Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat
Abstract
1. Introduction
2. Short Chain Peptide Generation
3. Taste Perception
4. Taste Evaluation Assessments
5. Taste Development during Dry Curing
6. Taste of Amino Acids
7. Taste of Di- and Tripeptides
7.1. Bitterness
7.2. Umami
7.3. Sweetness
7.4. Sourness
7.5. Saltiness
7.6. Kokumi Activity
8. Bioactivity of Di- and Tripeptides
| Sequence a | Origin (Curing Months) | Reference |
|---|---|---|
| Antihypertensive activity b | ||
| AA, GP, KA, RP, and VY | Spanish DCH (9 m) | [88,91] |
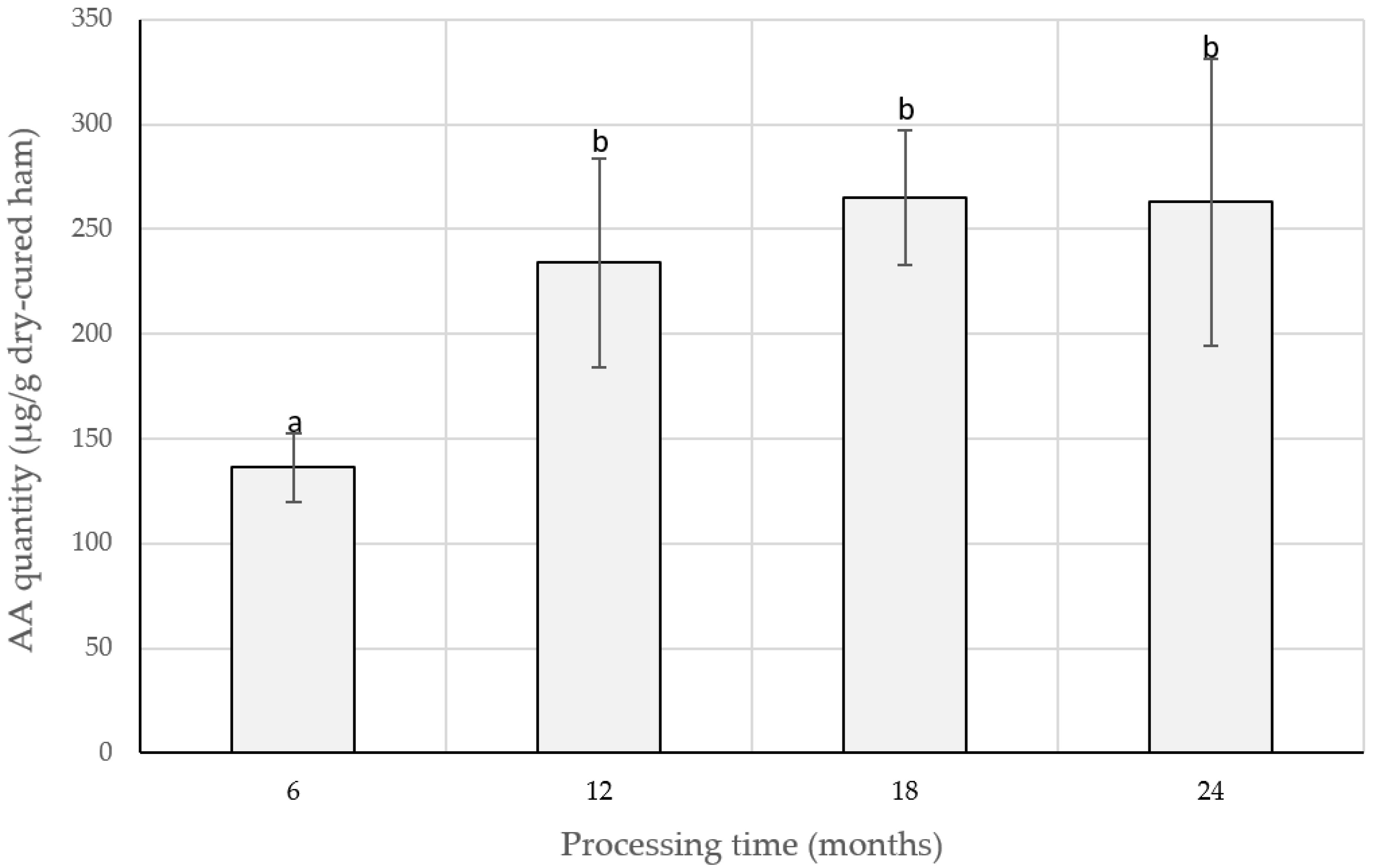
| AA | Traditional and low-salted Spanish DCH (6, 12, 18, and 24 m) | [22] |
| AKK, PAP, SGP, and TNP | Spanish DCH (2, 3.5, 5, 6.5, and 9 m) | [88,133,134] |
| LGL, ALM | Parma DCH (18 and 24 m) | [135] |
| EW, IF, GA, PL, and VF | Iberian DCH (24 m) | [122] |
| GA and VF | Traditional and low-salted Spanish DCH (6, 12, 18, and 24 m) | [87,100] |
| EL, EV, RL, EEL, and ESV | Jinhua DCH (6 m) | [15] |
| LR, NR, and EF | Spanish DCH (9 m) | [88,136] |
| YA | Laowo DCH (12, 24, and 36 m) | [137,138] |
| AW | Spanish DCH (6, 12, 18, and 24 m) | [90] |
| Antioxidant activity | ||
| AY, EL, KP, VY, and EAK | Spanish DCH (2, 3.5, 5, 6.5, and 9 m) | [88,133] |
| AW | Spanish DCH (6, 12, 18, and 24 m) | [90] |
| Anserine and carnosine | Spanish DCH (10 m) | [120,139] |
| Antidiabetic activity | ||
| KA, AA, GP, and PL | Spanish DCH (10 m) | [120] |
| II, IL, IV, LI, and LL | Spanish DCH (9 m) | [40,88,136] |
| GA, GP, and PG | Spanish DCH (9 m) Spanish DCH (6, 12, 18, and 24 m) In silico | [88,100,140] |
| VD, VDY, WK, VV, IE, and SI | Iberian DCH (24 m) | [122] |
| VF | Spanish DCH (18 and 24 m) | [87] |
| AS, QN, and YA | Laowo DCH (12, 24, and 36 m) | [137,141] |
| AD, EA, PE, PP, and VE | Iberian DCH (24 m) | [122] |
| STY | Spanish DCH (9 m) | [88,120] |
| Immunomodulatory activity | ||
| LL, RL, and EKL | Spanish DCH (9 m) | [133,142,143] |
| AL and VH | Spanish DCH (9 m) Spanish DCH (12 m) | [3,69,87,120] |
| QPL, EK, YP, DLE, LGD, DSN, EAD, AAP, LGT, TGL, GQP, LV, PE, MV, LAP, LM, IGA, LTN, MSL, and ENP | Panxian DCH (36 m) | [144] |
| AQ | Spanish DCH (2 m) | [22,89,145] |
| γ-EF, γ-EI, γ-EL and γ-EY | Parma DCH (18 and 24 m) | [81,92] |
| γ-EF, γ-EW, and γ-EY | Prosciutto DCH (14, 21, and 34 m) | [93] |
| Lipid metabolism-modulating activity | ||
| KA and VK | Spanish DCH (2 and 9 m) | [86,91,133,134] |
| DA, DD, EE, ES, and LL | Spanish DCH (6, 12, 18, and 24 m) | [87,146] |
| Brain health promoting and neuronal-related activities | ||
| γ-EW | Parma DCH (18, and 24 m) | [81,92] |
| Prosciutto DCH (14, 21, and 34 m) | [93] | |
| HK, HP, LR, and VY | Spanish DCH (12 m) Jinhua DCH (8 m) | [3,22,83,133] |
8.1. Antihypertensive Activity
8.2. Antioxidant Activity
8.3. Antidiabetic Activity
8.4. Immunomodulatory Activity
8.5. Antimicrobial Activity
8.6. Other Activities
8.6.1. Antithrombotic Activity
8.6.2. Lipid Metabolism-Modulating Activity
8.6.3. Brain Health Promoting and Neuronal-Related Activities
9. Experimental Relationships between Sensory and Bioactive Peptides
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.W.; Kim, C.W.; Noh, C.W.; Kim, S.W.; Kim, I.S. Identification of association between supply of pork and production of meat products in Korea by canonical correlation analysis. Korean J. Food Sci. Anim. Res. 2018, 38, 794–805. [Google Scholar]
- Toldrá, F. Dry-cured ham. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press/Elsevier Science Ltd.: London, UK, 2016; Volume 3, pp. 307–310. [Google Scholar]
- Mora, L.; Escudero, E.; Toldrá, F. Characterization of the peptide profile in Spanish Teruel, Italian Parma and Belgian dry-cured hams and its potential bioactivity. Food Res. Int. 2016, 89, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.; Tolstorebrov, I.; Mora, L.; Toldrá, F.; Eikevik, T.M. Evolution of proteolytic and physico-chemical characteristics of Norwegian dry-cured ham during its processing. Meat Sci. 2016, 121, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Physicochemical changes during manufacture and final sensory characteristics of dry-cured Celta ham. Effect of muscle type. Food Contr. 2014, 43, 263–269. [Google Scholar] [CrossRef]
- Carcò, G.; Schiavon, S.; Casiraghi, E.; Grassi, S.; Sturaro, E.; Bona, M.D.; Novelli, E.; Gallo, L. Influence of dietary protein content on the chemico-physical profile of dry-cured hams produced by pigs of two breeds. Sci. Rep. 2019, 9, 19608. [Google Scholar] [CrossRef]
- Toldrá, F. The role of muscle enzymes in dry-cured meat products with different drying conditons. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Tomažin, U.; Škrlep, M.; Prevolnik, M.; Batorek, N.; Karolyi, D. The effect of salting time and sex on chemical and textural properties of dry cured ham. Meat Sci. 2020, 161, 107990. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M.; Sanz, Y. Dry-cured ham flavour: Enzymatic generation and process influence. Food Chem. 1997, 59, 523–530. [Google Scholar] [CrossRef]
- Jünger, M.; Mittermeier-Kleßinger, V.K.; Farrenkopf, A.; Dunkel, A.; Stark, T.; Fröhlich, S.; Somoza, V.; Dawid, C.; Hofmann, T. Sensoproteomic Discovery of Taste-Modulating Peptides and Taste Re-engineering of Soy Sauce. J. Agric. Food Chem. 2022, 70, 6503–6518. [Google Scholar] [CrossRef]
- Hrynkiewicz, M.; Iwaniak, A.; Bucholska, J.; Minkiewicz, P.; Darewicz, M. Structure-activity prediction of ACE inhibitory/bitter dipeptides – a chemometric approach based on stepwise regression. Molecules 2019, 24, 950. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.-C.; Mora, L. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Escudero, E.; Toldrá, F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microbiol. 2018, 276, 71–78. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Reig, M.; Toldrá, F. Challenges in the quantitation of naturally generated bioactive peptides in processed meats. Trends Food Sci.Technol. 2017, 69, 306–314. [Google Scholar] [CrossRef]
- Hao, L.; Gao, X.; Zhou, T.; Cao, J.; Sun, Y.; Dang, Y.; Pan, D. Angiotensin I-Converting Enzyme (ACE) Inhibitory and Antioxidant Activity of Umami Peptides after In Vitro Gastrointestinal Digestion. J. Agric. Food Chem. 2020, 68, 8232–8241. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Dashdorj, D.; Tripathi, V.K.; Cho, S.; Kim, Y.; Hwang, I. Dry aging of beef; Review. J. Anim. Sci. Technol. 2016, 58, 20. [Google Scholar] [CrossRef]
