P2 Receptor Signaling in Motor Units in Muscular Dystrophy
Abstract
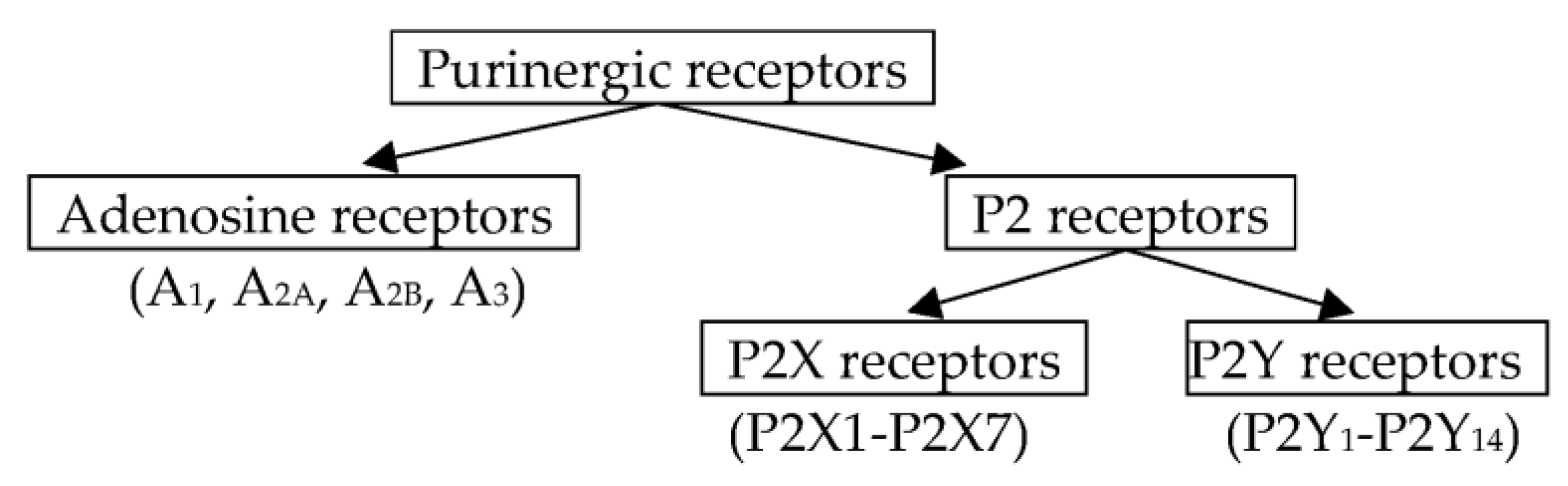
1. Introduction
2. Purinergic Myoneural Modulation
3. Purinergic Synaptic Modulation in Experimental Muscle Mass Reduction
4. Purinergic Signaling in Duchenne and Becker Dystrophies
5. P2 Signaling in Limb Girdle Muscular Dystrophy Type 2B and Miyoshi Myopathy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Zhang, J.; Shi, K.; Liu, Z. Drug development progress in Duchenne muscular dystrophy. Front. Pharmacol. 2022, 13, 950651. [Google Scholar] [CrossRef] [PubMed]
- Erkut, E.; Yokota, T. CRISPR Therapeutics for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2022, 23, 1832. [Google Scholar] [CrossRef] [PubMed]
- Ohlendieck, K.; Swandulla, D. Complexity of skeletal muscle degeneration: Multi-systems pathophysiology and organ crosstalk in dystrophinopathy. Pflug. Arch. 2021, 473, 1813–1839. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Schätzl, T.; Kaiser, L.; Deigner, H.P. Facioscapulohumeral muscular dystrophy: Genetics, gene activation and downstream signalling with regard to recent therapeutic approaches: An update. Orphanet. J. Rare. Dis. 2021, 16, 129. [Google Scholar] [CrossRef]
- Heller, S.A.; Shih, R.; Kalra, R.; Kang, P.B. Emery-Dreifuss muscular dystrophy. Muscle Nerve 2020, 61, 436–448. [Google Scholar] [CrossRef]
- Wicklund, M.P. The Limb-Girdle Muscular Dystrophies. Continuum 2019, 25, 1599–1618. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; Spitzer, N.C. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2007, 104, 335–340. [Google Scholar] [CrossRef]
- Burnstock, G. Adenosine Triphosphate (ATP). In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: Boston, MA, USA, 2009; pp. 105–113. [Google Scholar]
- Burnstock, G. Purines and Purinoceptors: Molecular Biology Overview. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: Boston, MA, USA, 2009; pp. 1253–1262. [Google Scholar]
- Ziganshin, A.U.; Khairullin, A.E.; Hoyle, C.H.V.; Grishin, S.N. Modulatory roles of ATP and adenosine in cholinergic neuromuscular transmission. Int. J. Mol. Sci. 2020, 21, 6423. [Google Scholar] [CrossRef]
- Grishin, S.N.; Gabdrakhmanov, A.I.; Khairullin, A.E.; Ziganshin, A.U. The Influence of glucocorticoids and catecholamines on the neuromuscular transmission. Biochem. Mosc. Suppl. Ser. A 2017, 11, 253–260. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Kamaliev, R.R.; Gabdrakhmanov, A.I.; Khairullin, A.E.; Grishin, S.N. Foot-shock stimulation decreases the inhibitory action of ATP on contractility and end-plate current of frog sartorius muscle. Int. J. Pharmacol. 2018, 14, 1198–1202. [Google Scholar]
- Khairullin, A.E.; Ziganshin, A.U.; Grishin, S.N. Motor units at various temperatures. Biochem. Mosc. Suppl. Ser. A 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Khairullin, A.E.; Zobov, V.V.; Ziganshina, L.E.; Gabdrakhmanov, A.I.; Ziganshin, B.A.; Grishin, S.N. Effects of ATP and adenosine on contraction amplitude of rat soleus muscle at different temperatures. Muscle Nerve 2017, 55, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.U.; Khairullin, A.E.; Teplov, A.Y.; Gabdrakhmanov, A.I.; Ziganshina, L.E.; Hoyle, C.H.V.; Ziganshin, B.A.; Grishin, S.N. The effects of ATP on the contractions of rat and mouse fast skeletal muscle. Muscle Nerve 2019, 59, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Khairullin, A.E.; Teplov, A.Y.; Grishin, S.N.; Farkhutdinov, A.M.; Ziganshin, A.U. The thermal sensitivity of purinergic modulation of contractile activity of locomotor and respiratory muscles in mice. Biophysics 2019, 64, 812–817. [Google Scholar] [CrossRef]
