ProNGF Expression and Targeting in Glioblastoma Multiforme
Abstract
1. Introduction
2. Results
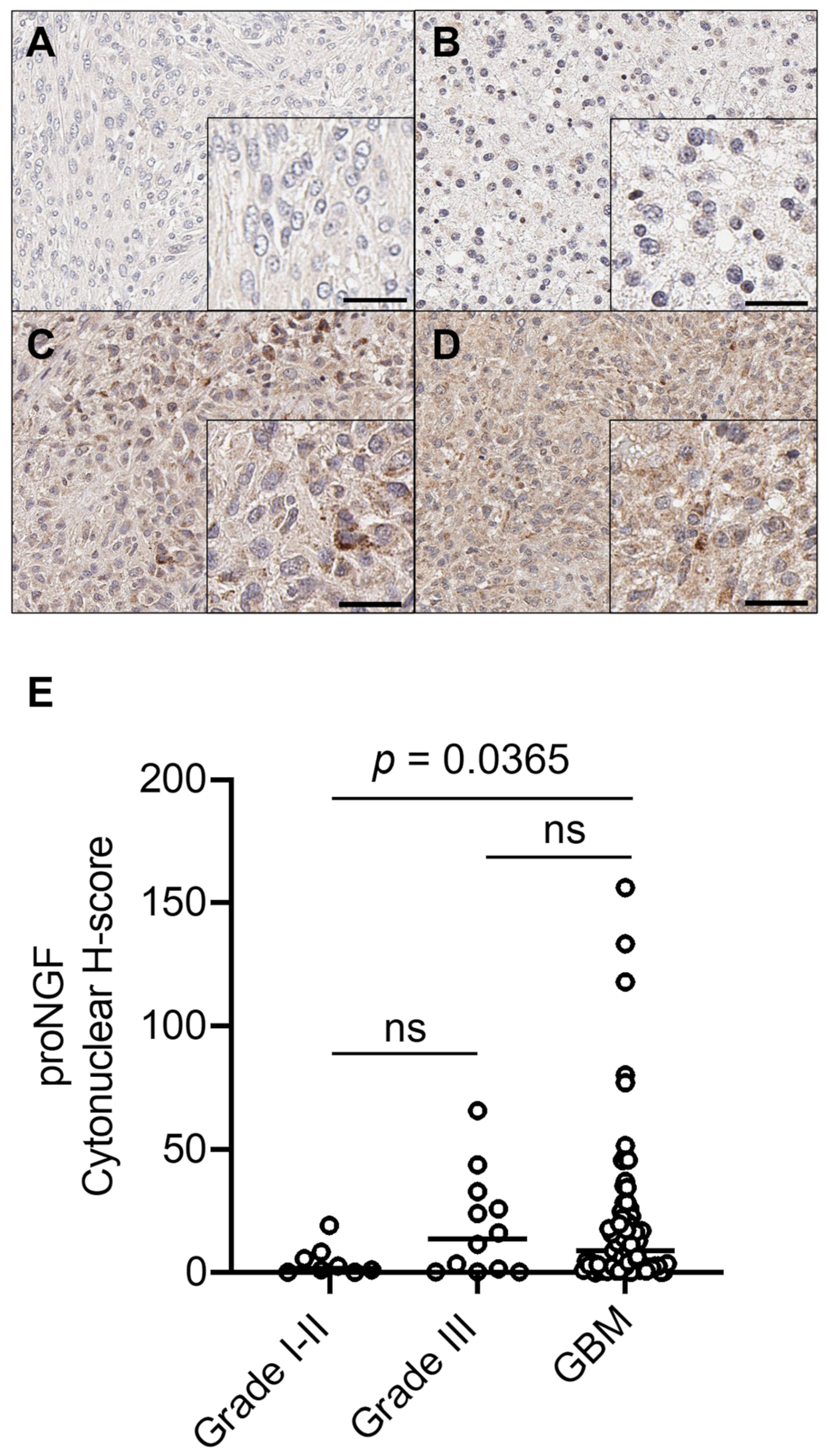
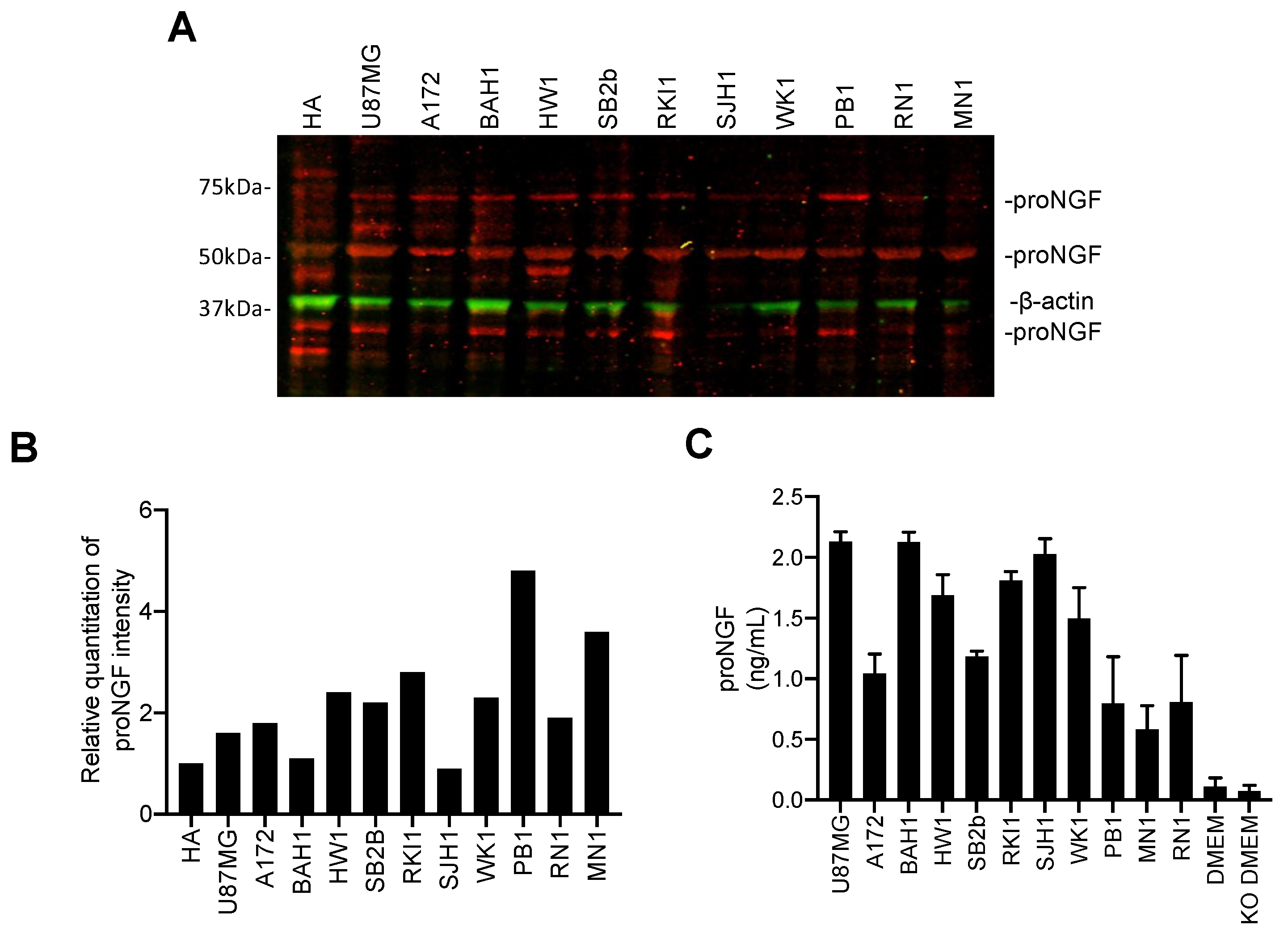
2.1. ProNGF Expression in GBM Tissues
2.2. Quantification of proNGF in Plasma from GBM Patients
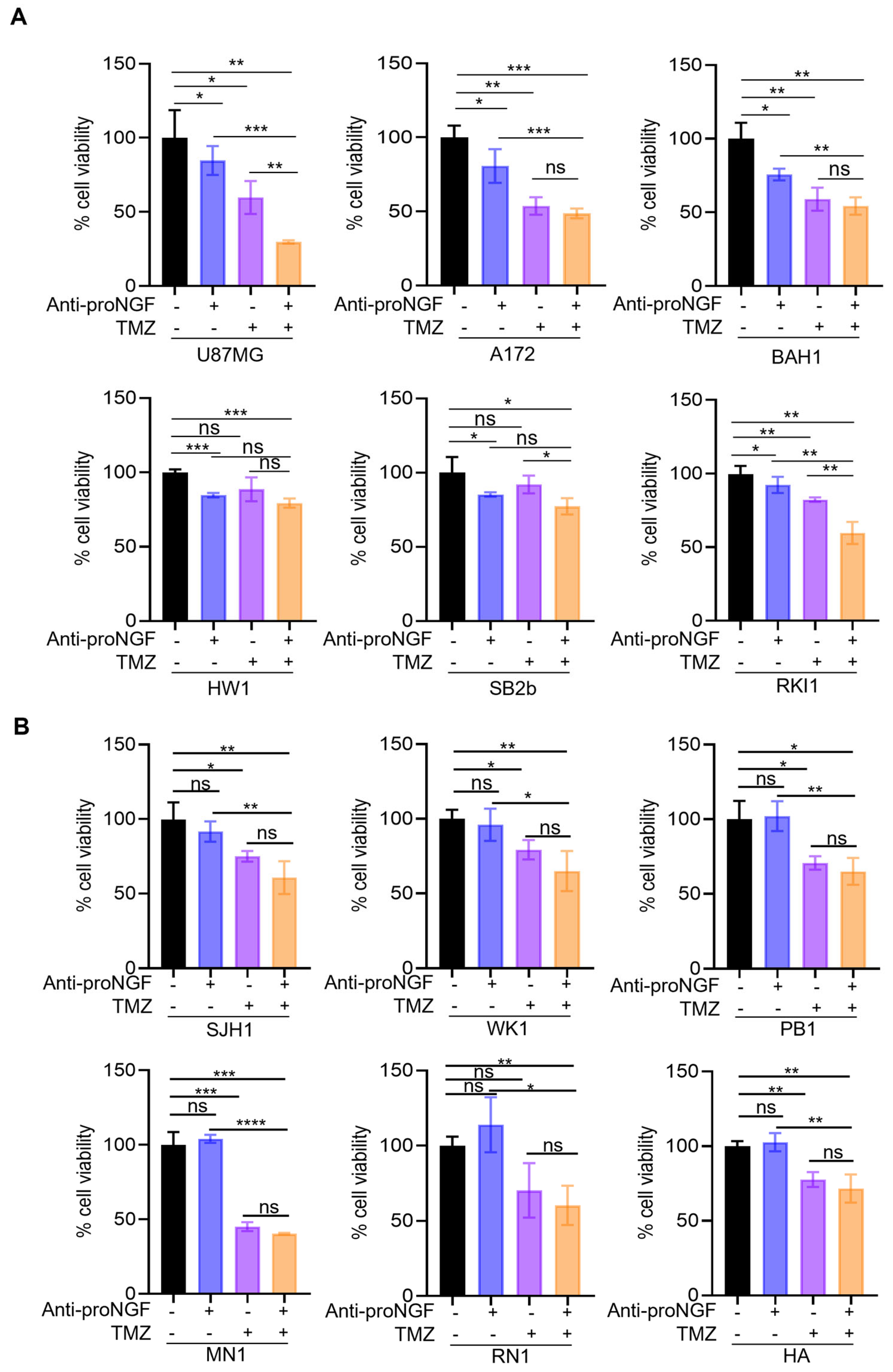
2.3. Targeting proNGF Inhibits GBM Cell Growth In Vitro
2.4. Targeting proNGF Inhibits GBM Tumor Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. GBM Tissues and Plasma Cohort
4.2. Immunohistochemistry
4.3. Digital Quantification of Immunohistochemistry
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Cell Culture
4.6. Preparation of Conditioned Medium
4.7. Protein Extraction and Western Blotting
4.8. In Vitro Cell Growth and Invasion Assays
4.9. In Vivo Experiments in Murine Xenograft Model
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skaga, E.; Skretteberg, M.A.; Johannesen, T.B.; Brandal, P.; Vik-Mo, E.O.; Helseth, E.; Langmoen, I.A. Real-world validity of randomized controlled phase III trials in newly diagnosed glioblastoma: To whom do the results of the trials apply? Neurooncol. Adv. 2021, 3, vdab008. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Friess, H.; Ceyhan, G.O. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim. Biophys. Acta 2016, 1866, 37–50. [Google Scholar] [CrossRef]
- Oelmann, E.; Sreter, L.; Schuller, I.; Serve, H.; Koenigsmann, M.; Wiedenmann, B.; Oberberg, D.; Reufi, B.; Thiel, E.; Berdel, W.E. Nerve growth factor stimulates clonal growth of human lung cancer cell lines and a human glioblastoma cell line expressing high-affinity nerve growth factor binding sites involving tyrosine kinase signaling. Cancer Res. 1995, 55, 2212–2219. [Google Scholar]
- Singer, H.S.; Hansen, B.; Martinie, D.; Karp, C.L. Mitogenesis in glioblastoma multiforme cell lines: A role for NGF and its TrkA receptors. J. Neuro-Oncol. 1999, 45, 1–8. [Google Scholar] [CrossRef]
