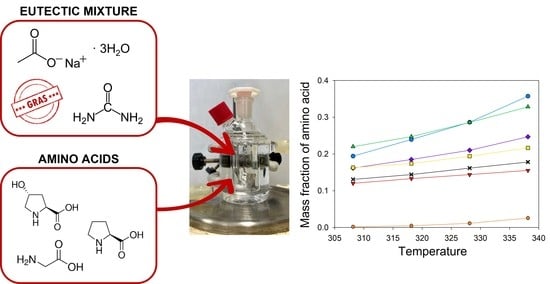
Solubility of Amino Acids in the Eutectic Solvent Constituted by Sodium Acetate Trihydrate and Urea and in Its Mixture with Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of the Eutectic
2.2. Solubility Studies
Apparent Properties of Dissolution
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Determination of the Eutectic
3.2.2. Solubility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. World Fisheries and Aquaculture; FAO: Rome, Italy, 2022; ISBN 9789251072257. [Google Scholar]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- European Commission. The EU Blue Economy Report; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Heidari, M.G.; Rezaei, M. Extracted pepsin of trout waste and ultrasound-promoted method for green recovery of fish collagen. Sustain. Chem. Pharm. 2022, 30, 100854. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Della Posta, S.; Gallo, V.; Gentili, A.; Fanali, C. Strategies for the recovery of bioactive molecules from deep eutectic solvents extracts. TrAC-Trends Anal. Chem. 2022, 157, 116798. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; the “Gold, Book”; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Com. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Coutinho, J.A.P.; Pinho, S.P. Special Issue on Deep Eutectic Solvents: A foreword. Fluid Phase Equilibr. 2017, 448, 1. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Schaeffer, N.; Silva, L.P.; Coutinho, J.A.P. Comment on “Structural Study of a Eutectic Solvent Reveals Hydrophobic Segregation and Lack of Hydrogen Bonding between the Components”. ACS Sus. Chem. Eng. 2022, 10, 8669–8670. [Google Scholar] [CrossRef]
- Bradić, B.; Novak, U.; Likozar, B. Crustacean shell bio-refining to chitin by natural. Green Process. Synth. 2020, 9, 13–25. [Google Scholar] [CrossRef]
- Bai, C.; Wei, Q.; Ren, X. Selective Extraction of Collagen Peptides with High Purity from Cod Skins by Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 7220–7227. [Google Scholar] [CrossRef]
- Bisht, M.; Martins, M.; Dias, A.C.R.V.; Ventura, S.P.M.; Coutinho, J.A.P. Uncovering the potential of aqueous solutions of deep eutectic solvents on the extraction and purification of collagen type I from Atlantic codfish (Gadus morhua). Green Chem. 2021, 23, 8940–8948. [Google Scholar] [CrossRef]
- Nuutinen, E.M.; Willberg-Keyriläinen, P.; Virtanen, T.; Mija, A.; Kuutti, L.; Lantto, R.; Jääskeläinen, A.S. Green process to regenerate keratin from feathers with an aqueous deep eutectic solvent. RSC Adv. 2019, 9, 19720–19728. [Google Scholar] [CrossRef]
- Rico, X.; Nuutinen, E.M.; Gullón, B.; Pihlajaniemi, V.; Yáñez, R. Application of an eco-friendly sodium acetate/urea deep eutectic solvent in the valorization of melon by-products. Food Bioprod. Process. 2021, 130, 216–228. [Google Scholar] [CrossRef]
- Muhammad, N.; Gonfa, G.; Rahim, A.; Ahmad, P.; Iqbal, F.; Sharif, F.; Khan, A.S.; Khan, F.U.; Khan, Z.U.H.; Rehman, F.; et al. Investigation of ionic liquids as a pretreatment solvent for extraction of collagen biopolymer from waste fish scales using COSMO-RS and experiment. J. Mol. Liq. 2017, 232, 258–264. [Google Scholar] [CrossRef]
- Muhammad, N.; Gao, Y.; Iqbal, F.; Ahmad, P.; Ge, R.; Nishan, U.; Rahim, A.; Gonfa, G.; Ullah, Z. Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 2016, 161, 129–135. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Gurau, G.; Griggs, C.S.; Rogers, R.D. Pulping of Crustacean Waste Using Ionic Liquids: To Extract or Not to Extract. ACS Sustain. Chem. Eng. 2016, 4, 6072–6081. [Google Scholar] [CrossRef]