- Khairullin, A.E.; Ziganshin, A.U.; Grishin, S.N. The influence of hypothermia on purinergic synaptic modulation in the rat diaphragm. Biophysics 2020, 65, 858–862. [Google Scholar] [CrossRef]
- Teplov, A.Y.; Grishin, S.N.; Mukhamedyarov, M.A.; Zefirov, A.L.; Ziganshin, A.U.; Palotás, A. Ovalbumin-induced sensitization affects non-quantal acetylcholine release from motor nerve terminals and alters contractility of skeletal muscles in mice. Exp. Physiol. 2009, 94, 264–268. [Google Scholar] [CrossRef]
- Khairullin, A.E.; Teplov, A.Y.; Grishin, S.N.; Ziganshin, A.U. Purinergic Mechanisms in the Adaptation of the Mouse Diaphragm to Allergic Disorders. Biophysics 2022, 67, 474–476. [Google Scholar] [CrossRef]
- Khairullin, A.E.; Grishin, S.N.; Eremeev, A.A. Synaptic aspects of hypogravity motor syndrome. Biophysics 2019, 64, 828–835. [Google Scholar] [CrossRef]
- Khairullin, A.E.; Efimova, D.V.; Markosyan, V.A.; Grishin, S.N.; Teplov, A.Y.; Ziganshin, A.U. The effect of acute unilateral denervation injury on purinergic signaling in the cholinergic synapse. Biophysics 2021, 66, 483–486. [Google Scholar] [CrossRef]
- Ziganshin, A.U.; Ziganshina, L.E. P2 Receptors: A Promising Target for Future Drugs; GeotarMedia: Moscow, Russia, 2009. (In Russian) [Google Scholar]
- Burnstock, G. Purinergic nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Burnstock, G. Purinergic receptors. J. Theor. Biol. 1976, 62, 491–503. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2021/22: G protein-coupled receptors. Br. J. Pharmacol. 2021, 178, 27–156. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2021/22: Ion channels. Br. J. Pharmacol. 2021, 178, 157–245. [Google Scholar] [CrossRef]
- Redman, R.S.; Silinsky, E.M. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J. Physiol. 1994, 477, 117–127. [Google Scholar] [CrossRef]
- Scefner, S.A.; Chiu, T.H. Adenosine inhibits locus coeruleus neurons: An intracellular study in a rat brain slice preparation. Brain Res. 1986, 366, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Krishtal, O.A.; Osipchuk, Y.V.; Shelest, T.N.; Smirnoff, S.V. Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res. 1987, 436, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.J.; Derkach, V.; Surprenant, A. ATP mediates fast synaptic transmission in mammalian neurons. Nature 1992, 357, 503–505. [Google Scholar] [CrossRef]
- Silinsky, E.M.; Gerzanich, V.; Vanner, S.M. ATP mediates excitatory synaptic transmission in mammalian neurons. Br. J. Pharmacol. 1992, 106, 762–763. [Google Scholar] [CrossRef]
- Fu, W.M. Potentiation by ATP of the postsynaptic acetylcholine response at developing neuromuscular synapses in Xenopus cell cultures. J. Physiol. 1994, 477, 449–458. [Google Scholar] [CrossRef]
- Liou, J.C.; Fu, W.M. Additive effect of ADP and CGRP in modulation of the acetylcholine receptor channel in Xenopus embryonic myocytes. Br. J. Pharmacol. 1995, 115, 563–568. [Google Scholar] [CrossRef]
- Lu, B.; Fu, W.M. Regulation of postsynaptic responses by calcitonin gene related peptide and ATP at developing neuromuscular junctions. Can. J. Physiol. Pharmacol. 1995, 73, 1050–1056. [Google Scholar] [CrossRef]
