Disomic Substitution of 3D Chromosome with Its Homoeologue 3E in Tetraploid Thinopyrum elongatum Enhances Wheat Seedlings Tolerance to Salt Stress
Abstract
1. Introduction
2. Results
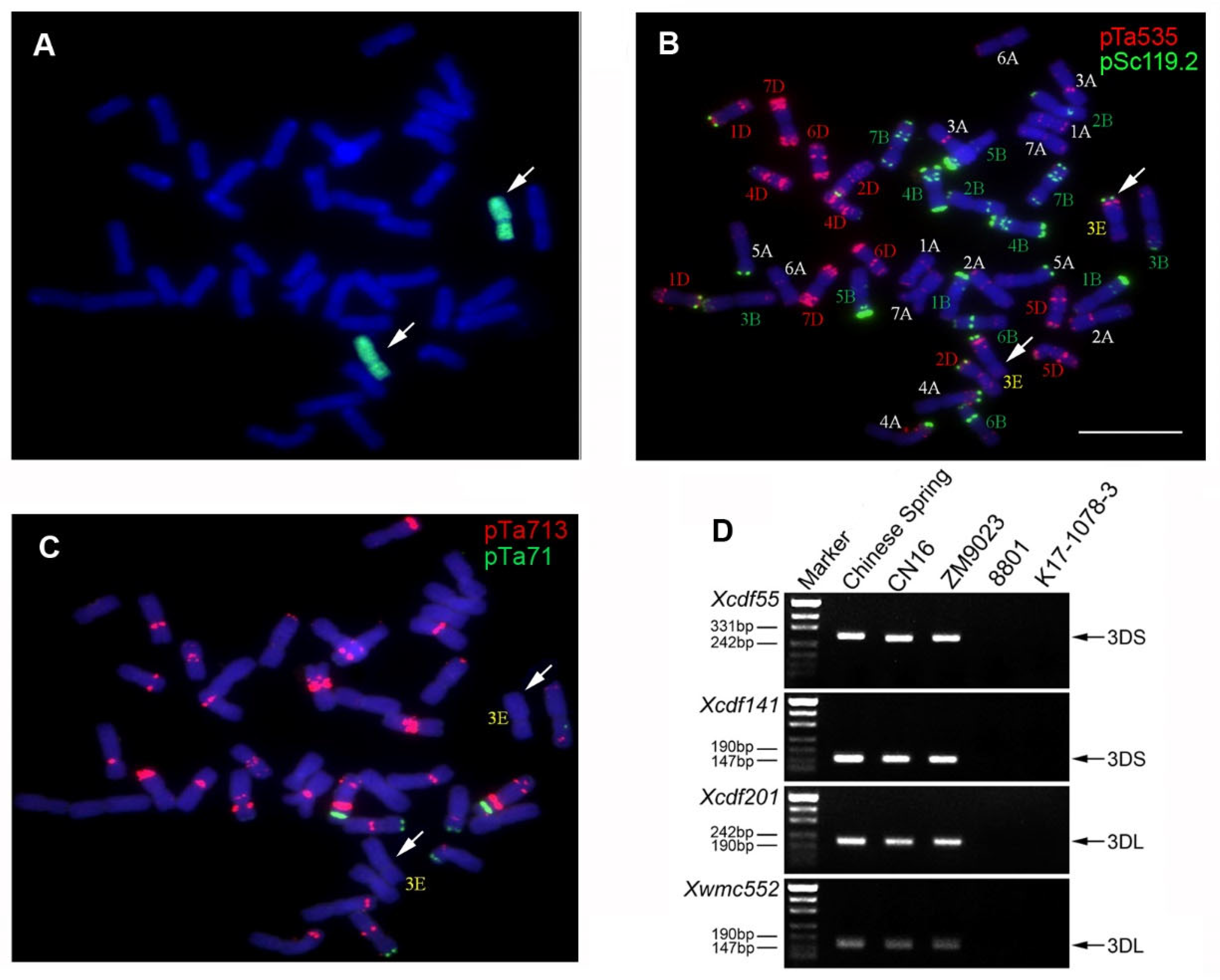
2.1. Genome Identification of K17-1078-3
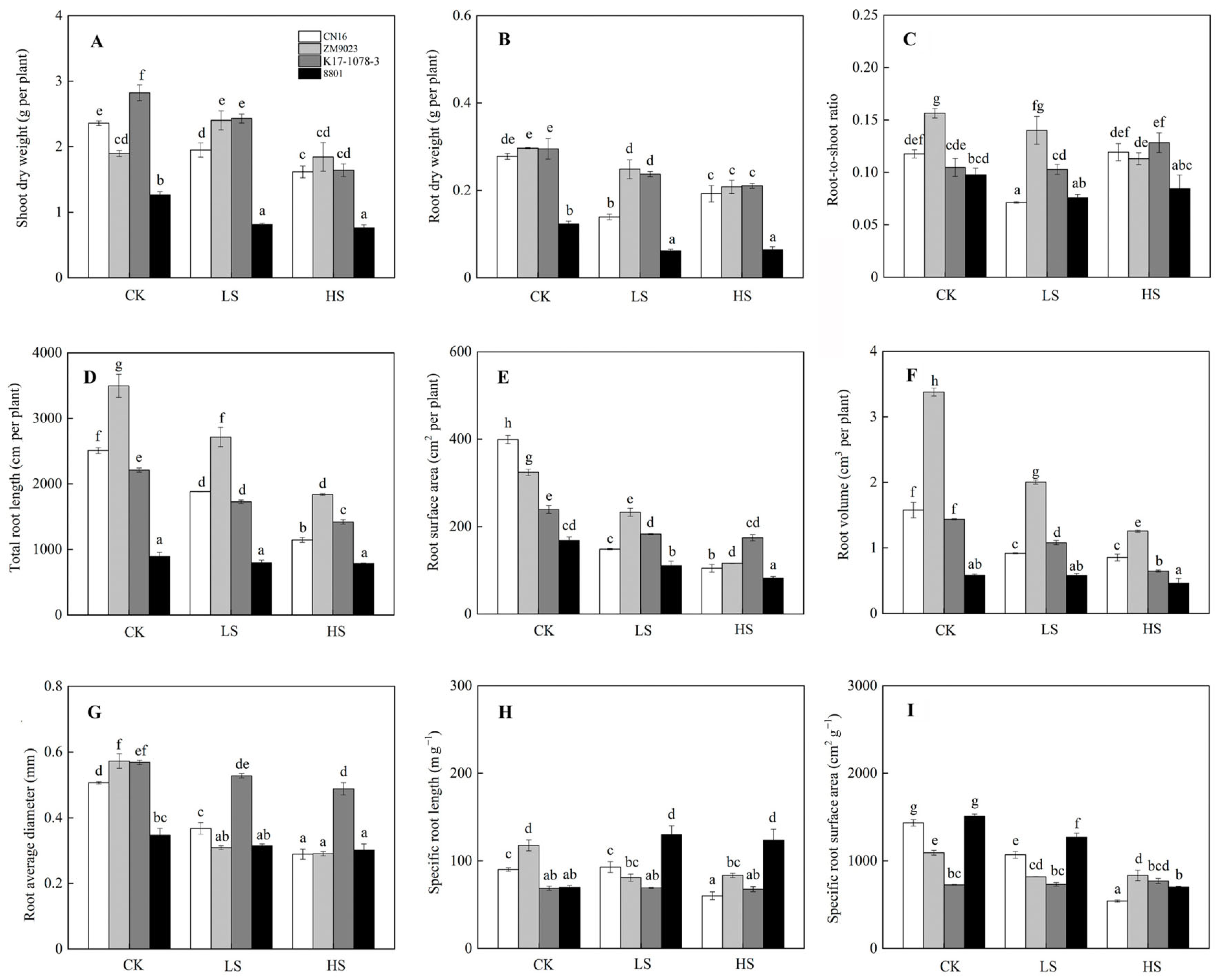
2.2. Biomass Accumulation in Response to Salinity
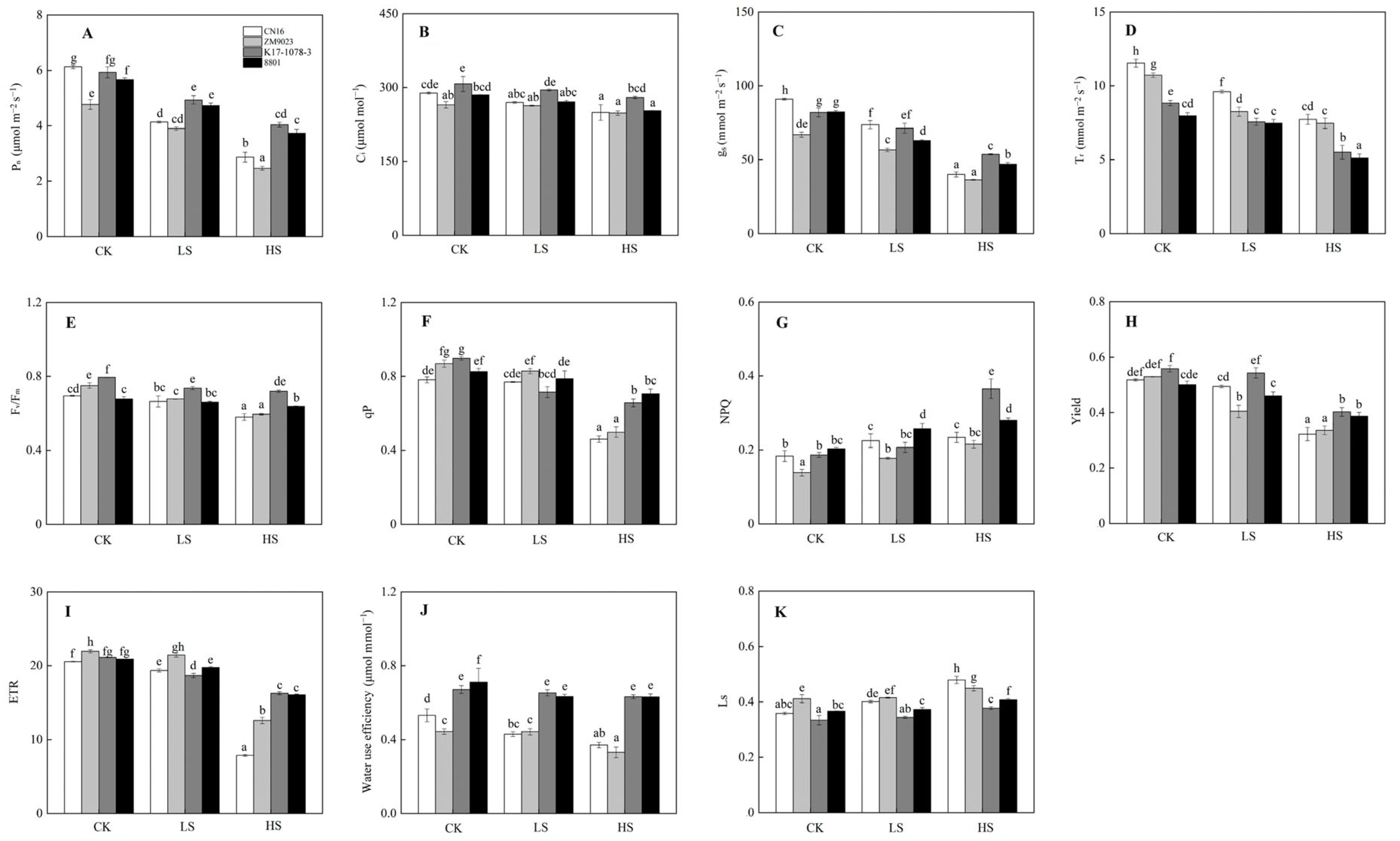
2.3. Photosynthetic Traits in Response to Salinity
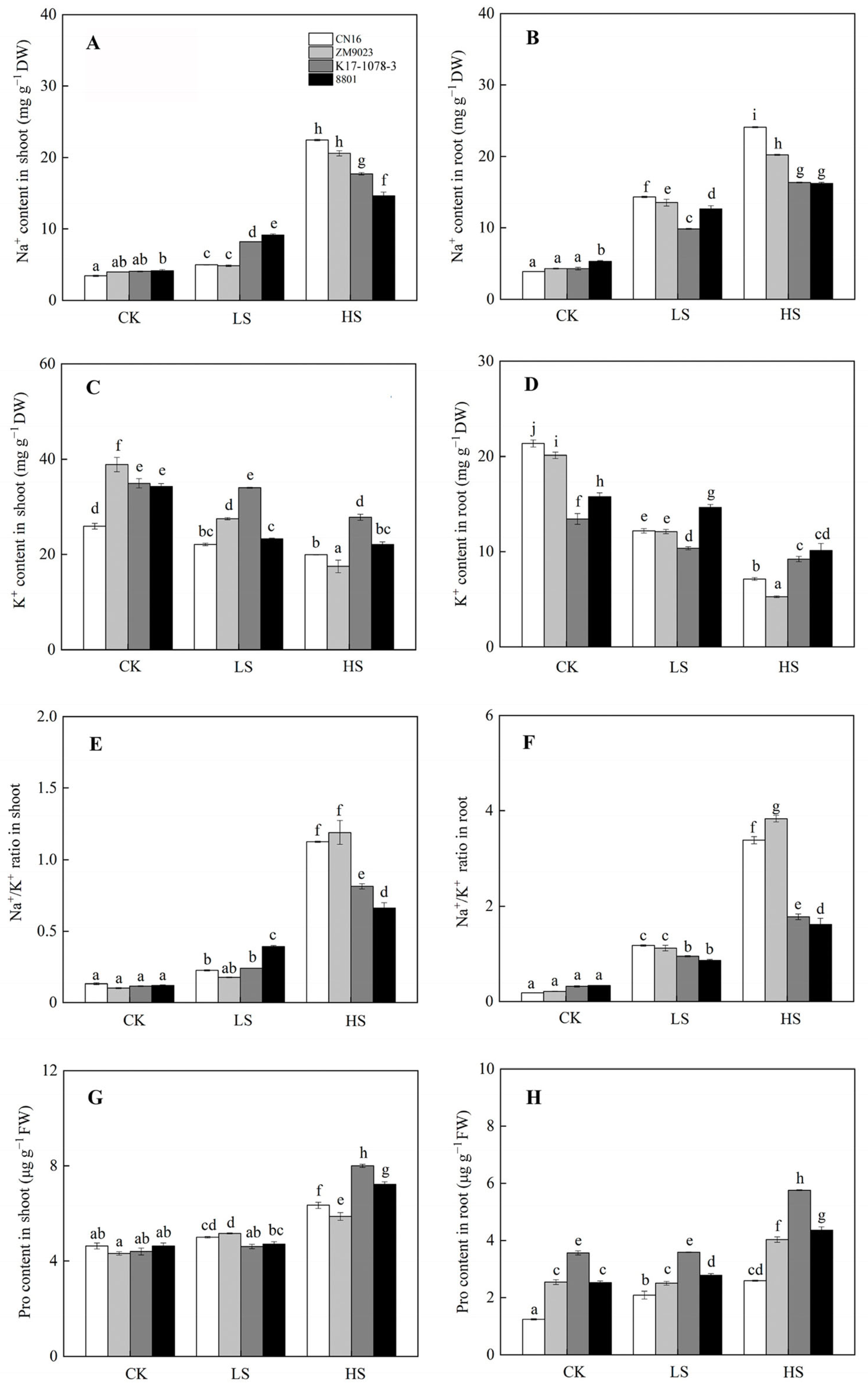
2.4. Osmotic Adjustment in Response to Salinity
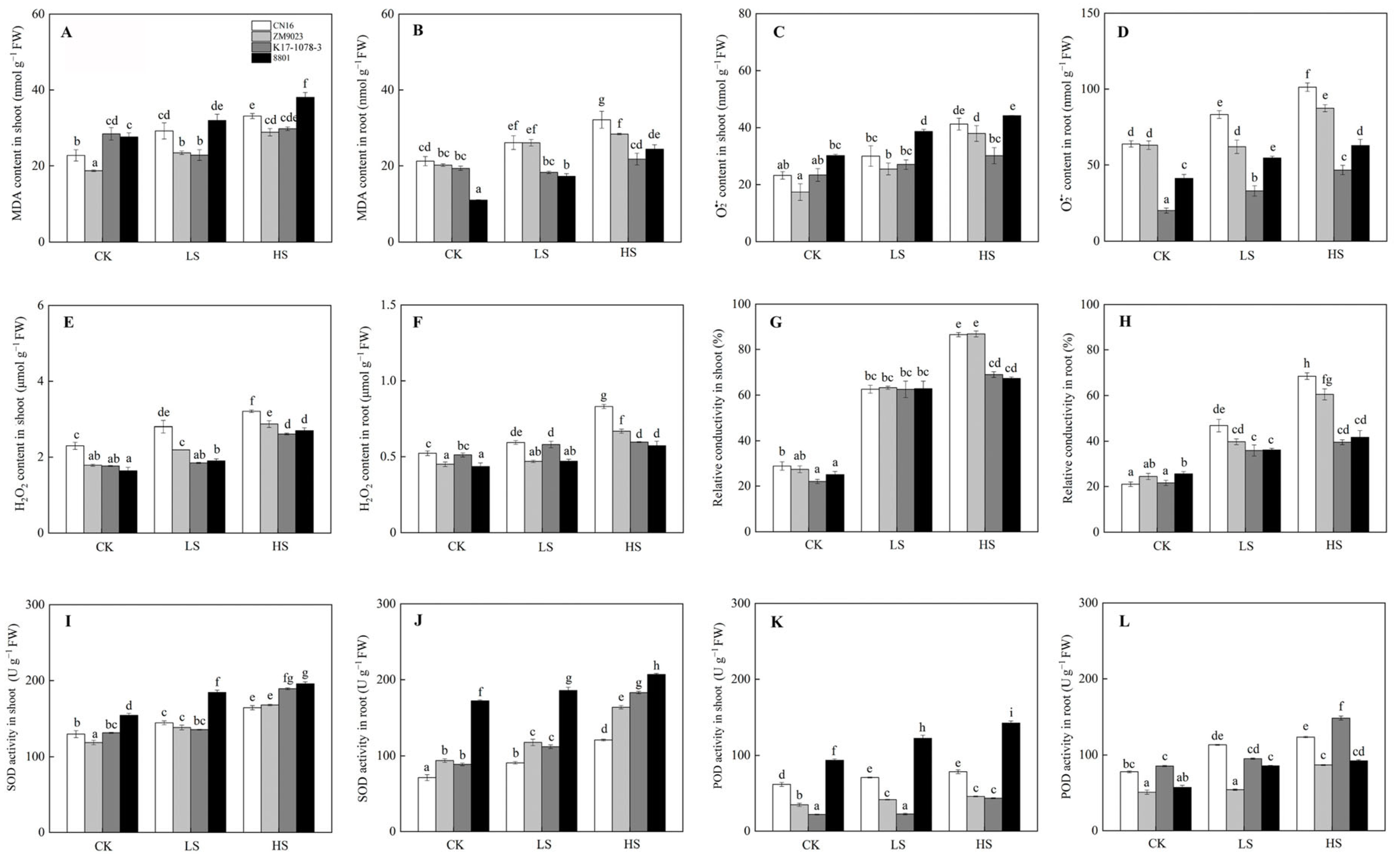
2.5. Antioxidant Defense in Response to Salinity
3. Discussion
3.1. Effect of Chromosome 3E on Root Growth and Photosynthesis
3.2. Effect of Chromosome 3E on Na+ Accumulation and K+ Acquisition
3.3. Effect of Chromosome 3E on Antioxidant Defense
4. Materials and Methods
4.1. Substitution Line Production
4.2. Genome Identification
4.3. Growth Conditions and Salt Treatments
4.4. Root Morphology and Photosynthesis Measurements
4.5. Antioxidant Defense Assays
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, S.J.; Negrao, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Demiral, T.; Türkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Ahmadi, N.; Negrao, S.; Katsantonis, D.; Frouin, J.; Ploux, J.; Letourmy, P.; Droc, G.; Babo, P.; Trindade, H.; Vruschi, G.; et al. Targeted association analysis identified japonica rice varieties achieving Na+/K+ homeostasis without the allelic make-up of the salt tolerant indica variety Nona Bokra. Theor. Appl. Genet. 