- Molina, I.; Toldrá, F. Detection of proteolytic activity in microorganisms isolated from dry-cured ham. J. Food Sci. 1992, 57, 1308–1310. [Google Scholar] [CrossRef]
- Zhou, G.H.; Zhao, G.M. Biochemical changes during processing of traditional Jinhua ham. Meat Sci. 2007, 77, 114–120. [Google Scholar] [CrossRef]
- Toldrá, F.; Aristoy, M.-C.; Part, C.; Cerveró, C.; Rico, E.; Motilva, M.-J.; Flores, J. Muscle and adipose tissue aminopeptidase activities in raw and dry-cured ham. J. Food Sci. 1992, 57, 816–818. [Google Scholar] [CrossRef]
- Rico, E.; Toldrá, F.; Flores, J. Cathepsin B, D, H and L activity in the processing of dry-cured-ham. J. Sci. Food Agric. 1993, 62, 157–161. [Google Scholar]
- Mora, L.; Gallego, M.; Toldrá, F. Degradation of myosin heavy chain and its potential as a source of natural bioactive peptides in dry-cured ham. Food Biosci. 2019, 30, 100416. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.C.; Mora, L. Recent Progress in Enzymatic Release of Peptides in Foods of Animal Origin and Assessment of Bioactivity. J. Agric. Food Chem. 2020, 68, 12842–12855. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Mora, L. Peptidomics as a useful tool in the follow-up of food bioactive peptides. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press/Elsevier: Amsterdam, The Netherlands, 2022; Volume 100, pp. 1–47. [Google Scholar]
- Alomirah, H.; Alli, I.; Konishi, Y. Applications of mass spectrometry to food proteins and peptides. J. Chrom. A 2000, 893, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Paulo, J.A.; Kadiyala, V.; Banks, P.A.; Steen, H.; Conwell, D.L. Mass spectrometry-based proteomics for translational research: A technical overview. Yale J. Biol. Med. 2012, 85, 59–73. [Google Scholar] [PubMed]
- Dupree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of This Field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Beauchamp, G.K. Taste Receptor Genes. Ann. Rev. Nutr. 2007, 27, 389–414. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling. Ann. Rev. Physiol. 2017, 79, 335–356. [Google Scholar] [CrossRef]
- Lushchak, O.; Strilbytska, O.M.; Yurkevych, I.; Vaiserman, A.M.; Storey, K.B. Implications of amino acid sensing and dietary protein to the aging process. Exp. Gerontol. 2019, 115, 69–78. [Google Scholar] [CrossRef]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sugawara, T.; Obiya, S.; Enomoto, A.; Kaneko, M.; Ota, S.; Soga, T.; Tomita, M. Sensory properties and metabolomic profiles of dry-cured ham during the ripening process. Food Res. Int. 2020, 129, 108850. [Google Scholar] [CrossRef]
- Choudhuri, S.P.; Delay, R.J.; Delay, E.R. L-Amino Acids Elicit Diverse Response Patterns in Taste Sensory Cells: A Role for Multiple Receptors. PLoS ONE 2015, 10, e0130088. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, M.; Su, G.; Lin, L. Identification and taste characteristics of novel umami and umami-enhancing peptides separated from peanut protein isolate hydrolysate by consecutive chromatography and UPLC–ESI–QTOF–MS/MS. Food Chem. 2019, 278, 674–682. [Google Scholar] [CrossRef]
- Lorido, L.; Pizarro, E.; Estévez, M.; Ventanas, S. Emotional responses to the consumption of dry-cured hams by Spanish consumers: A temporal approach. Meat Sci. 2019, 149, 126–133. [Google Scholar] [CrossRef]
- Guàrdia, M.D.; Aguiar, A.P.S.; Claret, A.; Arnau, J.; Guerrero, L. Sensory characterization of dry-cured ham using free-choice profiling. Food Qual. Pref. 2010, 21, 148–155. [Google Scholar] [CrossRef]
- Kavaliauskienė, I.; Domarkienė, I.; Ambrozaitytė, L.; Barauskienė, L.; Meškienė, R.; Arasimavičius, J.; Irnius, A.; Kučinskas, V. Association study of taste preference: Analysis in the Lithuanian population. Food Sci. Nutr. 2021, 9, 4310–4321. [Google Scholar] [CrossRef]
- González-Casado, A.; Jiménez-Carvelo, A.M.; Cuadros-Rodríguez, L. Sensory quality control of dry-cured ham: A comprehensive methodology for sensory panel qualification and method validation. Meat Sci. 2019, 149, 149–155. [Google Scholar] [CrossRef]
- Hernández-Ramos, P.; Vivar-Quintana, A.M.; Revilla, I.; González-Martín, M.I.; Hernández-Jiménez, M.; Martínez-Martín, I. Prediction of Sensory Parameters of Cured Ham: A Study of the Viability of the Use of NIR Spectroscopy and Artificial Neural Networks. Sensors 2020, 20, 5624. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Stoeva, S.; Aristoy, M.C.C.; Laib, K.; Voelter, W.; Toldra, E.; Toldrá, F.; Toldra, E. Identification of Small Peptides Generated in Spanish Dry-cured Ham. J. Food Sci. 2003, 68, 64–69. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M.; Protasiewicz, M.; Mogut, D. Chemometrics and cheminformatics in the analysis of biologically active peptides from food sources. J. Funct. Foods 2015, 16, 334–351. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M.; Hrynkiewicz, M. Food protein-originating peptides as tastants—Physiological, technological, sensory, and bioinformatic approaches. Food Res. Int. 2016, 89, 27–38. [Google Scholar] [CrossRef]
- Iwaniak, A.; Hrynkiewicz, M.; Bucholska, J.; Minkiewicz, P.; Darewicz, M. Understanding the nature of bitter-taste di- and tripeptides derived from food proteins based on chemometric analysis. J. Food Biochem. 2019, 43, e12500. [Google Scholar] [CrossRef] [PubMed]
- Charoenkwan, P.; Yana, J.; Nantasenamat, C.; Hasan, M.M.; Shoombuatong, W. iUmami-SCM: A Novel Sequence-Based Predictor for Prediction and Analysis of Umami Peptides Using a Scoring Card Method with Propensity Scores of Dipeptides. J. Chem. Inform. Model. 2020, 60, 6666–6678. [Google Scholar] [CrossRef] [PubMed]
- Charoenkwan, P.; Nantasenamat, C.; Hasan, M.M.; Moni, M.A.; Lio, P.; Shoombuatong, W. iBitter-Fuse: A Novel Sequence-Based Bitter Peptide Predictor by Fusing Multi-View Features. Int. J. Mol. Sci. 2021, 22, 8958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun-Waterhouse, D.; Su, G.; Zhao, M. New insight into umami receptor, umami/umami-enhancing peptides and their derivatives: A review. Trends Food Sci. Technol. 2019, 88, 429–438. [Google Scholar] [CrossRef]
- Medler, K.F. Calcium signaling in taste cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2025–2032. [Google Scholar] [CrossRef]
- Di Pizio, A.; Waterloo, L.A.W.; Brox, R.; Löber, S.; Weikert, D.; Behrens, M.; Gmeiner, P.; Niv, M.Y. Rational design of agonists for bitter taste receptor TAS2R14: From modeling to bench and back. Cell. Mol. Life Sci. 2020, 77, 531–542. [Google Scholar] [CrossRef]
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stöger, V.; Reiner, A.; Hochkogler, C.M.; Köck, E.; Marchiori, A.; Hans, J.; et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc. Nat. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef]