- Mozrzymas, J.W.; Ruzzier, F. ATP activates junctional and extrajunctional acetylcholine receptor channels in isolated adult rat muscle fibres. Neurosci. Lett. 1992, 139, 217–220. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Veggetti, M.; Muchnik, S.; Losavio, A. Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience 2006, 142, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Galkin, A.V.; Giniatullin, R.A.; Mukhtarov, M.R.; Svandova, I.; Grishin, S.N.; Vyskocil, F. ATP but not adenosine inhibits nonquantal acetylcholine release at the mouse neuromuscular junction. Eur. J. Neurosci. 2001, 13, 2047–2053. [Google Scholar] [CrossRef]
- Giniatullin, R.A.; Sokolova, E.M. ATP and adenosine inhibit transmitter release at the frog neuromuscular junction through distinct presynaptic receptors. Br. J. Pharmacol. 1998, 124, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.M.; Grishin, S.N.; Shakirzyanova, A.V.; Talantova, M.V.; Giniatullin, R.A. Distinct receptors and different transduction mechanisms for ATP and adenosine at the frog motor nerve endings. Eur. J. Neurosci. 2003, 18, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.I.; Cunha, R.A.; Ribeiro, J.A. Facilitation by P(2) receptor activation of acetylcholine release from rat motor nerve terminals: Interaction with presynaptic nicotinic receptors. Brain Res. 2000, 877, 245–250. [Google Scholar] [CrossRef]
- Robertson, S.J.; Ennion, S.J.; Evans, R.J.; Edwards, F.A. Synaptic P2X receptors. Curr. Opin. Neurobiol. 2001, 11, 378–386. [Google Scholar] [CrossRef]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Knight, G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004, 240, 301–304. [Google Scholar]
- Smith, D.O. Sources of adenosine released during neuromuscular transmission in the rat. J. Physiol. 1991, 432, 343–354. [Google Scholar] [PubMed]
- Ribeiro, J.A.; Cunha, R.A.; Correia-de-Sa, P.; Sebastiao, A.M. Purinergic regulation of acetylcholine release. Prog. Brain. Res. 1996, 109, 231–241. [Google Scholar] [PubMed]
- Burnstock, G.; Arnett, T.R.; Orriss, I.R. Purinergic signalling in the musculoskeletal system. Purinergic Signal. 2013, 9, 541–572. [Google Scholar] [CrossRef] [PubMed]
- Gachet, C. P2 receptors, platelet function and pharmacological implications. Thromb. Haemost. 2008, 99, 466–472. [Google Scholar] [CrossRef]
- Morillas, A.G.; Besson, V.C.; Lerouet, D. Microglia and Neuroinflammation: What Place for P2RY12? Int. J. Mol. Sci. 2021, 22, 1636. [Google Scholar] [CrossRef]
- Quintas, C.; Fraga, S.; Gonçalves, J.; Queiroz, G. The P2Y1- and P2Y12-receptors mediate autoinhibition of transmitter release in sympathetic innervated tissues. Neurochem. Int. 2009, 55, 505–513. [Google Scholar] [CrossRef]
- Sophocleous, R.A.; Ooi, L.; Sluyter, R. The P2X4 Receptor: Cellular and Molecular Characteristics of a Promising Neuroinflammatory Target. Int. J. Mol. Sci. 2022, 23, 5739. [Google Scholar] [CrossRef]
- Sidoryk-Węgrzynowicz, M.; Strużyńska, L. Astroglial and Microglial Purinergic P2X7 Receptor as a Major Contributor to Neuroinflammation during the Course of Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 8404. [Google Scholar] [CrossRef]
- Grishin, S.N.; Ziganshin, A.U. Modulatory role of purines in neuromuscular transmission. Biochem. Mosc. Suppl. Ser. A 2013, 7, 183–191. [Google Scholar] [CrossRef]