2011, 123, 881–895. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Raza, A. Eco-physiological and biochemical responses of rapeseed (Brassica napus L.) to abiotic stresses: Consequences and mitigation strategies. J. Plant Growth Regul. 2020, 40, 1368–1388. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Fortmeier, R.; Schubert, S. Salt tolerance of maize (Zea mays L.): The role of sodium exclusion. Plant Cell Environ. 1995, 18, 1041–1047. [Google Scholar] [CrossRef]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Munns, R.; Sharp, R.E. Involvement of abscisic acid in controlling plant growth in soil of low water potential. Aust. J. Plant Physiol. 1993, 20, 425–437. [Google Scholar] [CrossRef]
- Jharna, D.; Hossain, M.; Chowdhury, B.; Lita, M.; Islam, M. Screening of rice genotypes based on root growth for salt tolerance at germination stage. J. Environ. Sci. Nat. Resour. 2017, 10, 45–53. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ge, J.; Li, R.; Zhang, R.; Zhang, Y.; Huo, Z.; Xu, K.; Wei, H.; Dai, Q. Improved physiological and morphological traits of root synergistically enhanced salinity tolerance in rice under appropriate nitrogen application rate. Front. Plant Sci. 2022, 13, 982637. [Google Scholar] [CrossRef]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 2019, 39, 999–1014. [Google Scholar] [CrossRef]