- Zopun, M.; Liszt, K.I.; Stoeger, V.; Behrens, M.; Redel, U.; Ley, J.P.; Hans, J.; Somoza, V. Human Sweet Receptor T1R3 is Functional in Human Gastric Parietal Tumor Cells (HGT-1) and Modulates Cyclamate and Acesulfame K-Induced Mechanisms of Gastric Acid Secretion. J. Agric. Food Chem. 2018, 66, 4842–4852. [Google Scholar] [CrossRef]
- Fowler, B.E.; Macpherson, L.J. In vivo Calcium Imaging of Mouse Geniculate Ganglion Neuron Responses to Taste Stimuli. J. Visual. Exp. 2021, 168, 62172. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Jiang, S.; Chen, G.; Liu, Y. Research on sensing characteristics of three human umami receptors via receptor-based biosensor. Flav. Fragr. J. 2020, 35, 695–702. [Google Scholar] [CrossRef]
- Bupesh, G.; Meenakumari, K.; Prabhu, J.; Prabhu, K.; Kalaiselvi, V.S.; Manikandan, E.; Krishnarao, M.R.; Sathyarajeswaran, P. Molecular Properties and Insilico Neuroprotective Activity of Eugenol Against Glutamate Metabotrophic Receptors. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 318–323. [Google Scholar]
- Zhang, J.; Qu, L.; Wu, L.; Tang, X.; Luo, F.; Xu, W.; Xu, Y.; Liu, Z.-J.; Hua, T. Structural insights into the activation initiation of full-length mGlu1. Prot. Cell 2021, 12, 662–667. [Google Scholar] [CrossRef]
- Levit, A.; Barak, D.; Behrens, M.; Meyerhof, W.; Niv, M.Y. Homology Model-Assisted Elucidation of Binding Sites in GPCRs BT. In Membrane Protein Structure and Dynamics: Methods and Protocols; Vaidehi, N., Klein-Seetharaman, J., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 179–205. [Google Scholar]
- Dang, Y.; Hao, L.; Cao, J.; Sun, Y.; Zeng, X.; Wu, Z.; Pan, D. Molecular docking and simulation of the synergistic effect between umami peptides, monosodium glutamate and taste receptor T1R1/T1R3. Food Chem. 2019, 271, 697–706. [Google Scholar] [CrossRef]
- Spaggiari, G.; Cavaliere, F.; Cozzini, P. The Application of In Silico Methods on Umami Taste Receptor. In The Pharmacology of Taste; Palmer, R.K., Servant, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 275, pp. 137–154. [Google Scholar]
- Yu, Z.; Kang, L.; Zhao, W.; Wu, S.; Ding, L.; Zheng, F.; Liu, J.; Li, J. Identification of novel umami peptides from myosin via homology modeling and molecular docking. Food Chem. 2021, 344, 128728. [Google Scholar] [CrossRef]
- Suh, J.-Y.; Kim, H.-S.; Kim, M.-C.; Kong, K.-H. Design and Evaluation of Synthetic Peptides Corresponding to the Sweetness Loop of the Sweet-Tasting Protein Brazzein. Bull. Kor. Chem. Soc. 2014, 35, 3353–3356. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, X.; Liu, B. Structure basis of the improved sweetness and thermostability of a unique double-sites single-chain sweet-tasting protein monellin (MNEI) mutant. Biochimie 2018, 154, 156–163. [Google Scholar] [CrossRef]
- Dang, Y.; Gao, X.; Xie, A.; Wu, X.; Ma, F. Interaction Between Umami Peptide and Taste Receptor T1R1/T1R3. Cell Biochem. Biophys. 2014, 70, 1841–1848. [Google Scholar] [CrossRef]
- Di Pizio, A.; Nicoli, A. In Silico Molecular Study of Tryptophan Bitterness. Molecules 2020, 25, 4623. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Zhao, W.; Li, J.; Shuian, D.; Liu, J. Identification of Oncorhynchus mykiss nebulin-derived peptides as bitter taste receptor TAS2R14 blockers by in silico screening and molecular docking. Food Chem. 2022, 368, 130839. [Google Scholar] [CrossRef]
- Grigorov, M.G.; Schlichtherle-Cerny, H.; Affolter, M.; Kochhar, S. Design of Virtual Libraries of Umami-Tasting Molecules. J. Chem. Inform. Comp. Sci. 2003, 43, 1248–1258. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Resano, H.; Sanjuán, A.I.I.; Cilla, I.; Roncalés, P.; Albisu, L.M.M. Sensory attributes that drive consumer acceptability of dry-cured ham and convergence with trained sensory data. Meat Sci. 2010, 84, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Y.; Wu, J.; Tang, C.; Li, G.; Dai, C.; Bai, Y.; Li, C.; Xu, X.-L.; Zhou, G.; Cao, J.-X. Comparing the proteomic profile of proteins and the sensory characteristics in Jinhua ham with different processing procedures. Food Control. 2019, 106, 106694. [Google Scholar] [CrossRef]
- Iwaniak, A.; Hrynkiewicz, M.; Bucholska, J.; Darewicz, M.; Minkiewicz, P. Structural characteristics of food protein-originating di- and tripeptides using principal component analysis. Eur. Food Res. Technol. 2018, 244, 1751–1758. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Zhang, X.L.; Xie, Q.F. Purification and identification of anti-inflammatory peptides derived from simulated gastrointestinal digests of velvet antler protein (Cervus elaphus Linnaeus). J. Food Drug Anal. 2016, 24, 376–384. [Google Scholar] [CrossRef]
- Khan, M.I.; Jung, S.; Nam, K.C.; Jo, C. Postmortem Aging of Beef with a Special Reference to the Dry Aging. Korean J. Food Sci. Anim. Res. 2016, 36, 159–169. [Google Scholar] [CrossRef]
- Delompré, T.; Guichard, E.; Briand, L.; Salles, C. Taste Perception of Nutrients Found in Nutritional Supplements: A Review. Nutrients 2019, 11, 2050. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Zhou, C.-Y.; Tang, C.-B.; Wang, C.; Dai, C.; Bai, Y.; Yu, X.-B.; Li, C.-B.; Xu, X.-L.; Zhou, G.-H.; Cao, J.-X. Insights into the evolution of myosin light chain isoforms and its effect on sensory defects of dry-cured ham. Food Chem. 2020, 315, 126318. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. The role of muscle proteases and lipases in flavor development during the processing of dry-cured ham. Crit. Rev. Food Sci. Nutr. 1998, 38, 331–352. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Taste-active peptides and amino acids of pork meat as components of dry-cured meat products: An in-ilico study. J. Sens. Stud. 2017, 32, e12301. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Potential cardioprotective peptides generated in Spanish dry-cured ham. J. Food Bioactiv. 2019, 6, 110–117. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Perspectives in the Use of Peptidomics in Ham. Proteomics 2018, 18, 1700422. [Google Scholar] [CrossRef]
- Mora, L.; Sentandreu, M.Á.; Koistinen, K.M.; Fraser, P.D.; Toldrá, F.; Bramley, P.M. Naturally Generated Small Peptides Derived from Myofibrillar Proteins in Serrano Dry-Cured Ham. J. Agric. Food Chem. 2009, 57, 3228–3234. [Google Scholar] [CrossRef]
- Ying, C.Z.; Pan, W.D. The changes in the proteolysis activity and the accumulation of free amino acids during chinese traditional dry-cured loins processing. Food Sci. Biotechnol. 2017, 26, 679–687. [Google Scholar]