- Giniatullin, A.; Petrov, A.; Giniatullin, R. The involvement of P2Y12 receptors, NADPH oxidase, and lipid rafts in the action of extracellular ATP on synaptic transmission at the frog neuromuscular junction. Neuroscience 2015, 285, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, J.F.; Cinalli, A.R.; Fernández, V.; Roquel, L.I.; Losavio, A.S. P2Y13 receptors mediate presynaptic inhibition of acetylcholine release induced by adenine nucleotides at the mouse neuromuscular junction. Neuroscience 2016, 326, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Cotrina, M.L.; Han, X.; Yu, H.; Bekar, L.; Blum, L.; Takano, T.; Tian, G.F.; Goldman, S.A.; Nedergaard, M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 106, 12489–12493. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Trujillo, C.A.; Lopes, C.G.; Resende, R.R.; Gomes, K.N.; Yuahasi, K.K.; Britto, L.R.; Ulrich, H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007, 3, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Nakada, T.; Kawagishi, H.; Kato, H.; Yamada, M. Increase in phospholamban content in mouse skeletal muscle after denervation. J. Muscle Res. Cell Motil. 2018, 39, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Tryon, L.D.; Crilly, M.J.; Hood, D.A. Effect of denervation on the regulation of mitochondrial transcription factor A expression in skeletal muscle. Am. J. Physiol. Cell Physiol. 2015, 309, 228–238. [Google Scholar] [CrossRef]
- Midrio, M. The denervated muscle: Facts and hypotheses. A historical review. Eur. J. Appl. Physiol. 2006, 98, 1–21. [Google Scholar] [CrossRef]
- Finol, H.J.; Lewis, D.M.; Owens, R. The effects of denervation on contractile properties or rat skeletal muscle. J. Physiol. 1981, 319, 81–92. [Google Scholar] [CrossRef]
- Ponomareva, E.V.; Kravtsova, V.V.; Kachaeva, E.V.; Altaeva, E.G.; Vikhliantsev, I.M.; Podlubnaia, Z.A.; Krivoĭ, I.I.; Shenkman, B.S. [Contractile properties of the isolated rat musculus soleus and single skinned soleus fibers at the early stage of gravitational unloading: Facts and hypotheses]. Biofizika 2008, 53, 1087–1094. (In Russian) [Google Scholar]
- Küllmer, K.; Reimers, C.D.; Eysel, P.; Harland, U. Sonographische Verlaufskontrolle nach experimenteller Muskeldenervierung [Ultrasound follow-up after experimental muscle denervation]. Ultraschall Med. 1996, 17, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.; Guseva, D.S.; Chelyshev, Y.A.; Valiullin, V.V. Immunohistochemical study of rat soleus muscle in various modes of denervation. Bull. Exp. Biol. Med. 2001, 131, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B.; Rosenblueth, A. The supersensitivityof denervated structures: A law of denervation. Quar. Rev. Biol. 1949, 24, 374. [Google Scholar] [CrossRef]
- Frigon, A.; Rossignol, S. Locomotor and reflex adaptation after partial denervation of ankle extensors in chronic spinal cats. J. Neurophysiol. 2008, 100, 1513–1522. [Google Scholar] [CrossRef]
- Kovrigina, T.R.; Filimonov, V.I. The Vascular Network and Neuromuscular Synapses in Skeletal Muscle in Combined Chemical Denervation. Neurosci. Behav. Physiol. 2015, 45, 996–1000. [Google Scholar] [CrossRef]
- Farkhutdinov, A.M. The effect of ATP on the contractile properties of m.EDL mice in normal and motor denervation. Bull. RSMU 2005, 3, 191. (In Russian) [Google Scholar]
- Shackelford, L.C. Musculoskeletal response to space flight. In Principles of Clinical Medicine for Space Flight; Barratt, M.R., Pool, S.L., Eds.; Springer Science and Business Media: New York, NY, USA, 2008; pp. 293–306. [Google Scholar]