- Brown, M.C.; Staniszewska, I.; Lazarovici, P.; Tuszynski, G.P.; Del Valle, L.; Marcinkiewicz, C. Regulatory effect of nerve growth factor in alpha9beta1 integrin-dependent progression of glioblastoma. Neuro-Oncology 2008, 10, 968–980. [Google Scholar] [CrossRef]
- Forsyth, P.A.; Krishna, N.; Lawn, S.; Valadez, J.G.; Qu, X.; Fenstermacher, D.A.; Fournier, M.; Potthast, L.; Chinnaiyan, P.; Gibney, G.T.; et al. p75 neurotrophin receptor cleavage by α- and γ-secretases is required for neurotrophin-mediated proliferation of brain tumor-initiating cells. J. Biol. Chem. 2014, 289, 8067–8085. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Fan, Y.; Zhang, X.; Tang, Z.; Qi, J.; Zhao, B.; Li, F.; Chen, X.; Liang, H.; et al. SorCS3 promotes the internalization of p75(NTR) to inhibit GBM progression. Cell Death Dis. 2022, 13, 313. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Fahnestock, M. ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reductions in TrkA Receptor Levels. Int. J. Mol. Sci. 2017, 18, 599. [Google Scholar] [CrossRef]
- Demont, Y.; Corbet, C.; Page, A.; Ataman-Önal, Y.; Choquet-Kastylevsky, G.; Fliniaux, I.; Le Bourhis, X.; Toillon, R.A.; Bradshaw, R.A.; Hondermarck, H. Pro-nerve growth factor induces autocrine stimulation of breast cancer cell invasion through tropomyosin-related kinase A (TrkA) and sortilin protein. J. Biol. Chem. 2012, 287, 1923–1931. [Google Scholar] [CrossRef]
- Tomellini, E.; Touil, Y.; Lagadec, C.; Julien, S.; Ostyn, P.; Ziental-Gelus, N.; Meignan, S.; Lengrand, J.; Adriaenssens, E.; Polakowska, R.; et al. Nerve growth factor and proNGF simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment. Stem Cells 2015, 33, 342–353. [Google Scholar] [CrossRef]
- Pundavela, J.; Demont, Y.; Jobling, P.; Lincz, L.F.; Roselli, S.; Thorne, R.F.; Bond, D.; Bradshaw, R.A.; Walker, M.M.; Hondermarck, H. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 2014, 184, 3156–3162. [Google Scholar] [CrossRef]