- Zhou, C.; Wang, C.; Tang, C.; Dai, C.; Bai, Y.; Yu, X. Label-free proteomics reveals the mechanism of bitterness and adhesiveness in Jinhua ham. Food Chem. 2019, 297, 125012. [Google Scholar] [CrossRef]
- Sforza, S.; Galaverna, G.; Schivazappa, C.; Marchelli, R.; Dossena, A.; Virgili, R. Effect of Extended Aging of Parma Dry-Cured Ham on the Content of Oligopeptides and Free Amino Acids. J. Agric. Food Chem. 2006, 54, 9422–9429. [Google Scholar] [CrossRef]
- Degnes, K.F.; Kvitvang, H.F.N.; Haslene-Hox, H.; Aasen, I.M. Changes in the Profiles of Metabolites Originating from Protein Degradation During Ripening of Dry Cured Ham. Food Bioproc. Technol. 2017, 10, 1122–1130. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Tian, W.; Li, M.-Y.; Liu, Y.-X.; Zhao, G.-M. Separation and identification of peptides from dry-cured Jinhua ham. Int. J. Food Prop. 2017, 20, S2980–S2989. [Google Scholar] [CrossRef]
- Gallego, M.; Toldrá, F.; Mora, L. Quantification and in silico analysis of taste dipeptides generated during dry-cured ham processing. Food Chem. 2022, 370, 130977. [Google Scholar] [CrossRef]
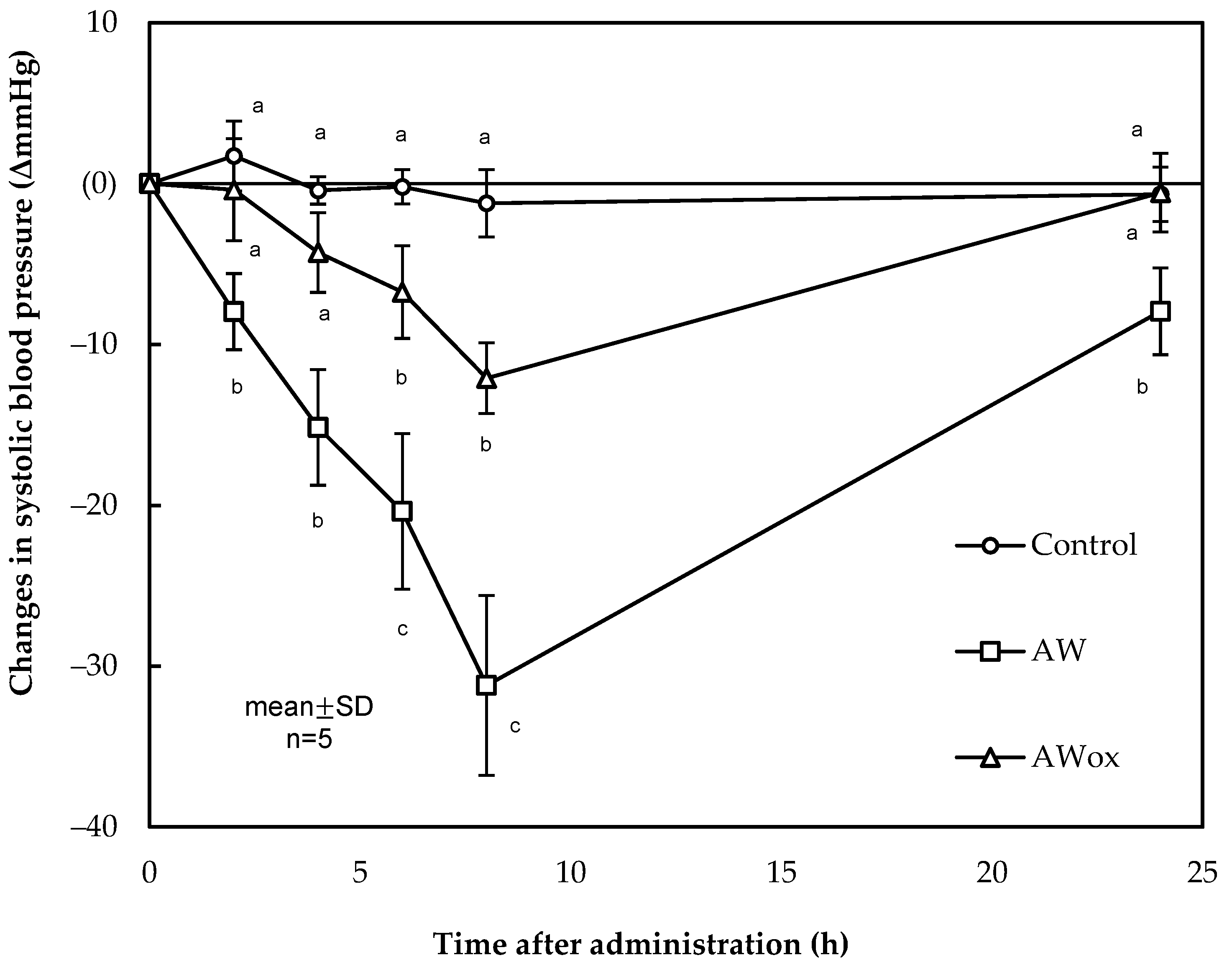
- Heres, A.; Yokoyama, I.; Gallego, M.; Toldrá, F.; Arihara, K.; Mora, L. Impact of oxidation on the cardioprotective properties of the bioactive dipeptide AW in dry-cured ham. Food Res. Int. 2022, 162, 112128. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Nagao, K.; Sakata, K.; Yamano, N.; Elgoda, P.; Gunawardena, R.; Han, S.; Matsui, T.; Nakamori, T.; Furuta, H.; et al. Screening of soy protein-derived hypotriglyceridemic di-peptides in vitro and in vivo. Lip. Health Dis. 2011, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Saldaña, C.; Toldrá, F.; Mora, L. Identification of dipeptides by MALDI-ToF mass spectrometry in long-processing Spanish dry-cured ham. Food Chem. Mol. Sci. 2021, 3, 100048. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldrá, F. The relevance of dipeptides and tripeptides in the bioactivity and taste of dry-cured ham. Food Prod. Proc. Nutr. 2019, 1, 2. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Escudero, E.; Reig, M.; Aristoy, M.-C.C.; Toldrá, F. Small peptides hydrolysis in dry-cured meats. Int. J. Food Microbiol. 2015, 212, 9–15. [Google Scholar] [CrossRef]
- Heres, A.; Yokoyama, I.; Gallego, M.; Toldrá, F.; Arihara, K.; Mora, L. Antihypertensive potential of sweet Ala-Ala dipeptide and its quantitation in dry-cured ham at different processing conditions. J. Funct. Foods 2021, 87, 104818. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Peptides with Potential Cardioprotective Effects Derived from Dry-Cured Ham Byproducts. J. Agric. Food Chem. 2019, 67, 1115–1126. [Google Scholar] [CrossRef]
- Paolella, S.; Prandi, B.; Falavigna, C.; Buhler, S.; Dossena, A.; Sforza, S.; Galaverna, G. Occurrence of non-proteolytic amino acyl derivatives in dry-cured ham. Food Res. Int. 2018, 114, 38–46. [Google Scholar] [CrossRef]
- Cerrato, A.; Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Laganà, A. Investigating the Short Peptidome Profile of Italian Dry-Cured Ham at Different Processing Times by High-Resolution Mass Spectrometry and Chemometrics. Int. J. Mol. Sci. 2022, 23, 3193. [Google Scholar] [CrossRef]
- Dang, Y.; Gao, X.; Ma, F.; Wu, X. Comparison of umami taste peptides in water-soluble extractions of Jinhua and Parma hams. LWT-Food Sci. Technol. 2015, 60, 1179–1186. [Google Scholar] [CrossRef]
- Ishibashi, N.; Sadamori, K.; Yamamoto, O.; Kanehisa, H.; Kouge, K.; Kikuchi, E.; Okai, H.; Fukui, S. Bitterness of Phenylalanine- and Tyrosine-containing Peptides. Agric. Biol. Chem. 1987, 51, 3309–3313. [Google Scholar] [CrossRef]
- Otagiri, K.; Nosho, Y.; Shinoda, I.; Fukui, H.; Okai, H. Studies on a model of bitter peptides including arginine, proline and phenylalanine residues. I. bitter taste of di- and tripeptides, and bitterness increase of the model peptides by extension of the peptide chain. Agric. Biol. Chem. 1985, 49, 1019–1026. [Google Scholar]
- Ishibashi, N.; Kubo, T.; Chino, M.; Fukui, H.; Shinoda, I.; Kikuchi, E.; Okai, H.; Fukui, S. Taste of Proline-containing Peptides. Agric. Biol. Chem. 1988, 52, 95–98. [Google Scholar] [CrossRef]
- Ishibashi, N.; Arita, Y.; Kanehisa, H.; Kouge, K.; Okai, H.; Fukui, S. Bitterness of Leucine-containing Peptides. Agric. Biol. Chem. 1987, 51, 2389–2394. [Google Scholar] [CrossRef]
- Ishibashi, N.; Ono, I.; Kato, K.; Shigenaga, T.; Shinoda, I.; Okai, H.; Fukui, S. Role of the Hydrophobic Amino Acid Residue in the Bitterness of Peptides. Agric. Biol. Chem. 1988, 52, 91–94. [Google Scholar] [CrossRef]
- Heres, A.; Gallego, M.; Mora, L.; Toldrá, F. Identification and Quantitation of Bioactive and Taste-Related Dipeptides in Low-Salt Dry-Cured Ham. Int. J. Mol. Sci. 2022, 23, 2507. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Liu, W.; Zhao, L. Novel Umami Ingredients: Umami Peptides and Their Taste. J. Food Sci. 2017, 82, 16–23. [Google Scholar] [CrossRef]
- Qi, L.; Gao, X.; Pan, D.; Sun, Y.; Cai, Z.; Xiong, Y.; Dang, Y. Research progress in the screening and evaluation of umami peptides. Comp. Rev. Food Sci. Food Saf. 2022, 21, 1462–1490. [Google Scholar] [CrossRef]
- Neacsu, M.; Fyfe, C.; Horgan, G.; Johnstone, A.M. Appetite control and biomarkers of satiety with Vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: A randomized crossover trial. Am. J. Clin. Nutr. 2014, 100, 548–558. [Google Scholar] [CrossRef]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tomé, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 2012, 25, 29–39. [Google Scholar] [CrossRef]
- Shen, D.-Y.; Begum, N.; Song, H.-L.; Zhang, Y.; Wang, L.-J.; Zhao, Y.-J.; Zhang, L.; Liu, P. In vitro and in vivo antioxidant activity and umami taste of peptides (<1 kDa) from porcine bone protein extract. Food Biosci. 2021, 40, 100901. [Google Scholar]
- Heres, A.; Toldrá, F.; Mora, L. Characterization of Umami Dry-Cured Ham-Derived Dipeptide Interaction with Metabotropic Glutamate Receptor (mGluR) by Molecular Docking Simulation. Appl. Sci. 2021, 11, 8268. [Google Scholar] [CrossRef]
- Mora, L.; Calvo, L.; Escudero, E.; Toldrá, F. Differences in pig genotypes influence the generation of peptides in dry-cured ham processing. Food Res. Int. 2016, 86, 74–82. [Google Scholar] [CrossRef]
