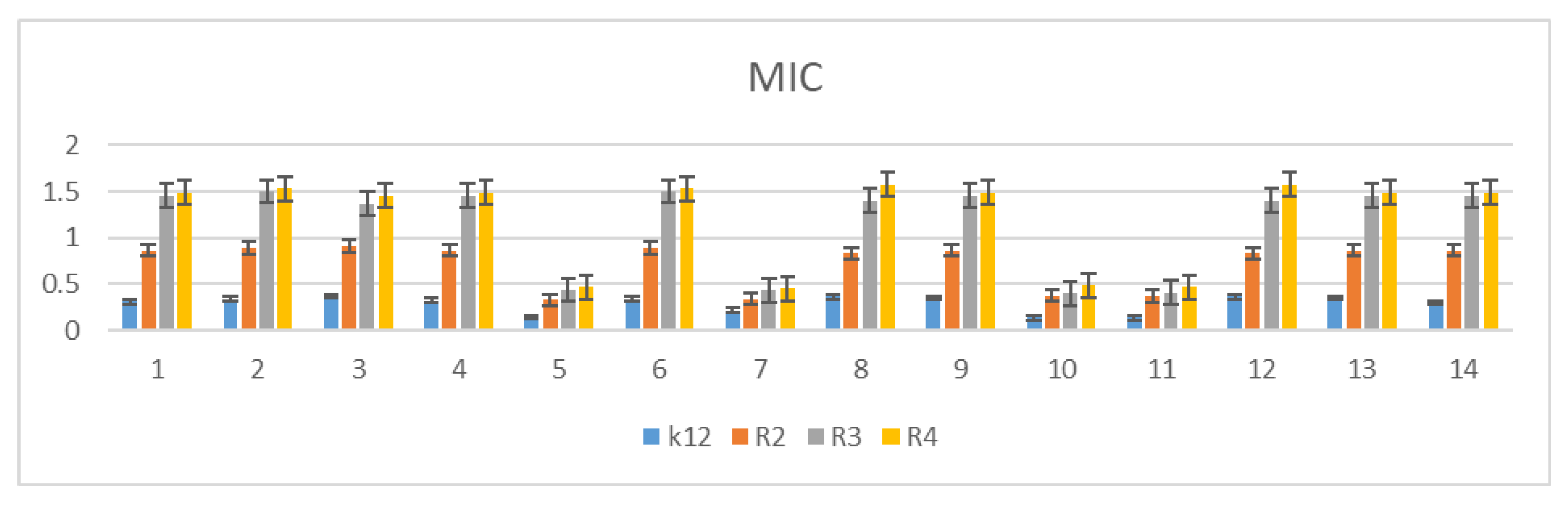
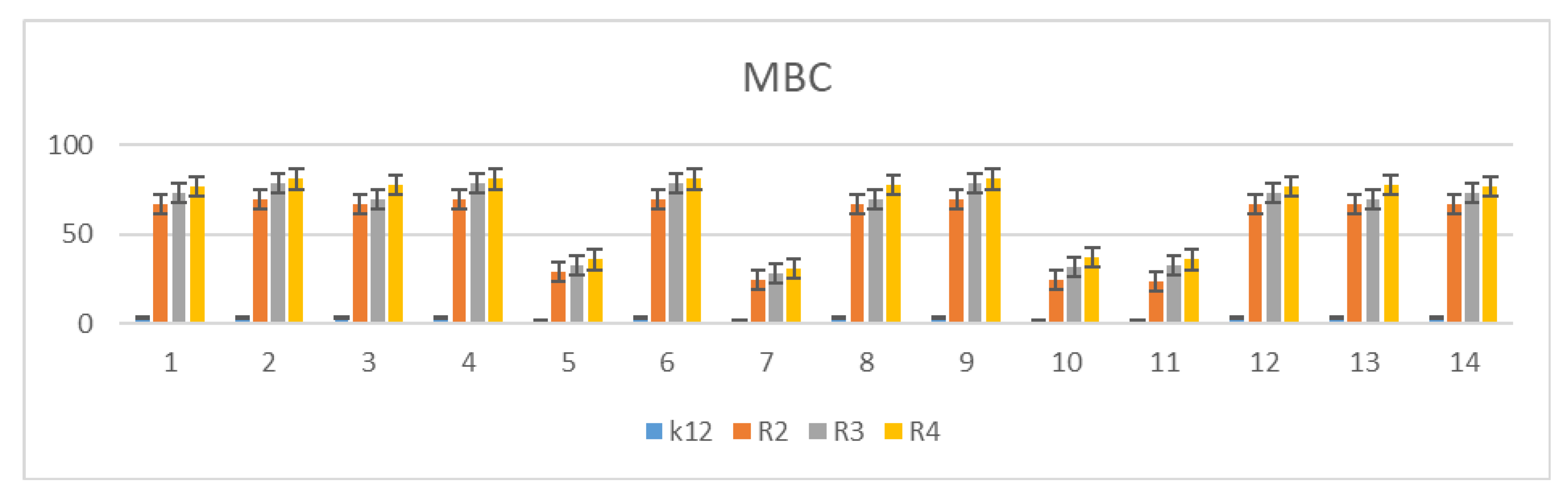
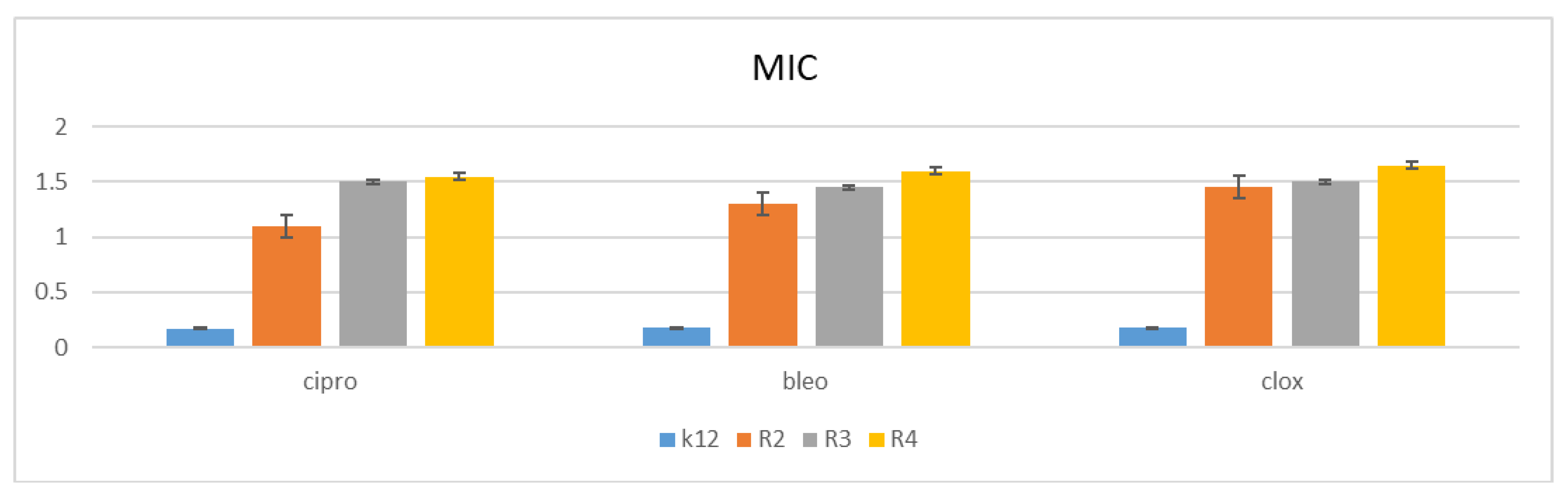
3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.3. Chemicals
3.4. General Procedure for the Synthesis of p-Quinols
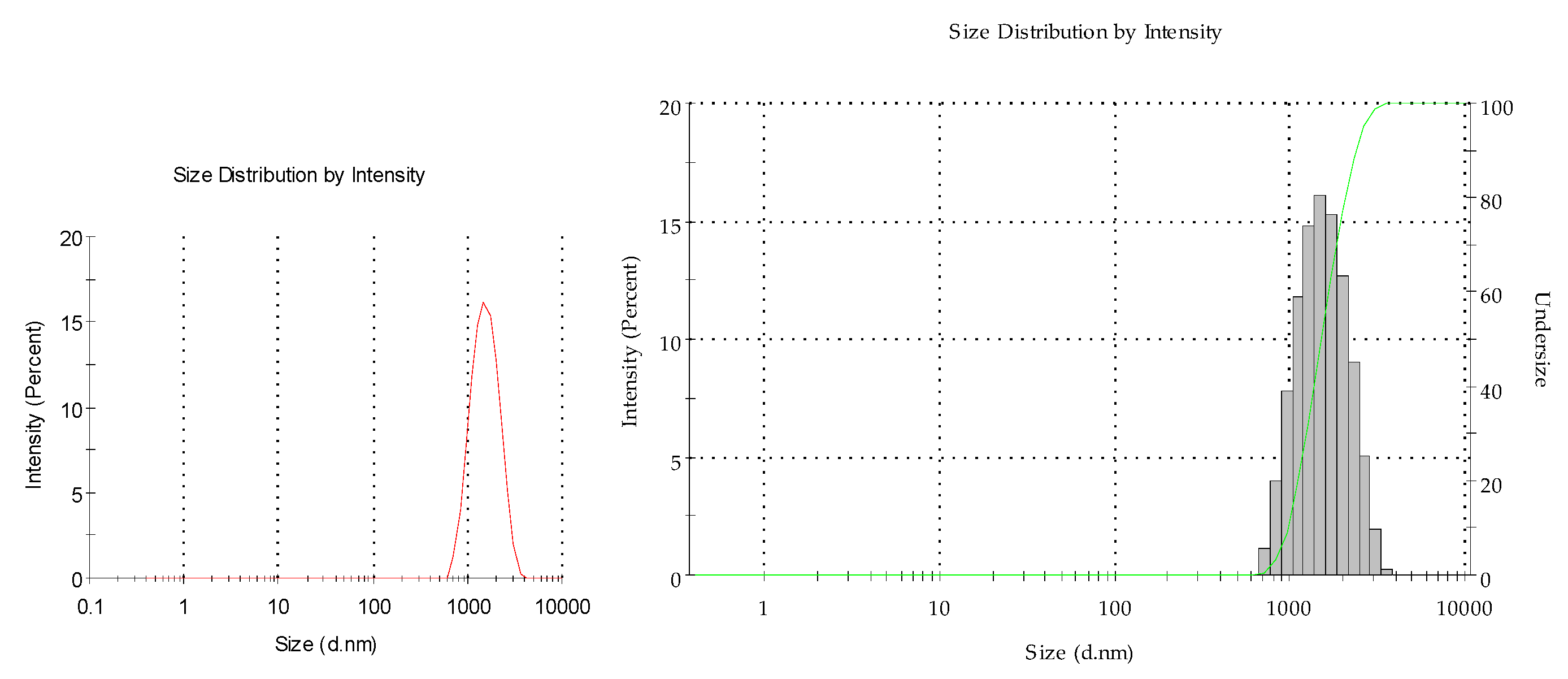
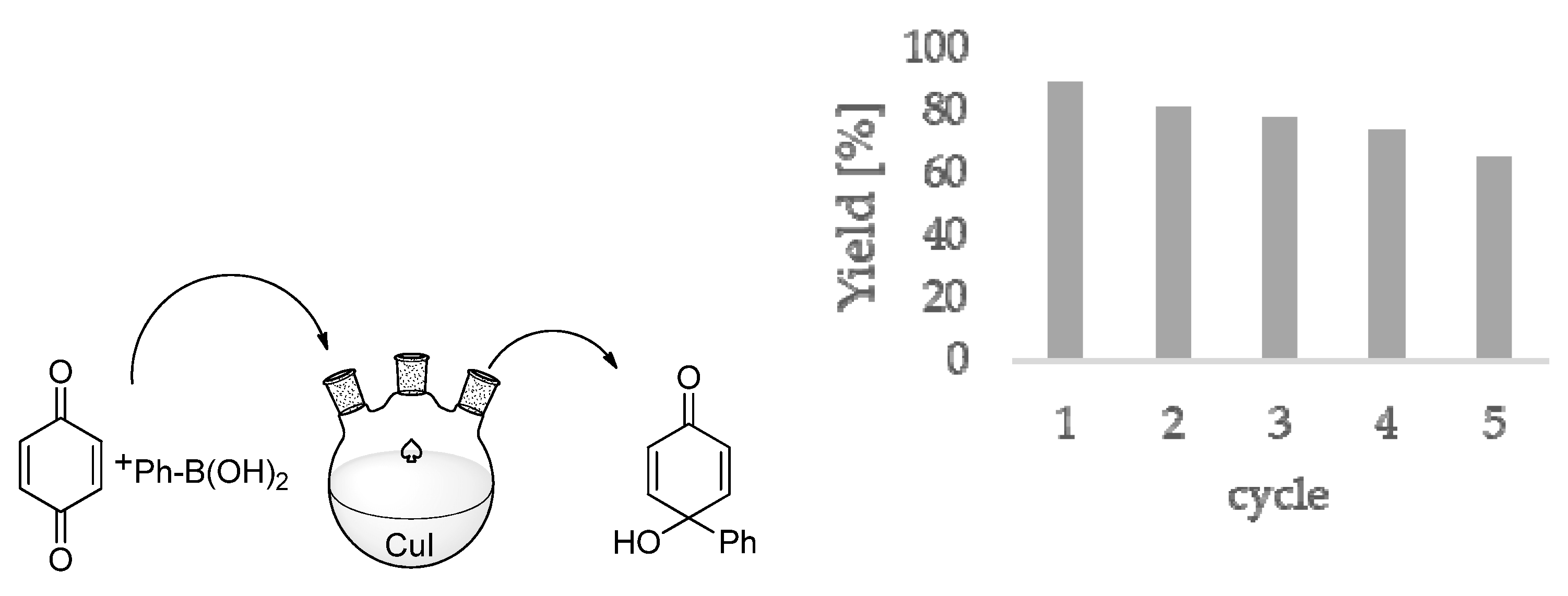
3.5. Preparation of Copper–PVP Colloids in Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| Oc | open circle |
| Ccc | covalently closed circle |
| BER | base excision repair |
| Fpg | DNA-formamidopyrimidine glycosylase |
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Sugimoto, O.; Tanji, K.; Hirota, A. Identification of the Quinol Metabolite “Sorbicillinol”, a Key Intermediate Postulated in Bisorbicillinoid Biosynthesis. J. Am. Chem. Soc. 2000, 122, 12606–12607. [Google Scholar] [CrossRef]
- Urban, S.; Blunt, J.W.; Munro, M.H.G. Coproverdine, a Novel, Cytotoxic Marine Alkaloid from a New Zealand Ascidian. J. Nat. Prod. 2002, 65, 1371–1373. [Google Scholar] [CrossRef]
- Chan, H.-H.; Hwang, T.-L.; Thang, T.D.; Leu, Y.-L.; Kuo, P.-C.; Nguyet, B.T.M.; Dai, D.N.; Wu, T.-S. Isolation and Synthesis of Melodamide A, a New Anti-inflammatory Phenolic Amide from the Leaves of Melodorum fruticosum. Planta Med. 2013, 79, 288–294. [Google Scholar] [CrossRef]
- Magdziak, D.; Meek, S.J.; Pettus, T.R.R. Cyclohexadienone ketals and quinols: Four building blocks potentially useful for enantioselective synthesis. Chem. Rev. 2004, 104, 1383–1429. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Molina, M.T.; Anjum, S. Naturally Occurring Cyclohexane Epoxides: Sources, Biological Activities, and Synthesis. Chem. Rev. 2004, 104, 2857–2900. [Google Scholar] [CrossRef]
- You, Z.; Hoveyda, A.H.; Snapper, M.L. Catalytic Enantioselective Silylation of Acyclic and Cyclic Triols: Application to Total Syntheses of Cleroindicins D, F, and C. Angew. Chem. Int. Ed. 2009, 48, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Tello-Aburto, R.; Kalstabakken, K.A.; Volp, K.A.; Harned, A.M. Regioselective and stereoselective cyclizations of cyclohexadienones tethered to active methylene groups. Org. Biomol. Chem. 2011, 9, 7849–7859. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yin, Z.; Zeng, H.; Khanna, K.; Huc, C.; Zheng, S. An expedient stereoselective and chemoselective synthesis of bicyclic oxazolidinones from quinols and isocyanates. Org. Biomol. Chem. 2013, 11, 2939–2942. [Google Scholar] [CrossRef]
- Xie, L.; Dong, S.; Zhang, Q.; Feng, X.; Liu, X. Asymmetric construction of dihydrobenzofuran- 2,5-dione derivatives via desymmetrization of p-quinols with azlactones. Chem. Commun. 2019, 55, 87–90. [Google Scholar] [CrossRef]
- Kitson, R.R.A.; Taylor, R.J.K.; Wood, J.L. A One-Pot, Base-Free Annelation Approach to r-Alkylidene-γ-butyrolactones. Org. Lett. 2009, 11, 5338–5341. [Google Scholar] [CrossRef]
- Moon, N.G.; Harned, A.M. A concise synthetic route to the stereotetrad core of the briarane diterpenoids. Org. Lett. 2015, 17, 2218–2221. [Google Scholar] [CrossRef] [PubMed]