- Truzzi, F.; Marconi, A.; Lotti, R.; Dallaglio, K.; French, L.E.; Hempstead, B.L.; Pincelli, C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J. Investig. Dermatol. 2008, 128, 2031–2040. [Google Scholar] [CrossRef]
- Faulkner, S.; Roselli, S.; Demont, Y.; Pundavela, J.; Choquet, G.; Leissner, P.; Oldmeadow, C.; Attia, J.; Walker, M.M.; Hondermarck, H. ProNGF is a potential diagnostic biomarker for thyroid cancer. Oncotarget 2016, 7, 28488–28497. [Google Scholar] [CrossRef]
- Rowe, C.W.; Dill, T.; Faulkner, S.; Gedye, C.; Paul, J.W.; Tolosa, J.M.; Jones, M.; King, S.; Smith, R.; Hondermarck, H. The Precursor for Nerve Growth Factor (proNGF) in Thyroid Cancer Lymph Node Metastases: Correlation with Primary Tumour and Pathological Variables. Int. J. Mol. Sci. 2019, 20, 5924. [Google Scholar] [CrossRef]
- Rowe, C.W.; Faulkner, S.; Paul, J.W.; Tolosa, J.M.; Gedye, C.; Bendinelli, C.; Wynne, K.; McGrath, S.; Attia, J.; Smith, R.; et al. The precursor for nerve growth factor (proNGF) is not a serum or biopsy-rinse biomarker for thyroid cancer diagnosis. BMC Endocr. Disord. 2019, 19, 128. [Google Scholar] [CrossRef]
- Zabłocka, A.; Mitkiewicz, M.; Macała, J.; Janusz, M. Neurotrophic Activity of Cultured Cell Line U87 is Up-Regulated by Proline-Rich Polypeptide Complex and Its Constituent Nonapeptide. Cell Mol. Neurobiol. 2015, 35, 977–986. [Google Scholar] [CrossRef]
- Trabjerg, E.; Kartberg, F.; Christensen, S.; Rand, K.D. Conformational characterization of nerve growth factor-β reveals that its regulatory pro-part domain stabilizes three loop regions in its mature part. J. Biol. Chem. 2017, 292, 16665–16676. [Google Scholar] [CrossRef] [PubMed]
- Lobos, E.; Gebhardt, C.; Kluge, A.; Spanel-Borowski, K. Expression of nerve growth factor (NGF) isoforms in the rat uterus during pregnancy: Accumulation of precursor proNGF. Endocrinology 2005, 146, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Reinshagen, M.; Geerling, I.; Eysselein, V.E.; Adler, G.; Huff, K.R.; Moore, G.P.; Lakshmanan, J. Commercial recombinant human beta-nerve growth factor and adult rat dorsal root ganglia contain an identical molecular species of nerve growth factor prohormone. J. Neurochem. 