- Colmer, T.D.; Munns, R.; Flowers, T.J. Improving salt tolerance of wheat and barley: Future prospects. Aust. J. Exp. Agric. 2005, 45, 1425–1443. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, L.; Zhang, H.; Yang, Z.; Wang, H.; Wen, S.; Zhang, C.; Rustgi, S.; von Wettstein, D.; Liu, B. Evolution of physiological responses to salt stress in hexaploid wheat. Proc. Natl. Acad. Sci. USA 2014, 111, 11882–11887. [Google Scholar] [CrossRef]
- Dvořák, J.; Edge, M.; Ross, K. On the evolution of the adaptation of Lophopyrum elongatum to growth in saline environments. Proc. Natl. Acad. Sci. USA 1988, 85, 3805–3809. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; He, F.; Cai, J.; Wang, H.; Li, A.; Wang, H.; Kong, L. Molecular and cytological comparisons of chromosomes 7el₁, 7el₂, 7E(e), and 7Eⁱ derived from Thinopyrum. Cytogenet. Genome Res. 2015, 145, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.; Bristol, A.; Young, E.M.; Wyn Jones, R.G.; Kashour, G. Salt tolerance in the Triticeae: K/Na discrimination in barley. J. Exp. Bot. 1990, 41, 1095–1101. [Google Scholar] [CrossRef]
- Omielan, J.A.; Epstein, E.; Dvořák, J. Salt tolerance and ionic relations of wheat as affected by individual chromosomes of salt tolerant Lophopyrum elongatum. Genome 1991, 34, 961–974. [Google Scholar] [CrossRef]