- García-García, C.; Ortiz-Rojano, L.; Álvarez, S.; Álvarez, R.; Ribagorda, M.; Carreño, M.C. Friedel–Crafts Alkylation of Indoles with p-Quinols: The Role of Hydrogen Bonding of Water for the Desymmetrization of the Cyclohexadienone System. Org. Lett. 2016, 18, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Imbos, R.; Minnaard, A.J.; Feringa, B.L. A Highly Enantioselective Intramolecular Heck Reaction with a Monodentate Ligand. J. Am. Chem. Soc. 2002, 124, 184–185. [Google Scholar] [CrossRef]
- Liu, Q.; Rovis, T. Asymmetric Synthesis of Hydrobenzofuranones via Desymmetrization of Cyclohexadienones Using the Intramolecular Stetter Reaction. J. Am. Chem. Soc. 2006, 128, 2552–2553. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.-J.; Gao, Y.-N.; Shi, M. Phosphine-Initiated Cascade Annulation of β′-Acetoxy Allenoate and p-Quinols: Access to Ring Fused Hexahydroindeno Furan Derivatives. Adv. Synth. Catal. 2018, 360, 2552–2559. [Google Scholar] [CrossRef]
- Zhao, F.; Li, N.; Zhu, Y.-F.; Han, Z.-Y. Enantioselective Construction of Functionalized Tetrahydrocarbazoles Enabled by Asymmetric Relay Catalysis of Gold Complex and Chiral Brønsted Acid. Org. Lett. 2016, 18, 1506–1509. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Xu, M.; Zhao, X.; Zhou, X.; Zhao, W.; Liu, L. Catalytic stereoselective cascade reactions of quinols with trifluoromethyl ketones: Direct access to CF3-containing 1,3-dioxolanes. Org. Biomol. Chem. 2016, 14, 3981–3988. [Google Scholar] [CrossRef]
- Berry, J.M.; Bradshaw, T.D.; Fichtner, I.; Ren, R.; Schwalbe, C.H.; Wells, G.; Chew, E.H.; Stevens, M.F.G.; Westwell, A.D. Quinols as Novel Therapeutic Agents. 2.1 4-(1-Arylsulfonylindol-2-yl)-4-hydroxycyclohexa-2,5-dien-1-ones and Related. Agents as Potent and Selective Antitumor Agents. J. Med. Chem. 2005, 48, 639–644. [Google Scholar] [CrossRef]
- Bradshaw, T.D.; Matthews, C.S.; Cookson, J.; Chew, E.H.; Shah, M.; Bailey, K.; Monks, A.; Harris, E.; Westwell, A.D.; Wells, G.; et al. Elucidation of Thioredoxin as a Molecular Target for Antitumor Quinols. Cancer Res. 2005, 65, 3911–3919. [Google Scholar] [CrossRef]
- Wells, G.; Berry, J.M.; Bradshaw, T.D.; Burger, A.M.; Seaton, A.; Wang, B.; Westwell, A.D.; Stevens, M.F.G. 4-Substituted 4-Hydroxycyclohexa-2,5-dien-1-ones with Selective Activities against Colon and Renal Cancer Cell Lines. J. Med. Chem. 2003, 46, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Pugh, C.W.; Wigfield, S.; Stevens, M.F.G.; Harris, A.L. Novel Thioredoxin Inhibitors Paradoxically Increase Hypoxia-Inducible Factor-A Expression but Decrease Functional Transcriptional Activity, DNABinding, and Degradation. Clin. Cancer Res. 2006, 12, 5384–5394. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.R.; Romoff, P.; Fávero, O.A.; Reimão, J.Q.; Lourenço, W.C.; Tempone, A.G.; Hristov, A.D.; Di Santi, S.M.; Lago, J.H.G.; Sartorelli, P.; et al. Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacalia desiderabilis (Vell.) Cuatrec. (Asteraceae). Parasitol. Res. 2012, 110, 95–101. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wyllie, S.; Wells, G.; Stevens, M.F.; Wyatt, P.G.; Fairlamb, A.H. Antitumor Quinol PMX464 Is a Cytocidal Anti-trypanosomal Inhibitor Targeting Trypanothione Metabolism. J. Biol. Chem. 2011, 286, 8523–8533. [Google Scholar] [CrossRef]