- Amino, Y.; Wakabayashi, H.; Akashi, S.; Ishiwatari, Y. Structural analysis and taste evaluation of γ-glutamyl peptides comprising sulfur-containing amino acids. Biosci. Biotech. Biochem. 2018, 82, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Miyamura, N. Mechanism of the perception of “kokumi” substances and the sensory characteristics of the “kokumi” peptide, γ -Glu-Val-Gly. Flavour 2015, 4, 11. [Google Scholar] [CrossRef]
- Rhyu, M.-R.; Lyall, V. Interaction of taste-active nutrients with taste receptors. Curr. Op. Physiol. 2021, 20, 64–69. [Google Scholar] [CrossRef]
- Yang, J.; Bai, W.; Zeng, X.; Cui, C. Gamma glutamyl peptides: The food source, enzymatic synthesis, kokumi-active and the potential functional properties—A review. Trends Food Sci. Technol. 2019, 91, 339–346. [Google Scholar] [CrossRef]
- Fu, Y.; Amin, M.S.; Li, Q.; Bak, K.H.; Lametsch, R. Applications in nutrition: Peptides as taste enhancers. In Biologically Active Peptides; Toldrá, F., Wu, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 569–580. [Google Scholar]
- Li, Q.; Liu, J.; De Gobba, C.; Zhang, L.; Bredie, W.L.P.; Lametsch, R. Production of Taste Enhancers from Protein Hydrolysates of Porcine Hemoglobin and Meat Using Bacillus amyloliquefaciens γ-Glutamyltranspeptidase. J. Agric. Food Chem. 2020, 68, 11782–11789. [Google Scholar] [CrossRef]
- Suzuki, H.; Kajimoto, Y.; Kumagai, H. Improvement of the Bitter Taste of Amino Acids through the Transpeptidation Reaction of Bacterial γ-Glutamyltranspeptidase. J. Agric. Food Chem. 2002, 50, 313–318. [Google Scholar] [CrossRef]
- Rico, A.G.; Braun, J.P.; Benard, P.; Thouvenot, J.P. Tissue and blood gamma-glutamyl transferase distribution in the pig. Res. Vet. Sci. 1977, 23, 395–396. [Google Scholar] [CrossRef]
- Xing, L.; Liu, R.; Cao, S.; Zhang, W.; Guanghong, Z. Meat protein based bioactive peptides and their potential functional activity: A review. Int. J. Food Sci. Technol. 2019, 54, 1956–1966. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- Li, C.; Mora, L.; Toldrá, F. Structure-function relationship of small peptides generated during the ripening of Spanish dry-cured ham: Peptidome, molecular stability and computational modelling. Food Chem. 2022, 375, 131673. [Google Scholar] [CrossRef]
- Castellano, P.; Mora, L.; Escudero, E.; Vignolo, G.; Aznar, R.; Toldrá, F. Antilisterial peptides from Spanish dry-cured hams: Purification and identification. Food Microbiol. 2016, 59, 133–141. [Google Scholar] [CrossRef]
- Gallego, M.; Aristoy, M.C.; Toldrá, F. Dipeptidyl peptidase IV inhibitory peptides generated in Spanish dry-cured ham. MESC 2014, 96, 757–761. [Google Scholar] [CrossRef]
- Gallego, M.; Grootaert, C.; Mora, L.; Aristoy, M.C.; Van Camp, J.; Toldrá, F. Transepithelial transport of dry-cured ham peptides with ACE inhibitory activity through a Caco-cell monolayer. J. Funct. Foods 2016, 21, 388–395. [Google Scholar] [CrossRef]
- Mora, L.; González-Rogel, D.; Heres, A.; Toldrá, F. Iberian dry-cured ham as a potential source of α-glucosidase-inhibitory peptides. Funct. Foods 2020, 67, 103840. [Google Scholar] [CrossRef]
- Escudero, E.; Aristoy, M.C.; Nishimura, H.; Arihara, K.; Toldrá, F. Antihypertensive effect and antioxidant activity of peptide fractions extracted from Spanish dry-cured ham. MESC 2012, 91, 306–311. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.C.; Arihara, K.; Toldrá, F. Purification and Identification of antihypertensive peptides in Spanish dry-cured ham. J. Proteom. 2012, 78, 499–507. [Google Scholar] [CrossRef]
- Martínez-Sánchez, S.M.; Minguela, A.; Prieto-Merino, D.; Zafrilla-Rentero, M.P.; Abellán-Alemán, J.; Montoro-García, S. The Effect of Regular Intake of Dry-Cured Ham Rich in Bioactive Peptides on Inflammation, Platelet and Monocyte Activation Markers in Humans. Nutrients 2017, 9, 321. [Google Scholar] [CrossRef]
- Martínez-Sánchez, S.M.; Pérez-Sánchez, H.; Antonio Gabaldón, J.; Abellán-Alemán, J.; Montoro-García, S. Multifunctional Peptides from Spanish Dry-Cured Pork Ham: Endothelial Responses and Molecular Modeling Studies. Int. J. Mol. Sci. 2019, 20, 4204. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Velasco-Soria, Á.; Mora, L.; Carazo-Díaz, C.; Prieto-Merino, D.; Avellaneda, A.; Miranzo, D.; Casas-Pina, T.; Toldrá, F.; Abellán-Alemán, J. Beneficial Impact of Pork Dry-Cured Ham Consumption on Blood Pressure and Cardiometabolic Markers in Individuals with Cardiovascular Risk. Nutrients 2022, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Li, G.; Toldrá, F.; Zhang, W. The physiological activity of bioactive peptides obtained from meat and meat by-products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press/Elsevier: Amsterdam, The Netherlands, 2021; Volume 97, pp. 147–185. [Google Scholar]
- Bouglé, D.; Bouhallab, S. Dietary bioactive peptides: Human studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Zhao, M.; Bi, L.; Bi, W.; Wang, C.; Yang, Z.; Ju, J.; Peng, S. Synthesis of new class dipeptide analogues with improved permeability and antithrombotic activity. Bioorg. Med. Chem. 2006, 14, 4761–4774. [Google Scholar] [CrossRef]
- Mora, L.; Valero, M.L.L.; Sánchez del Pino, M.M.M.; Sentandreu, M.Á.; Toldrá, F. Small peptides released from muscle glycolytic enzymes during dry-cured ham processing. J. Proteom. 2011, 74, 442–450. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Aristoy, M.C.; Toldrá, F. Titin-derived peptides as processing time markers in dry-cured ham. Food Chem. 2015, 167, 326–339. [Google Scholar] [CrossRef]
- Dellafiora, L.; Paolella, S.; Asta, C.D.; Dossena, A.; Cozzini, P.; Galaverna, G.; Dall’Asta, C.; Dossena, A.; Cozzini, P.; Galaverna, G. Hybrid in Silico/in Vitro Approach for the Identification of Angiotensin I Converting Enzyme Inhibitory Peptides from Parma Dry-Cured Ham. J. Agric. Food Chem. 2015, 63, 6366–6375. [Google Scholar] [CrossRef]
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Intense Degradation of Myosin Light Chain Isoforms in Spanish Dry-Cured Ham. J. Agric. Food Chem. 2011, 59, 3884–3892. [Google Scholar] [CrossRef]
- Lin, F.; Cai, F.; Luo, B.; Gu, R.; Ahmed, S.; Long, C. Variation of Microbiological and Biochemical Profiles of Laowo Dry-Cured Ham, an Indigenous Fermented Food, during Ripening by GC-TOF-MS and UPLC-QTOF-MS. J. Agric. Food Chem. 2020, 68, 8925–8935. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Rouvinen-Watt, K. The role of food peptides in lipid metabolism during dyslipidemia and associated health conditions. Int. J. Mol. Sci. 2015, 16, 9303–9313. [Google Scholar] [CrossRef]