- Colmer, T.D.; Epstein, E.; Dvořák, J. Differential solute regulation in leaf blades of various ages in salt-sensitive wheat and a salt-tolerant wheat × Lophopyrum elongatum (Host) A Love amphiploid. Plant Physiol. 1995, 108, 1715–1724. [Google Scholar] [CrossRef]
- Mullan, D.J.; Mirzaghaderi, G.; Walker, E.; Colmer, T.D.; Francki, M.G. Development of wheat-Lophopyrum elongatum recombinant lines for enhanced sodium ‘exclusion’ during salinity stress. Theor. Appl. Genet. 2009, 119, 1313–1323. [Google Scholar] [CrossRef]
- Li, D.; Long, D.; Li, T.; Wu, Y.; Wang, Y.; Zeng, J.; Xu, L.; Fan, X.; Sha, L.; Zhang, H.; et al. Cytogenetics and stripe rust resistance of wheat–Thinopyrum elongatum hybrid derivatives. Mol. Cytogenet. 2018, 11, 16. [Google Scholar] [CrossRef]
- Zhong, G.Y.; Dvořák, J. Chromosomal control of the tolerance of gradually and suddenly imposed salt stress in the Lophopyrum elongatum and wheat, Triticum aestivum L. genomes. Theor. Appl. Genet. 1995, 90, 229–236. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Matthew, C.; Uddin, M.J.; Bayazid, K.N. Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat. J. Exp. Bot. 2016, 67, 3719–3729. [Google Scholar] [CrossRef]