- Capes, A.; Patterson, S.; Wyllie, S.; Hallyburton, I.; Collie, I.T.; McCarroll, A.J.; Stevens, M.F.G.; Frearson, J.A.; Wyatt, P.G.; Fairlamb, A.H.; et al. Quinol derivatives as potential trypanocidal agents. Bioorg. Med. Chem. 2012, 20, 1607–1615. [Google Scholar] [CrossRef]
- Lajide, L.; Escoubas, P.; Mizutani, J. Cyclohexadienones-insect growth inhibitors from the foliar surface and tissue extracts of Senecio cannabifolius. Experientia 1996, 52, 259–263. [Google Scholar] [CrossRef]
- Lia, H.-X.; Xiaoa, C.-J.; Wangb, M.; Cuib, S.-J.; Lia, H.-F.; Wanga, K.-L.; Donga, X.; Jiang, B. Four new phenylethanoid glycosides from Ternstroemia gymnanthera and their analgesic activities. Phytochem. Lett. 2019, 34, 25–29. [Google Scholar] [CrossRef]
- Abraham, I.; Joshi, R.; Pardasani, P.; Pardasani, R.T. Recent Advances in 1,4-Benzoquinone Chemistry. J. Braz. Chem. Soc. 2011, 22, 385–421. [Google Scholar] [CrossRef]
- Singh, N.; Mishra, B.B.; Bajpai, S.; Singh, R.K.; Tiwari, V.K. Natural product based leads to fight against leishmaniasis. Bioorg. Med. Chem. 2014, 22, 18–45. [Google Scholar] [CrossRef]
- Drewes, S.E.; Khan, F.; van Vuuren, S.F.; Viljoen, A.M. Simple 1,4-benzoquinones with antibacterial activity from stems and leaves of Gunnera perpensa. Phytochemistry 2005, 66, 1812–1816. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jo, S.-H.; Ha, K.-S.; Song, J.-H.; Jang, H.-D.; Kwon, Y.-I. Antimicrobial Activities of 1,4-Benzoquinones and Wheat Germ Extract. J. Microbiol. Biotechnol. 2010, 20, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Carcamo-Noriegaa, E.N.; Sathyamoorthib, S.; Banerjeeb, S.; Gnanamanib, E.; Mendoza-Trujillod, M.; Mata-Espinosad, D.; Hernández-Pandod, R.; Veytia-Buchelia, J.I.; Possania, L.D.; Zare, R.N. 1,4-Benzoquinone antimicrobial agents against Staphylococcus aureus and Mycobacterium tuberculosis derived from scorpion venom. Proc. Natl. Acad. Sci. USA 2019, 116, 12642–12647. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial Mechanism of Hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Yakura, T.; Omoto, M. Efficient Synthesis of P-Quinols Using Catalytic Hypervalent Iodine Oxidation of 4-arylphenols with 4-iodophenoxyacetic Acid and Oxone. Chem. Pharm. Bull. 2009, 57, 643–645. [Google Scholar] [CrossRef]
- Felpin, F.-X. Oxidation of 4-arylphenol trimethylsilyl ethers to p-arylquinols using hypervalent iodine(III) reagents. Tetrahedron Lett. 2007, 48, 409–412. [Google Scholar] [CrossRef]
- Fischer, A.; Henderson, G.N. Reactions of organolithium reagents with p-benzoquinones and cyclohexadienones. Synthesis of 4-alkyl-4-hydroxycyclohexa-2,5-dien-1-ones and 1,4-dialkylcyclohexa-2,5-diene-1,4-diols. Tetrahedron Lett. 1980, 21, 701–704. [Google Scholar] [CrossRef]