2000, 74, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, C.E.; Podlesniy, P.; Vidal, N.; Arévalo, J.C.; Lee, R.; Hempstead, B.; Ferrer, I.; Iglesias, M.; Espinet, C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am. J. Pathol. 2005, 166, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [CrossRef]
- Burgess, D.J. Reaching completion for GTEx. Nat. Rev. Genet. 2020, 21, 717. [Google Scholar] [CrossRef]
- Gygi, S.P.; Rochon, Y.; Franza, B.R.; Aebersold, R. Correlation between protein and mRNA abundance in yeast. Mol. Cell Biol. 1999, 19, 1720–1730. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Griffin, C.P.; Paul, C.L.; Alexander, K.L.; Walker, M.M.; Hondermarck, H.; Lynam, J. Postmortem brain donations vs premortem surgical resections for glioblastoma research: Viewing the matter as a whole. Neurooncol. Adv. 2022, 4, vdab168. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wang, X.; Zhu, J.; Liu, Q.; Shi, Z.; Chambers, M.C.; Zimmerman, L.J.; Shaddox, K.F.; Kim, S.; et al. Proteogenomic characterization of human colon and rectal cancer. Nature 2014, 513, 382–387. [Google Scholar] [CrossRef]
- Roselli, S.; Pundavela, J.; Demont, Y.; Faulkner, S.; Keene, S.; Attia, J.; Jiang, C.C.; Zhang, X.D.; Walker, M.M.; Hondermarck, H. Sortilin is associated with breast cancer aggressiveness and contributes to tumor cell adhesion and invasion. Oncotarget 2015, 6, 10473–10486. [Google Scholar] [CrossRef]
- Faulkner, S.; Jobling, P.; Rowe, C.W.; Rodrigues Oliveira, S.M.; Roselli, S.; Thorne, R.F.; Oldmeadow, C.; Attia, J.; Jiang, C.C.; Zhang, X.D.; et al. Neurotrophin Receptors TrkA, p75(NTR), and Sortilin Are Increased and Targetable in Thyroid Cancer. Am. J. Pathol. 2018, 188, 229–241. [Google Scholar] [CrossRef]