- Rawson, H.M.; Richards, R.A.; Munns, R. An examination of selection criteria for salt tolerance in wheat, barley and triticale genotypes. Crop Pasture Sci. 1988, 39, 759–772. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.; Niyogi, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops-what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Genc, Y.; McDonald, G.K.; Tester, M. Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ. 2007, 30, 1486–1498. [Google Scholar] [CrossRef]
- Babgohari, M.Z.; Niazi, A.; Moghadam, A.A.; Deihimi, T.; Ebrahimie, E. Genome-wide analysis of key salinity-tolerance transporter (HKT1;5) in wheat and wild wheat relatives (A and D genomes). In Vitro Cell. Dev. Biol.-Plant 2013, 49, 97–106. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Hoefsloot, H.C.; Mol, S.; Feron, R.; de Boer, G.J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stress that affect plant water stress. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Amor, N.B.; Hamed, K.B.; Debez, A.; Grignon, C.; Abdelly, C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 2005, 168, 889–899. [Google Scholar] [CrossRef]
- Asrar, H.; Hussain, T.; Qasim, M.; Nielsen, B.; Gul, B.; Khan, M.A. Salt induced modulations in antioxidative defense system of Desmostachya bipinnata. Plant Physiol. Biochem. 2020, 147, 113–124. [Google Scholar] [CrossRef]