- Ghandi, M.; Shahidzadeh, M. Experimental and semiemprical stud-ies of chemical reactivity of dialkylcadmium reagents addition to α,β-enones. J. Organometal. Chem. 2006, 691, 4918–4925. [Google Scholar] [CrossRef]
- Muthusamy, S.; Krishnamurthi, J. Multicomponent reactions involving p-benzoquinones, diazo esters, titanium(IV) isopropoxide and alcohol in the presence of rhodium(II) acetate as catalyst. Tetrahedron Lett. 2007, 48, 6692–6695. [Google Scholar] [CrossRef]
- Krause, K.P.; Kayser, O.; Mäder, K.; Gust, R.; Müller, R.H. Heavy metal contamination of nanosuspensions produced by high-pressure homogenisation. Int. J. Pharmaceut. 2000, 196, 169–172. [Google Scholar] [CrossRef]
- Koszelewski, D.; Paprocki, D.; Brodzka, A.; Kęciek, A.; Wilk, M.; Ostaszewski, R. The sustainable copper-catalyzed direct formation of highly functionalized p-quinols in water. Sustain. Chem. Pharm. 2022, 25, 100576. [Google Scholar] [CrossRef]
- Ranu, B.C.; Dey, R.; Chatterjee, T.; Ahammed, S. Copper Nanoparticle-Catalyzed Carbon-Carbon and Carbon-Heteroatom Bond Formation with a Greener Perspective. ChemSusChem 2012, 5, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Iwamatsu, S.-i.; Matsubara, K.; Nagashima, H. Synthetic Studies of cis-3a-Aryloctahydroindole Derivatives by Copper-Catalyzed Cyclization of N-Allyltrichloroacetamides: Facile Construction of Benzylic Quaternary Carbons by Carbon−Carbon Bond-Forming Reactions. J. Org. Chem. 1999, 64, 9625–9631. [Google Scholar] [CrossRef]
- Shilpa, T.; Neetha, M.; Anilkumara, G. Recent Trends and Prospects in the Copper-Catalysed “on Water” Reactions. Adv. Synth. Catal. 2021, 363, 1559–1582. [Google Scholar] [CrossRef]
- Villalobos, J.M.; Srogl, J.; Liebeskind, L.S. A New Paradigm for Carbon−Carbon Bond Formation: Aerobic, Copper-Templated Cross-Coupling. J. Am. Chem. Soc. 2007, 129, 15734–15735. [Google Scholar] [CrossRef] [PubMed]
- Prokopcová, H.; Kappe, C.O. Palladium(0)-Catalyzed, Copper(I)-Mediated Coupling of Boronic Acids with Cyclic Thioamides. Selective Carbon−Carbon Bond Formation for the Functionalization of Heterocycles. J. Org. Chem. 2007, 72, 4440–4448. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Yavari, A.; Bahadorikhalili, S.; Moazzam, A.; Hosseini, S.; Larijani, B.; Iraji, A.; Moradi, S.; Mahdavi, M. Copper Catalyst-Supported Modified Magnetic Chitosan for the Synthesis of Novel 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives via Chan–Lam Coupling. Inorganics 2022, 10, 231. [Google Scholar] [CrossRef]
- Wu, F.; Ma, M.; Xie, J. Additive Effects on Copper-Catalyzed Tandem Reactions. Asian J. Org. Chem. 2019, 8, 755–766. [Google Scholar] [CrossRef]
- Fihri, A.; Cha, D.; Bouhrara, M.; Almana, N.; Polshettiwar, V. Fibrous Nano-Silica (KCC-1)-Supported Palladium Catalyst: Suzuki Coupling Reactions Under Sustainable Conditions. ChemSusChem 2012, 5, 85–89. [Google Scholar] [CrossRef]
- Miao, T.; Wang, L. Regioselective synthesis of 1, 2, 3-triazoles by use of a silica-supported copper (I) catalyst. Synthesis 2008, 2008, 363–368. [Google Scholar] [CrossRef]