- Faulkner, S.; Jobling, P.; March, B.; Jiang, C.C.; Hondermarck, H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019, 9, 702–710. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917.e2831. [Google Scholar] [CrossRef]
- Horai, Y.; Mizukawa, M.; Nishina, H.; Nishikawa, S.; Ono, Y.; Takemoto, K.; Baba, N. Quantification of histopathological findings using a novel image analysis platform. J. Toxicol. Pathol. 2019, 32, 319–327. [Google Scholar] [CrossRef]
- Stringer, B.W.; Day, B.W.; D’Souza, R.C.J.; Jamieson, P.R.; Ensbey, K.S.; Bruce, Z.C.; Lim, Y.C.; Goasdoué, K.; Offenhäuser, C.; Akgül, S.; et al. A reference collection of patient-derived cell line and xenograft models of proneural, classical and mesenchymal glioblastoma. Sci. Rep. 2019, 9, 4902. [Google Scholar] [CrossRef]
- Festuccia, C.; Mancini, A.; Colapietro, A.; Gravina, G.L.; Vitale, F.; Marampon, F.; Delle Monache, S.; Pompili, S.; Cristiano, L.; Vetuschi, A.; et al. The first-in-class alkylating deacetylase inhibitor molecule tinostamustine shows antitumor effects and is synergistic with radiotherapy in preclinical models of glioblastoma. J. Hematol. Oncol. 2018, 11, 32. [Google Scholar] [CrossRef]


| Parameter | proNGF Intensity | p-Value | |
|---|---|---|---|
| Low | High | ||
| Sex | 0.2927 | ||
| Female | 17 (46%) | 20 (54%) | |
| Male | 29 (54%) | 25 (46%) | |
| Age | 0.9892 | ||
| ≤63 | 24 (51%) | 23 (49%) | |
| >63 | 22 (50%) | 22 (50%) | |
| Grade | |||
| I–II | 7 (87.5%) | 1 (12.5%) | <0.0001 |
| III | 5 (42%) | 7 (58%) | |
| GBM | 34 (48%) | 37 (52%) | |
| Tumor site | 0.0848 | ||
| Frontal | 19 (49%) | 20 (51%) | |
| Temporal | 19 (66%) | 10 (34%) | |
| Other | 7 (30%) | 16 (70%) | |
| Parameter | proNGF | p-Value | |
|---|---|---|---|
| Negative | Positive | ||
| Sex | 0.1562 | ||
| Female | 30 (81%) | 7 (19%) | |
| Male | 38 (69%) | 17 (31% | |
| Age | 0.9151 | ||
| ≤63 | 37 (79%) | 10 (21%) | |
| >63 | 31 (69%) | 14 (31%) | |