- Marušić, N.; Aristoy, M.-C.; Toldrá, F. Nutritional pork meat compounds as affected by ham dry-curing. Meat Sci. 2013, 93, 53–60. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J. Funct. Foods 2012, 4, 403–422. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ito, S.; Kawarasaki, Y. Peptides Trp-Arg-Xaa tripeptides act as uncompetitive-type inhibitors of human dipeptidyl peptidase IV. Peptides 2014, 54, 166–170. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of anti-inflammatory proteins/peptides: An insilico approach. J. Trans. Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-based prediction of anti-inflammatory peptides using random forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Wan, J.; Zhou, Y.; Zhu, Q. Extraction and identification of bioactive peptides from Panxian dry-cured ham with multifunctional activities. LWT 2022, 160, 113326. [Google Scholar] [CrossRef]
- Mitsuhashi, S. Current topics in the biotechnological production of essential amino acids, functional amino acids, and dipeptides. Curr. Op. Biotechnol. 2014, 26, 38–44. [Google Scholar] [CrossRef]
- Heres, A.; Mora, L.; Toldrá, F. Inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase enzyme by dipeptides identified in dry-cured ham. Food Prod. Proc. Nutr. 2021, 3, 18. [Google Scholar] [CrossRef]
- WHO. World Health Statistics: Monitoring Health for the SDGs, Sustainable Development Goals. 2022; 125p. Available online: https://www.who.int/publications/i/item/9789240051157 (accessed on 15 November 2022).
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J.F. Improving health-promoting effects of food-derived bioactive peptides through rational design and oral delivery strategies. Nutrients 2019, 11, 2545. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Mason, S.; Morton, J.D.; Bekhit, A.E.-D.A.; Bhat, H.F. Antihypertensive Peptides from Animal Proteins. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 319–353. [Google Scholar]
- Deng, B.; Ni, X.; Zhai, Z.; Tang, T.; Tan, C.; Yan, Y.; Deng, J.; Yin, Y. New Quantitative Structure—Activity Relationship Model for Angiotensin-Converting Enzyme Inhibitory Dipeptides Based on Integrated Descriptors. J. Agric. Food Chem. 2017, 65, 9774–9781. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of Di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Benito, M.J.; Martín, A.; Casquete, R.; Córdoba, J.J.; Córdoba, M.G. Influence of starter culture and a protease on the generation of ACE-inhibitory and antioxidant bioactive nitrogen compounds in Iberian dry-fermented sausage “salchichón”. Heliyon 2016, 2, 00093. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Toldrá, F.; Sentandreu, M.A.; Nishimura, H.; Arihara, K. Antihypertensive activity of peptides identified in the in vitro gastrointestinal digest of pork meat. Meat Sci. 2012, 91, 382–384. [Google Scholar] [CrossRef]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of Valyl-Tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Human Hyper. 2000, 14, 519–523. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Toldrá, F. Evaluation of ACE inhibitory activity of dipeptides generated by the action of porcine muscle dipeptidyl peptidases. Food Chem. 2007, 102, 511–515. [Google Scholar] [CrossRef]
- Zhou, Z.; Cheng, C.; Li, Y. Structure-based design and optimization of antihypertensive peptides to obtain high inhibitory potency against both renin and angiotensin I-converting enzyme. SAR QSAR Environ. Res. 2015, 26, 1001–1016. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Li, H.; Aluko, R.E. Quantitative structure—Activity relationship modeling of renin-inhibiting dipeptides. Amino Acids 2012, 42, 1379–1386. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Evolution of a new class of antihypertensive drugs. In Angiotensin Converting Enzyme Inhibitors: Mechanism of Action and Clinical Implications; Horovitz, Z.P., Ed.; Urban & Schwarzenberg: Munich, Germany, 1981; pp. 1–25. [Google Scholar]
- Li, C.; Mora, L.; Gallego, M.; Aristoy, M.-C.; Toldrá, F. Evaluation of main post-translational modifications occurring in naturally generated peptides during the ripening of Spanish dry-cured ham. Food Chem. 2020, 332, 127388. [Google Scholar] [CrossRef]
- Maestri, E.; Pavlicevic, M.; Montorsi, M.; Marmiroli, N. Meta-Analysis for Correlating Structure of Bioactive Peptides in Foods of Animal Origin with Regard to Effect and Stability. Comp. Rev. Food Sci. Food Saf. 2019, 18, 3–30. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Gu, L.; Zhao, M.; Li, W.; You, L.; Wang, J.; Wang, H.; Ren, J. Chemical and cellular antioxidant activity of two novel peptides designed based on glutathione structure. Food Chem. Toxicol. 2012, 50, 4085–4091. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.H.; Zhang, W.G. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef]
- Xing, L.J.; Hu, Y.Y.; Hu, H.Y.; Ge, Q.F.; Zhou, G.H.; Zhang, W.G. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016, 194, 951–958. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food Funct. 2013, 4, 1843–1849. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Evolution of oxidised peptides during the processing of 9 months Spanish dry-cured ham. Food Chem. 2018, 239, 823–830. [Google Scholar] [CrossRef]
- Ohata, M.; Uchida, S.; Zhou, L.; Arihara, K. Antioxidant activity of fermented meat sauce and isolation of an associated antioxidant peptide. Food Chem. 2016, 194, 1034–1039. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Li, P.; Liu, Q.; Kong, B.; Huang, X.; Wu, Z. Physicochemical changes of antioxidant peptides hydrolyzed from porcine plasma protein subject to free hydroxyl radical system. Adv. J. Food Sci. Technol. 2013, 5, 14–18. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.C.; Toldrá, F. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Escudero, E.; Fraser, P.D.; Aristoy, M.C.; Toldrá, F. Proteomic identification of antioxidant peptides from 400 to 2500 Da generated in Spanish dry-cured ham contained in a size-exclusion chromatography fraction. Food Res. Int. 2014, 56, 68–76. [Google Scholar] [CrossRef]
- Xing, L.; Liu, R.; Gao, X.; Zheng, J.; Wang, C.; Zhou, G.; Zhang, W. The proteomics homology of antioxidant peptides extracted from dry-cured Xuanwei and Jinhua ham. Food Chem. 2018, 266, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Kim, D.; Yoon, S.K.; Ham, J.S.; Jang, A. Anti-oxidative and anti-inflammation activities of pork extracts. Korean J. Food Sci. Anim. Res. 2016, 36, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Saunders, B.; Harris, R.C.; Bicudo, J.E.P.W.; Bishop, D.J.; Sale, C.; Gualano, B. Comparative physiology investigations support a role for histidine-containing dipeptides in intracellular acid–base regulation of skeletal muscle. Comp. Biochem. Physiol.-Part A Mol. Integr. Physiol. 2019, 234, 77–86. [Google Scholar] [CrossRef]