- Chassaing, S.; Sido, A.S.; Alix, A.; Kumarraja, M.; Pale, P.; Sommer, J. “Click Chemistry” in Zeolites: Copper (I) Zeolites as New Heterogeneous and Ligand-Free Catalysts for the Huisgen [3 + 2] Cycloaddition. Chem. Eur. J. 2008, 14, 6713–6721. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Taft, B.R. Heterogeneous Copper-in-Charcoal-Catalyzed Click Chemistry. Angew. Chem. Int. Ed. 2006, 45, 8235–8238. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Onen, E.; Aufort, M.; Beauviere, S.; Samson, E.; Herscovici, J. Reusable polymer-supported catalyst for the [3 + 2] Huisgen cycloaddition in automation protocols. Org. Lett. 2006, 8, 1689–1692. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lu, X. Cationic Palladium Complex Catalyzed Highly Enantioselective Intramolecular Addition of Arylboronic Acids to Ketones. A Convenient Synthesis of Optically Active Cycloalkanols. J. Am. Chem. Soc. 2006, 128, 16504–16505. [Google Scholar] [CrossRef] [PubMed]
- Bosica, G.; Zammit, R. One-pot multicomponent nitro-Mannich reaction using a heterogeneous catalyst under solvent-free conditions. PeerJ 2018, 6, e5065. [Google Scholar] [CrossRef] [PubMed]
- Sirion, U.; Bae, Y.J.; Lee, B.S.; Chi, D.Y. Ionic Polymer Supported Copper(I): A Reusable Catalyst for Huisgen’s 1,3-Dipolar Cycloaddition. Synlett 2008, 2008, 2326–2330. [Google Scholar] [CrossRef]
- Chavan, P.V.; Pandit, K.S.; Desai, U.V.; Kulkarni, M.A.; Wadgaonkar, P.P. Cellulose supported cuprous iodide nanoparticles (Cell-CuI NPs): A new heterogeneous and recyclable catalyst for the one pot synthesis of 1,4-disubstituted–1,2,3-triazoles in water. RSC Adv. 2014, 4, 42137–42146. [Google Scholar] [CrossRef]
- Subudhi, S.; Rath, D.; Parida, K. A mechanistic approach towards the photocatalytic organic transformations over functionalised metal organic frameworks: A review. Catal. Sci. Technol. 2018, 8, 679–696. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Gong, S.; Xie, J. MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction. Nanomaterials 2022, 12, 1070. [Google Scholar] [CrossRef]
- Mollabagher, H.; Taheri, S.; Mojtahedi, M.; Seyedmousavi, S.A. Cu-metal organic frameworks (Cu-MOF) as an environment-friendly and economical catalyst for one pot synthesis of tacrine derivatives. RSC Adv. 2020, 10, 1995–2003. [Google Scholar] [CrossRef]
- Ullah, S.; Akram, B.; Ali, H.; Zhang, H.; Yang, H.; Liu, Q.; Wang, X. 2-Methylimidazole assisted ultrafast synthesis of carboxylate-based metal–organic framework nano-structures in aqueous medium at room temperature. Sci. Bull. 2019, 64, 1103–1109. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Gniewek, A.; Trzeciak, A.M.; Ziółkowski, J.J.; Kępiński, L.; Wrzyszcz, J.; Tylus, W. Pd-PVP colloid as catalyst for Heck and carbonylation reactions: TEM and XPS studies. J. Catal. 2005, 229, 332–343. [Google Scholar] [CrossRef]