- Kalb, R.; Solomon, D. Space exploration, Mars, and the nervous system. Arch. Neurol. 2007, 64, 485–490. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Kurushin, V.A.; Ponomareva, E.V.; Altaeva, E.G.; Shenkman, B.S.; Glashev, M.M.; Mikhailova, E.V.; Krivoi, I.I. Comparative analysis of structural and functional characteristics of soleus muscle in rats and mongolian gerbils during gravitational unloading of different duration. Biophysics 2010, 55, 1013–1018. [Google Scholar] [CrossRef]
- Sandonà, D.; Desaphy, J.F.; Camerino, G.M.; Bianchini, E.; Ciciliot, S.; Danieli-Betto, D.; Dobrowolny, G.; Furlan, S.; Germinario, E.; Goto, K.; et al. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS ONE 2012, 7, 33232. [Google Scholar] [CrossRef]
- O’Brien, K.F.; Kunkel, L.M. Dystrophin and muscular dystrophy: Past, present, and future. Mol. Genet. Metab. 2001, 74, 75–88. [Google Scholar] [CrossRef]
- Emery, A.E.H. The muscular dystrophies. Lancet 2002, 357, 687–695. [Google Scholar] [CrossRef]
- Radojevic, V.; Oppliger, C.; Gaschen, F.; Burgunder, J.M. Restoration of dystrophin expression in cultured hybrid myotubes. Neuropathol. Appl. Neurobiol. 2002, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P. Duchenne muscular dystrophy-what causes the increased membrane permeability in skeletal muscle? Int. J. Biochem. Cell Biol. 2011, 43, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Ryten, M.; Yang, S.Y.; Dunn, P.M.; Goldspink, G.; Burnstock, G. Purinoceptor expression in regenerating skeletal muscle in the mdx mouse model of muscular dystrophy and in satellite cell cultures. FASEB J. 2004, 18, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Sandonà, D.; Gastaldello, S.; Martinello, T.; Betto, R. Characterization of the ATP-hydrolysing activity of alpha-sarcoglycan. Biochem. J. 2004, 381, 105–112. [Google Scholar] [CrossRef]
- Betto, R.; Senter, L.; Ceoldo, S.; Tarricone, E.; Biral, D.; Salviati, G. Ecto-ATPase activity of α-sarcoglycan (adhalin). J. Biol. Chem. 1999, 274, 7907–7912. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, E.; Róg, J.; Sinadinos, A.; Young, C.N.; Górecki, D.C.; Zabłocki, K. Purinergic receptors in skeletal muscles in health and in muscular dystrophy. Postepy Biochem. 2014, 60, 483–489. [Google Scholar]
- Yeung, D.; Kharidia, R.; Brown, S.C.; Górecki, D.C. Enhanced expression of the P2X4 receptor in Duchenne muscular dystrophy correlates with macrophage invasion. Neurobiol. Dis. 2004, 15, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Brutkowski, W.; Lien, C.F.; Arkle, S.; Lochmüller, H.; Zabłocki, K.; Górecki, D.C. P2X7 purinoceptor alterations in dystrophic mdx mouse muscles: Relationship to pathology and potential target for treatment. J. Cell. Mol. Med. 2012, 16, 1026–1037. [Google Scholar] [CrossRef]
- Górecki, D.C. P2X7 purinoceptor as a therapeutic target in muscular dystrophies. Curr. Opin. Pharmacol. 2019, 47, 40–45. [Google Scholar] [CrossRef]
- Mallouk, N.; Jacquemond, V.; Allard, B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4950–4955. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Kennedy, C. P2X receptors in health and disease. Adv. Pharmacol. 2011, 61, 333–372. [Google Scholar] [PubMed]
- Jiang, L.H. P2X receptor-mediated ATP purinergic signaling in health and disease. Cell Health Cytoskel. 2012, 4, 83–101. [Google Scholar] [CrossRef]
- Altamirano, F.; Valladares, D.; Henríquez-Olguín, C.; Casas, M.; López, J.R.; Allen, P.D.; Jaimovich, E. Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS ONE 2013, 8, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Valladares, D.; Casas, M.; Figueroa, R.; Leyton, A.; Buvinic, S.; Jaimovich, E. ATP sensitivity and IP3-dependent calcium transients which regulate gene expression in adult muscle fibres are altered in Mdx mice. Biophys. J. 2011, 100, 592a. [Google Scholar] [CrossRef]