| Grade | 0.3415 | ||
| I–II | 7 (87.5%) | 1 (12.5%) | |
| III | 8 (67%) | 4 (33%) | |
| GBM | 53 (74%) | 19 (26%) | |
| Tumor site | 0.6417 | ||
| Frontal | 28 (72%) | 11 (28%) | |
| Temporal | 23 (77%) | 7 (23%) | |
| Other | 17 (55%) | 6 (45%) | |
| Characteristic | Subgroup | Total |
|---|---|---|
| Participants | N | 92 |
| Sex | Female | 37 (40%) |
| Male | 55 (60%) | |
| Age at diagnosis | Median (min, max) | 63 (17, 82) |
| Median (Q1, Q3) | 63 (56.5, 72) | |
| Grade | I | 2 (2.2%) |
| II | 6 (6.5%) | |
| III | 12 (13%) | |
| GBM | 72 (78%) | |
| Tumor site | Frontal | 39 (42%) |
| Temporal | 30 (33%) | |
| Parietal | 15 (16%) | |
| Other | 8 (9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsland, M.; Dowdell, A.; Faulkner, S.; Jobling, P.; Rush, R.A.; Gedye, C.; Lynam, J.; Griffin, C.P.; Baker, M.; Marsland, J.; et al. ProNGF Expression and Targeting in Glioblastoma Multiforme. Int. J. Mol. Sci. 2023, 24, 1616. https://doi.org/10.3390/ijms24021616
Marsland M, Dowdell A, Faulkner S, Jobling P, Rush RA, Gedye C, Lynam J, Griffin CP, Baker M, Marsland J, et al. ProNGF Expression and Targeting in Glioblastoma Multiforme. International Journal of Molecular Sciences. 2023; 24(2):1616. https://doi.org/10.3390/ijms24021616
Chicago/Turabian StyleMarsland, Mark, Amiee Dowdell, Sam Faulkner, Phillip Jobling, Robert A. Rush, Craig Gedye, James Lynam, Cassandra P. Griffin, Mark Baker, Joanne Marsland, and et al. 2023. "ProNGF Expression and Targeting in Glioblastoma Multiforme" International Journal of Molecular Sciences 24, no. 2: 1616. https://doi.org/10.3390/ijms24021616
APA StyleMarsland, M., Dowdell, A., Faulkner, S., Jobling, P., Rush, R. A., Gedye, C., Lynam, J., Griffin, C. P., Baker, M., Marsland, J., Jiang, C. C., & Hondermarck, H. (2023). ProNGF Expression and Targeting in Glioblastoma Multiforme. International Journal of Molecular Sciences, 24(2), 1616. https://doi.org/10.3390/ijms24021616