- Joshi, N.; Banerjee, S. PVP coated copper–iron oxide nanocomposite as an efficient catalyst for Click reactions. Tetrahedron Lett. 2015, 56, 4163–4169. [Google Scholar] [CrossRef]
- Raut, D.; Wankhede, K.; Vaidya, V.; Bhilare, S.; Darwatkar, N.; Deorukhkar, A.; Trivedi, G.; Salunkhe, M. Copper nanoparticles in ionic liquids: Recyclable and efficient catalytic system for 1,3-dipolar cycloaddition reaction. Catal. Commun. 2009, 10, 1240–1243. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, C.; Yang, C.; Hu, D.; Long, J.; Wang, L.; Li, H.; Chen, Y.; Kongthe, D. Stabilized Copper(I) Oxide Nanoparticles Catalyze Azide-Alkyne Click Reactions in Water. Adv. Synth. Catal. 2010, 352, 1600–1604. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R. Biocatalytic Promiscuity of Lipases in Carbon-Phosphorus Bond Formation. ChemCatChem 2019, 11, 2554–2558. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R.; Smigielski, P.; Hrunyk, A.; Kramkowski, K.; Laskowski, Ł.; Laskowska, M.; Lizut, R.; Szymczak, M.; Michalski, J.; et al. Pyridine Derivatives—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 5401. [Google Scholar] [CrossRef] [PubMed]
- Koszelewski, D.; Kowalczyk, P.; Smigielski, P.; Samsonowicz-Górski, J.; Kramkowski, K.; Wypych, A.; Szymczak, M.; Ostaszewski, R. Relationship between Structure and Antibacterial Activity of α-Aminophosphonate Derivatives Obtained via Lipase-Catalyzed Kabachnik-Fields Reaction. Materials 2022, 15, 3846. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz-Górski, J.; Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Szymczak, M.; Kramkowski, K.; Ostaszewski, R. The Synthesis and Evaluation of Amidoximes as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 7577. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Kramkowski, K.; Ostaszewski, R. 1,2-Diarylethanols—A New Class of Compounds that Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 1025. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Madej, A.; Szymczak, M.; Ostaszewski, R. α-Amidoamids as New Replacements of Antibiotics—Research on the Chosen K12, R2–R4 E. coli Strains. Materials 2020, 13, 5169. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Gawdzik, B.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Raj, S.; Kramkowski, K.; Lizut, R.; Ostaszewski, R. δ-Lactones—A New Class of Compounds that Are Toxic to E. coli K12 and R2–R4 Strains. Materials 2021, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Gawdzik, B.; Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Masternak, J.; Kramkowski, K.; Wypych, A.; Ostaszewski, R. The Evaluation of DHPMs as Biotoxic Agents on Pathogen Bacterial Membranes. Membranes 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Sahrawat, P.; Kowalczyk, P.; Koszelewski, D.; Szymczak, M.; Kramkowski, K.; Wypych, A.; Ostaszewski, R. Influence of Open Chain and Cyclic Structure of Peptidomimetics on Antibacterial Activity in E. coli Strains. Molecules 2022, 27, 3633. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Koszelewski, D.; Gawdzik, B.; Samsonowicz-Górski, J.; Kramkowski, K.; Wypych, A.; Lizut, R.; Ostaszewski, R. Promiscuous Lipase-Catalyzed Markovnikov Addition of H-Phosphites to Vinyl Esters for the Synthesis of Cytotoxic α-Acyloxy Phosphonate Derivatives. Materials 2022, 15, 1975. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Borkowski, A.; Czerwonka, G.; Cłapa, T.; Cieśla, J.; Misiewicz, A.; Borowiec, M.; Szala, M. The microbial toxicity of quaternary ammonium ionic liquids is dependent on the type of lipopolysaccharide. J. Mol. Liq. 2018, 266, 540–547. [Google Scholar] [CrossRef]
- Borkowski, A.; Kowalczyk, P.; Czerwonka, G.; Ciésla, J.; Cłapa, T.; Misiewicz, A.; Szala, M.; Drabik, M. Interaction of quaternary ammonium ionic liquids with bacterial membranes—Studies with Escherichia coli R1–R4-type lipopolysaccharides. J. Mol. Liq. 2017, 246, 282–289. [Google Scholar] [CrossRef]
- Maciejewska, A.; Kaszowska, M.; Jachymek, W.; Lugowski, C.; Lukasiewicz, J. Lipopolysaccharide-linked Enterobacterial Common Antigen (ECALPS) Occurs in Rough Strains of Escherichia coli R1, R2, and R4. Int. J. Mol. Sci. 2020, 21, 6038. [Google Scholar] [CrossRef]
- Prost, M.E.; Prost, R. Basic parameters of evaluation of the effectiveness of antibiotic therapy. Ophtha Ther. 2017, 4, 233–236. [Google Scholar] [CrossRef]
- Ramnial, T.; Taylor, S.A.; Clyburne, J.A.C.; Walsby, C.J. Grignard reagents in ionic solvents: Electron transfer reactions and evidence for facile Br–Mg exchange. Chem. Commun. 2007, 20, 2066–2068. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Soto-Acosta, R.; Mao, L.; Lian, J.; Chen, K.; Pillon, G.; Zhang, G.; Geraghty, R.J.; Zheng, S. One-Pot Synthesis of 1-Hydroxyacridones from para-Quinols and ortho-Methoxycarbonylaryl Isocyanates. J. Org. Chem. 2020, 85, 4515–4524. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Poturalski, M.J.; Johnson, W.L.; Jones, M.P.; Wang, Y.; Glover, S.A. 4‘-Substituted-4-biphenylyloxenium Ions: Reactivity and Selectivity in Aqueous Solution. J. Org. Chem. 2006, 71, 3778–3785. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Harned, A.M. Experimental evidence for the formation of cationic intermediates during iodine(III)-mediated oxidative dearomatization of phenols. Org. Biomol. Chem. 2018, 16, 6871–6874. [Google Scholar] [CrossRef]
- Li, P.; Sisto, T.J.; Darzi, E.R.; Jasti, R. The Effects of Cyclic Conjugation and Bending on the Optoelectronic Properties of Paraphenylenes. Org. Lett. 2014, 16, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Yakura, T.; Omoto, M.; Yamauchi, Y.; Tian, Y.; Ozono, A. Hypervalent iodine oxidation of phenol derivatives using a catalytic amount of 4-iodophenoxyacetic acid and Oxone® as a co-oxidant. Tetrahedron 2010, 66, 5833–5840. [Google Scholar] [CrossRef]
- Yang, B.; Yao, W.; Xia, X.-F.; Wang, D. Mn-Catalyzed 1,6-conjugate addition/aromatization of para-quinone methides. Org. Biomol. Chem. 2018, 16, 4547–4557. [Google Scholar] [CrossRef]
- Zhang, S.; Song, F.; Zhao, D.; You, J. Tandem oxidation–oxidative C–H/C–H cross-coupling: Synthesis of arylquinones from hydroquinones. Chem. Commun. 2013, 49, 4558–4560. [Google Scholar] [CrossRef]