- Bengmark, S. Advanced glycation and lipoxidation end products-amplifiers of inflammation: The role of food. J. Parent. Enter. Nutr. 2007, 31, 430–440. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Straniero, V.; Pedretti, A.; Pallavicini, M.; Valoti, E.; Carini, M.; Testa, B.; Aldini, G. Exploring the space of histidine containing dipeptides in search of novel efficient RCS sequestering agents. Eur. J. Med. Chem. 2013, 66, 153–160. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Ventanas, J.; Toldrá, F. Nutritional composition of dry-cured ham and its role in a healthy diet. Meat Sci. 2010, 84, 585–593. [Google Scholar] [CrossRef]
- Geissler, S.; Zwarg, M.; Knütter, I.; Markwardt, F.; Brandsch, M. The bioactive dipeptide anserine is transported by human proton-coupled peptide transporters. FEBS J. 2010, 277, 790–795. [Google Scholar] [CrossRef]
- Kondrashina, A.; Brodkorb, A.; Giblin, L. Dairy-derived peptides for satiety. J. Funct. Foods 2020, 66, 103801. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J Diab. 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef]
- Liu, R.; Cheng, J.; Wu, H. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kawakami, K.; Uraji, M. Inhibitory effect of collagen-derived tripeptides on dipeptidylpeptidase-IV activity. J. Enz. Inhib. Med. Chem. 2014, 29, 823–828. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.H.; Gaspar, A.R.M. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Design 2018, 91, 370–379. [Google Scholar] [CrossRef]
- Montoro-García, S.; Zafrilla-Rentero, M.P.; Celdrán-de Haro, F.M.; Piñero-de Armas, J.J.; Toldrá, F.; Tejada-Portero, L.; Abellán-Alemán, J. Effects of dry-cured ham rich in bioactive peptides on cardiovascular health: A randomized controlled trial. J. Funct. Foods 2017, 38, 160–167. [Google Scholar] [CrossRef]
- Rueda, Á.A.; Jurado, J.M.; de Pablos, F.; León-Camacho, M. Differentiation between Ripening Stages of Iberian Dry-Cured Ham According to the Free Amino Acids Content. Foods 2020, 9, 82. [Google Scholar] [CrossRef]
- Smith, D.E.; Clémençon, B.; Hediger, M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Asp. Med. 2013, 34, 323–336. [Google Scholar] [CrossRef]
- Ayyadurai, S.; Charania, M.A.; Xiao, B.; Viennois, E.; Merlin, D. PepT1 expressed in immune cells has an important role in promoting the immune response during experimentally induced colitis. Lab. Invest. 2013, 93, 888–899. [Google Scholar] [CrossRef]
- Fürst, P.; Alteheld, B.; Stehle, P. Why should a single nutrient—glutamine—improve outcome ? The remarkable story of glutamine dipeptides. Clinical Nutr. Suppl. 2004, 1, 3–15. [Google Scholar] [CrossRef]
- Wang, H.; Jia, G.; Chen, Z.L.; Huang, L.; Wu, C.M.; Wang, K.N. The effect of glycyl-glutamine dipeptide concentration on enzyme activity, cell proliferation and apoptosis of jejunal tissues from weaned piglets. Agric. Sci. China 2011, 10, 1088–1095. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Jiang, Z.M.; Sun, Y.H.; He, G.Z.; Shu, H. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: A randomized, double-blind, controlled clinical trial. Clin. Nutr. Suppl. 2004, 1, 55–60. [Google Scholar] [CrossRef]
- Raizel, R.; Leite, J.S.M.; Hypólito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Brit. J. Nutr. 2016, 116, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl valine supplementation-induced mitigation of gut inflammation in a porcine model of colitis. J. Funct. Foods 2016, 24, 558–567. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys Acta-Mol. Basis Dis. 2015, 1852, 792–804. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, M.; Yu, C.; Li, J.; Zhang, L.; Zhou, P.; Yang, W.; Gao, F.; Zhou, G. Alanyl-glutamine supplementation regulates mTOR and ubiquitin proteasome proteolysis signaling pathways in piglets. Nutrition 2016, 32, 1123–1131. [Google Scholar] [CrossRef]
- Strøm, M.B.; Haug, B.E.; Skar, M.L.; Stensen, W.; Stiberg, T.; Svendsen, J.S. The Pharmacophore of Short Cationic Antibacterial Peptides. J. Med. Chem. 2003, 46, 1567–1570. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Porcine myofibrillar proteins as potential precursors of bioactive peptides—An in silico study. Food Funct. 2016, 7, 2878–2885. [Google Scholar] [CrossRef]
- Shimizu, M.; Sawashita, N.; Morimatsu, F.; Ichikawa, J.; Taguchi, Y. Antithrombotic papain-hydrolyzed peptides isolated from pork meat. Thromb. Res. 2009, 123, 753–757. [Google Scholar] [CrossRef]
- Nasri, M. Protein Hydrolysates and Biopeptides. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press/Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–159. [Google Scholar]
- Morimatsu, F.; Kimura, S. Hypocholesterolemic Effect of Partial Hydrolyzates of Pork Meat in Rats. Nippon Shok. Kog. Gakk. 1992, 39, 770–777. [Google Scholar] [CrossRef]
- Morimatsu, F.; Ito, M.; Budijanto, S.; Watanabe, I.; Furukawa, Y.; Kimura, S. Plasma cholesterol-suppressing effect of papain-hydrolyzed pork meat in rats fed hypercholesterolemic diet. J. Nutr. Sci. Vit. 1996, 42, 145–153. [Google Scholar] [CrossRef]
- Katsuda, S.; Ito, M.; Waseda, Y.; Morimatsu, F.; Taguchi, Y.; Hasegawa, M.; Takaichi, S.; Yamada, R.; Shimizui, T. Papain-Hydrolyzed and Premature Pork Meat Reduces Serum Rabbits Cholesterol Level Atherosclerosis in Dietary-Induced Hypercholesterolemic 1 Department of Physiology, School of Medicine, Fukushima Medical University, 2 Department of Science of Biologica. J. Nutr. Sci. Vit. 2000, 46, 180–187. [Google Scholar] [CrossRef]
- Caron, J.; Domenger, D.; Dhulster, P.; Ravallec, R.; Cudennec, B. Protein digestion-derived peptides and the peripheral regulation of food intake. Front. Endocrinol. 2017, 8, 85. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Miralles, B.; Amigo, L.; Recio, I. Intestinal Signaling of Proteins and Digestion-Derived Products Relevant to Satiety. J. Agric. Food Chem. 2018, 66, 10123–10131. [Google Scholar] [CrossRef]
- Sufian, M.K.N.B.; Hira, T.; Miyashita, K.; Nishi, T.; Asano, K.; Hara, H. Pork peptone stimulates cholecystokinin secretion from enteroendocrine cells and suppresses appetite in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1869–1874. [Google Scholar] [CrossRef]
- Stock, S.; Leichner, P.; Wong, A.C.K.; Ghatei, M.A.; Kieffer, T.J.; Bloom, S.R.; Chanoine, J.P. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J. Clin. Endocrinol. Metab. 2005, 90, 2161–2168. [Google Scholar] [CrossRef]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef]