- Han, F.; Lamb, J.; Birchler, J. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.J.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, K.; Baker, N.R. An instrument capable of imaging chlorophyll a fluorescence from intact leaves at very low irradiance and at cellular and subcellular levels of organization. Plant Cell Environ. 1997, 20, 1473–1483. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Egashira, T. Peroxidase in vacuoles of Vicia faba leaves. Phytochemistry 1991, 30, 73–77. [Google Scholar] [CrossRef]
- Wang, A.G.; Luo, G.H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commun. 1990, 26, 55–57. [Google Scholar]
- Bernt, E.; Bergmeyer, H. Inorganic peroxides. Methods Enzym. Anal. 1974, 4, 2246–2248. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreases levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Singh, T.N.; Paleg, L.G.; Aspinall, D. Stress metabolism. I. Nitrogen metabolism and growth in the barley during water stress. Aust. J. Biol. Sci. 1973, 26, 45–56. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
| Marker | Primer Sequences (5′-3′) | Annealing Temperature (°C) | Chromosomal Arm Location | Amplification Size (bp) |
|---|---|---|---|---|
| Xcfd55 | F: CCAGTAGCCGGCCCTACTAT R: GCACGAGATACGGACAATCA | 57 | 3DS | 269 |
| Xcfd141 | F: CGTAAAGATCCGAGAGGGTG R: TCCGAGGTGCTACCTACCAG | 58 | 3DS | 152 |
| Xcfd201 | F: ACAAGACCACACCTCCAAGG R: CGGTTTGGGTTTTGTGATCT | 55 | 3DL | 206 |