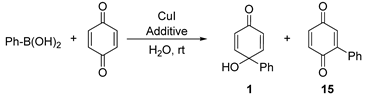 | |||||
|---|---|---|---|---|---|
| Entry | Additive | T (°C) | Solvent | Yield 1 [%] d | Yield 15 [%] d |
| 1 | None | 20 | H2O | 51 | 9 |
| 2 | Amberlite IRA-400 | 20 | H2O | 58 | 7 |
| 3 | Montmorillonite | 20 | H2O | 64 | <1 |
| 4 | Amberlyst | 20 | H2O | 18 | <1 |
| 5 | Dowex-1 | 20 | H2O | 68 | 6 |
| 6 | Silica gel | 20 | H2O | 50 | 8 |
| 7 | Al2O3 | 20 | H2O | 54 | 11 |
| 8 | Cellulose | 20 | H2O | 49 | <1 |
| 9 | MOF-1 | 20 | H2O | 38 | 11 |
| 10 | MOF-2 | 20 | H2O | 42 | <1 |
| 11 | PVP 8000 | 20 | H2O | 74 | <1 |
| 12 | PVP 3500 | 20 | H2O | 84 | <1 |
| 13 | PVP 24000 | 20 | H2O | 62 | <1 |
| 14 | PVP 3500 [b] | 20 | H2O | 81 | <1 |
| 15 | PVP 3500 [c] | 20 | H2O | 79 | <1 |
| 16 | PVP 3500 | 30 | H2O | 89 | <1 |
| 17 | PVP 3500 | 40 | H2O | 83 | <1 |
| 18 | PVP 3500 | 30 | Methanol | 71 | <1 |
| No. of Samples | 5 | 7 | 10, 11 | Type of Test |
|---|---|---|---|---|
| K12 | ** | ** | ** | MIC |
| R2 | ** | ** | ** | MIC |
| R3 | ** | ** | ** | MIC |
| R4 | ** | ** | ** | MIC |
| K12 | ** | ** | *** | MBC |
| R2 | ** | ** | *** | MBC |
| R3 | ** | ** | *** | MBC |
| R4 | ** | ** | *** | MBC |
| K12 | *** | *** | * | MBC/MIC |
| R2 | *** | *** | * | MBC/MIC |
| R3 | *** | *** | * | MBC/MIC |
| R4 | *** | *** | * | MBC/MIC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koszelewski, D.; Kowalczyk, P.; Samsonowicz-Górski, J.; Hrunyk, A.; Brodzka, A.; Łęcka, J.; Kramkowski, K.; Ostaszewski, R. Synthesis and Antimicrobial Activity of the Pathogenic E. coli Strains of p-Quinols: Additive Effects of Copper-Catalyzed Addition of Aryl Boronic Acid to Benzoquinones. Int. J. Mol. Sci. 2023, 24, 1623. https://doi.org/10.3390/ijms24021623
Koszelewski D, Kowalczyk P, Samsonowicz-Górski J, Hrunyk A, Brodzka A, Łęcka J, Kramkowski K, Ostaszewski R. Synthesis and Antimicrobial Activity of the Pathogenic E. coli Strains of p-Quinols: Additive Effects of Copper-Catalyzed Addition of Aryl Boronic Acid to Benzoquinones. International Journal of Molecular Sciences. 2023; 24(2):1623. https://doi.org/10.3390/ijms24021623
Chicago/Turabian StyleKoszelewski, Dominik, Paweł Kowalczyk, Jan Samsonowicz-Górski, Anastasiia Hrunyk, Anna Brodzka, Justyna Łęcka, Karol Kramkowski, and Ryszard Ostaszewski. 2023. "Synthesis and Antimicrobial Activity of the Pathogenic E. coli Strains of p-Quinols: Additive Effects of Copper-Catalyzed Addition of Aryl Boronic Acid to Benzoquinones" International Journal of Molecular Sciences 24, no. 2: 1623. https://doi.org/10.3390/ijms24021623
APA StyleKoszelewski, D., Kowalczyk, P., Samsonowicz-Górski, J., Hrunyk, A., Brodzka, A., Łęcka, J., Kramkowski, K., & Ostaszewski, R. (2023). Synthesis and Antimicrobial Activity of the Pathogenic E. coli Strains of p-Quinols: Additive Effects of Copper-Catalyzed Addition of Aryl Boronic Acid to Benzoquinones. International Journal of Molecular Sciences, 24(2), 1623. https://doi.org/10.3390/ijms24021623