- Huang, S.-L.; Hung, C.-C.; Jao, C.-L.; Tung, Y.-S.; Hsu, K.-C. Porcine skin gelatin hydrolysate as a dipeptidyl peptidase IV inhibitor improves glycemic control in streptozotocin-induced diabetic rats. J. Funct. Foods 2014, 11, 235–242. [Google Scholar] [CrossRef]
- Bernard, C.; Sutter, A.; Vinson, C.; Ratineau, C.; Chayvialle, J.A.; Cordier-Bussat, M. Peptones stimulate intestinal cholecystokinin gene transcription via cyclic adenosine monophosphate response element-binding factors. Endocrinology 2001, 142, 721–729. [Google Scholar] [CrossRef]
- Cordier-Bussat, M.; Bernard, C.; Haouche, S.; Roche, C.; Abello, J.; Chayvialle, J.A.; Cuber, J.C. Peptones stimulate cholecystokinin secretion and gene transcription in the intestinal cell line STC-I. Endocrinology 1997, 138, 1137–1144. [Google Scholar] [CrossRef]
- Matsui, T.; Yoshino, A.; Tanaka, M. A trip of peptides to the brain. Food Prod. Proc. Nutr. 2020, 2, 30. [Google Scholar] [CrossRef]
- Wang, X.-X.; Hu, Y.; Keep, R.F.; Toyama-Sorimachi, N.; Smith, D.E. A novel role for PHT1 in the disposition of l-histidine in brain: In vitro slice and in vivo pharmacokinetic studies in wildtype and Pht1 null mice. Biochem. Pharmacol. 2017, 124, 94–102. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Degradation of LIM domain-binding protein three during processing of Spanish dry-cured ham. Food Chem. 2014, 149, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Maebuchi, M.; Kishi, Y.; Koikeda, T.; Furuya, S. Soy peptide dietary supplementation increases serum dopamine level and improves cognitive dysfunction in subjects with mild cognitive impairment. Jap. Pharm. Therap. 2013, 41, 67–73. [Google Scholar]
- Katayama, S.; Imai, R.; Sugiyama, H.; Nakamura, S. Oral Administration of Soy Peptides Suppresses Cognitive Decline by Induction of Neurotrophic Factors in SAMP8 Mice. J. Agric. Food Chem. 2014, 62, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sun-Waterhouse, D.; Tao, Q.; Li, W.; Shu, D.; Cui, C. The enhanced serotonin (5-HT) synthesis and anti-oxidative roles of Trp oligopeptide in combating anxious depression C57BL/6 mice. J. Funct. Foods 2020, 67, 103859. [Google Scholar] [CrossRef]
- Khlebnikova, N.N.; Krupina, N.A.; Kushnareva, E.Y.; Zolotov, N.N.; Kryzhanovskii, G.N. Effect of imipramine and prolyl endopeptidase inhibitor benzyloxycarbonyl-methionyl-2(S)-cyanopyrrolidine on activity of proline-specific peptidases in the brain of ratswith experimental anxious-depressive syndrome. Bull. Exp. Biol. Med. 2012, 152, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Arısoy, S.; Üstün-Aytekin, Ö. Hydrolysis of food-derived opioids by dipeptidyl peptidase IV from Lactococcus lactis spp. lactis. Food Res. Int. 2018, 111, 574–581. [Google Scholar] [CrossRef]
- Khaket, T.P.; Redhu, D.; Dhanda, S.; Singh, J.; Khaket, T.P.; Redhu, D.; Dhanda, S.; Singh, J. In Silico Evaluation of Potential DPP-III Inhibitor Precursors from Dietary Proteins In Silico Evaluation of Potential DPP-III Inhibitor Precursors from Dietary Proteins. Int. J. Food Prop. 2015, 18, 499–507. [Google Scholar] [CrossRef]
- Prajapati, S.C.; Chauhan, S.S. Dipeptidyl peptidase III: A multifaceted oligopeptide N-end cutter. FEBS J. 2011, 278, 3256–3276. [Google Scholar] [CrossRef]

| Amino Acid | Abbreviation a | Threshold (μM) | Taste |
|---|---|---|---|
| Isoleucine | I | 7.51 | Bitter |
| Valine | V | 4.76 | Sweet and bitter |
| Leucine | L | 6.45 | Bitter |
| Phenylalanine | F | 6.61 | Bitter |
| Cysteine | C | 0.063 | Sulfurous |
| Methionine | M | 3.72 | Sweet and bitter |
| Alanine | A | 16.2 | Sweet |
| Glycine | G | 30.9 | Sweet |
| Threonine | T | 25.7 | Sweet and bitter |
| Serine | S | 0.029 | Sweet |
| Tryptophan | W | 2.29 | Bitter |
| Tyrosine | Y | ND | Bitter |
| Proline | P | 15 | Sweet and bitter |
| Histidine | H | 1.23 | Bitter |
| Glutamate | E | 0.063 | Umami, bitter, salty, sour |
| Glutamic acid | Q | 9.77 | Sweet |
| Aspartic acid | D | 0.182 | Umami, bitter, salty, sour |
| Asparagine | N | 1.62 | Bitter |
| Lysine | K | 0.708 | Salty, sweet, bitter |
| Arginine | R | 1.20 | Sweet and bitter |
| Sequence a | Origin (Curing Months) | Reference |
|---|---|---|
| Bitter b | ||
| IV, LE, ID, and PL | Serrano DCH (8 m) | [40] |
| GF and LL | Parma DCH (12 m) | [81] |
| AD, DL, EA, EE, EF, EI, EL, GP, IF, IL, KP, LA, LG, LL, PA, PK, PL, PP, RG, VE, VF, VY | In silico | [75] |
| PG and VG | Norwegian DCH (21 m) | [82] |
| PL | Jinhua (DCH) (8 m) | [83] |
| GL | Prosciutto-like DCH (22 m) | [32] |
| PA and VG | Traditional and low-salted Spanish DCH (6, 12, 18, and 24 m) | [84,85] |
| Umami | ||
| DE, EA, EE, EK, EL, VE | In silico | [75,86] |
| EE, EF, EK, and DA | Jinhua DCH (6 m) | [73] |
| EL, EV, RL, EEL, and ESV | Jinhua DCH (6 m) | [15] |
| DA, DG, EE, ES, EV, and VG | Traditional and low-salted Spanish DCH (6, 12, 18, and 24 m) | [84,85] |
| AH | Spanish DCH (18 m) Jinhua (6 m) | [83,87] |
| EE | Prosciutto-like DCH (22 m) | [32] |
| DK | Spanish DCH (9 m) | [22] |
| Sweet | ||
| AA, AAA, AGA, AGG, EV, GAG, and GGA | In silico | [75] |
| AA and GAG | Spanish DCH (2 m) | [88,89] |
| AA | Traditional and low-salted Spanish DCH (6, 12, 18, and 24 m) Prosciutto-like DCH (22 m) | [32,90] |
| Sour | ||
| AED, DD, DE, DV, DEE, DES, ED, EV, VD, and VE | In silico | [75] |
| VE, GE, and DV | Serrano DCH (8 m) | [40] |
| DE | Spanish DCH (12 m) | [88,91] |
| Salty | ||
| DE | Spanish DCH (12 m) | [22,88] |
| AR | Jinhua (6 m) | [83] |
| Kokumi | ||
| γ-EI, γ-EL, γ-EF and γ-EY | Parma DCH (18, and 24 m) | [81,92] |
| γ-EF, γ-EW and γ-EY | Prosciutto DCH (14, 21, and 34 m) | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heres, A.; Mora, L.; Toldrá, F. Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat. Int. J. Mol. Sci. 2023, 24, 1574. https://doi.org/10.3390/ijms24021574
Heres A, Mora L, Toldrá F. Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat. International Journal of Molecular Sciences. 2023; 24(2):1574. https://doi.org/10.3390/ijms24021574
Chicago/Turabian StyleHeres, Alejandro, Leticia Mora, and Fidel Toldrá. 2023. "Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat" International Journal of Molecular Sciences 24, no. 2: 1574. https://doi.org/10.3390/ijms24021574
APA StyleHeres, A., Mora, L., & Toldrá, F. (2023). Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat. International Journal of Molecular Sciences, 24(2), 1574. https://doi.org/10.3390/ijms24021574