| Xwmc552 | F: ACTAAGGAGTGTGAGGGCTGTG R: CTCTCGCGCTATAAAAGAAGGA | 58 | 3DL | 149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Zhou, C.; He, Z.; Wang, Y.; Xu, L.; Chen, G.; Zhu, W.; Zhou, Y.; Kang, H. Disomic Substitution of 3D Chromosome with Its Homoeologue 3E in Tetraploid Thinopyrum elongatum Enhances Wheat Seedlings Tolerance to Salt Stress. Int. J. Mol. Sci. 2023, 24, 1609. https://doi.org/10.3390/ijms24021609
Zeng J, Zhou C, He Z, Wang Y, Xu L, Chen G, Zhu W, Zhou Y, Kang H. Disomic Substitution of 3D Chromosome with Its Homoeologue 3E in Tetraploid Thinopyrum elongatum Enhances Wheat Seedlings Tolerance to Salt Stress. International Journal of Molecular Sciences. 2023; 24(2):1609. https://doi.org/10.3390/ijms24021609
Chicago/Turabian StyleZeng, Jian, Chunli Zhou, Zaimei He, Yi Wang, Lili Xu, Guangdeng Chen, Wei Zhu, Yonghong Zhou, and Houyang Kang. 2023. "Disomic Substitution of 3D Chromosome with Its Homoeologue 3E in Tetraploid Thinopyrum elongatum Enhances Wheat Seedlings Tolerance to Salt Stress" International Journal of Molecular Sciences 24, no. 2: 1609. https://doi.org/10.3390/ijms24021609
APA StyleZeng, J., Zhou, C., He, Z., Wang, Y., Xu, L., Chen, G., Zhu, W., Zhou, Y., & Kang, H. (2023). Disomic Substitution of 3D Chromosome with Its Homoeologue 3E in Tetraploid Thinopyrum elongatum Enhances Wheat Seedlings Tolerance to Salt Stress. International Journal of Molecular Sciences, 24(2), 1609. https://doi.org/10.3390/ijms24021609