- Valladares, D.; Almarza, G.; Pavez, M.; Jaimovich, E. ATP release is altered in a mouse model for Duchenne muscular dystrophy and signals for proteins that promote cell death. FASEB J. 2012, 26, 798–823. [Google Scholar] [CrossRef]
- De Oliveira Moreira, D.; Santo Neto, H.; Marques, M.J. P2Y2 purinergic receptors are highly expressed in cardiac and diaphragm muscles of mdx mice, and their expression is decreased by suramin. Muscle Nerve 2017, 55, 116–121. [Google Scholar] [CrossRef]
- Iwata, Y.; Katanosaka, Y.; Hisamitsu, T.; Wakabayashi, S. Enhanced Na+/H+ exchange activity contributes to the pathogenesis of muscular dystrophy via involvement of P2 receptors. Am. J. Pathol. 2007, 171, 1576–1587. [Google Scholar] [CrossRef]
- Bertorini, T.E.; Palmieri, G.M.; Griffin, J.; Chesney, C.; Pifer, D.; Verling, L.; Airozo, D.; Fox, I.H. Chronic allopurinol and adenine therapy in Duchenne muscular dystrophy: Effects on muscle function, nucleotide degradation, and muscle ATP and ADP content. Neurology 1985, 35, 61–65. [Google Scholar] [CrossRef]
- Ferrari, D.; Munerati, M.; Melchiorri, L.; Hanau, S.; Di Virgilio, F.; Baricordi, O.R. Responses to extracellular ATP of lymphoblastoid cell lines from Duchenne muscular dystrophy patients. Am. J. Physiol. 1994, 267, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.; Zablocki, K.; Lien, C.F.; Jiang, T.; Arkle, S.; Brutkowski, W.; Brown, J.; Lochmuller, H.; Simon, J.; Barnard, E.A.; et al. Increased susceptibility to ATP via alteration of P2X receptor function in dystrophic mdx mouse muscle cells. FASEB J. 2006, 20, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Cohen, T.V.; Ampong, B.; Francia, D.; Henriques-Pons, A.; Hoffman, E.P.; Nagaraju, K. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am. J. Pathol. 2010, 176, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, K.; Rawat, R.; Veszelovszky, E.; Thapliyal, R.; Kesari, A.; Sparks, S.; Raben, N.; Plotz, P.; Hoffman, E.P. Dysferlin deficiency enhances monocyte phagocytosis: A model for the inflammatory onset of limbgirdle muscular dystrophy 2B. Am. J. Pathol. 2008, 172, 774–785. [Google Scholar] [CrossRef] [PubMed]

| Type of Muscular Dystrophy | Incidence |
|---|---|
| Duchenne dystrophy | 1 per 3500–5000 men [1,2,3] |
| Landouzy-Dejerine dystrophy | 0.9–2 per 100,000 people [5] |
| Emery-Dreyfus dystrophy | 0.39 per 100,000 people [6] |
| Muscular dystrophy of the limb girdle type 2B | 2.27–10 per 100,000 people [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairullin, A.E.; Grishin, S.N.; Ziganshin, A.U. P2 Receptor Signaling in Motor Units in Muscular Dystrophy. Int. J. Mol. Sci. 2023, 24, 1587. https://doi.org/10.3390/ijms24021587
Khairullin AE, Grishin SN, Ziganshin AU. P2 Receptor Signaling in Motor Units in Muscular Dystrophy. International Journal of Molecular Sciences. 2023; 24(2):1587. https://doi.org/10.3390/ijms24021587
Chicago/Turabian StyleKhairullin, Adel E., Sergey N. Grishin, and Ayrat U. Ziganshin. 2023. "P2 Receptor Signaling in Motor Units in Muscular Dystrophy" International Journal of Molecular Sciences 24, no. 2: 1587. https://doi.org/10.3390/ijms24021587
APA StyleKhairullin, A. E., Grishin, S. N., & Ziganshin, A. U. (2023). P2 Receptor Signaling in Motor Units in Muscular Dystrophy. International Journal of Molecular Sciences, 24(2), 1587. https://doi.org/10.3390/ijms24021587