- Lundström, A.; Andersson, B.; Olsson, L. Urea thermolysis studied under flow reactor conditions using DSC and FT-IR. Chem. Eng. J. 2009, 150, 544–550. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, G.-E.; Wang, J.Y. Investigation of a eutectic mixture of sodium acetate trihydrate and urea as latent heat storage. Sol. Energy 1991, 47, 443–445. [Google Scholar] [CrossRef]
- Stradella, L.; Argentero, M. A study of the thermal decomposition of urea, of related compounds and thiourea using DSC and TG-EGA. Thermochim. Acta 1993, 219, 315–323. [Google Scholar] [CrossRef]
- Yang, H.; Bao, X.; Cui, H.; Lo, T.Y.; Chen, X. Optimization of supercooling, thermal conductivity, photothermal conversion, and phase change temperature of sodium acetate trihydrate for thermal energy storage applications. Energy 2022, 254, 124280. [Google Scholar] [CrossRef]
- Guion, J.; Physique, L.D.C.; De Nice, U.; Antipolis, S.; Sauzade, J.D.; Laugt, M.; Antipolis, S. Critical examination and experimental determination of melting enthalpies and entropies of salt hydrates. Thermochim. Acta 1983, 67, 167–179. [Google Scholar] [CrossRef]
- Wada, T.; Kimura, F.; Yamamoto, R. Studies on salt hydrates for latent heat storage. II. Eutectic mixture of pseudo-binary system. Bull. Chem. Soc. Jpn. 1983, 56, 1223–1226. [Google Scholar] [CrossRef]
- Li, X.; Fu, Z.; Qiao, Y.; Zhang, Z.; Zhou, Y.; Hai, C.; Shen, Y.; Sun, Y.; Zeng, J.; Ren, X. Preparation, Characterization, and Modification of Sodium Acetate Trihydrate-Urea Binary Eutectic Mixtures as Phase Change Material. Energy Fuels 2020, 34, 6439–6447. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Dai, R.; Shan, Z.; Yi, J.; Chen, H. The solubility and interactions of gelatin in “water-in-sodium acetate trihydrate/urea DES” system. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126916. [Google Scholar] [CrossRef]
- Scheiner, S.; Kar, T.; Pattanayak, J. Comparison of various types of hydrogen bonds involving aromatic amino acids. J. Am. Chem. Soc. 2002, 124, 13257–13264. [Google Scholar] [CrossRef]
- Rose, G.D.; Geselowitz, A.R.; Lesser, G.J.; Lee, R.H.; Zehfus, M.H. Hydrophobicity of amino acid residues in globular proteins. Science 1985, 229, 834–838. [Google Scholar] [CrossRef]
- Nagano, N.; Ota, M.; Nishikawa, K. Strong hydrophobic nature of cysteine residues in proteins. FEBS Lett. 1999, 458, 69–71. [Google Scholar] [CrossRef]
- Cohn, E.J.; Edsall, J.T. Proteins, Amino Acids and Peptides as Ions and Dipolar Ions; Renhold Publ. Corp.: New York, NY, USA, 1943. [Google Scholar]
- Krug, R.R.; Hunter, W.G.; Grieger, R.A. Enthalpy-Entropy Compensation. 2. Separation of the Chemical from the Statistical Effect. J. Phys. Chem. 1976, 80, 2341–2351. [Google Scholar] [CrossRef]
- Blanco-Márquez, J.H.; Caviedes Rubio, D.I.; Ortiz, C.P.; Cerquera, N.E.; Martínez, F.; Delgado, D.R. Thermodynamic analysis and preferential solvation of sulfamethazine in acetonitrile + water cosolvent mixtures. Fluid Phase Equilib. 2020, 505, 112361. [Google Scholar] [CrossRef]
- Tinjacá, D.A.; Martínez, F.; Almanza, O.A.; Jouyban, A.; Acree, W.E. Effect of N-Methyl-pyrrolidone (NMP) on the Equilibrium Solubility of Meloxicam in Aqueous Media: Correlation, Dissolution Thermodynamics, and Preferential Solvation. ACS Omega 2022, 7, 37988–38002. [Google Scholar] [CrossRef] [PubMed]
 ) are compared to literature data:
) are compared to literature data:  , ref. [24];
, ref. [24];  , ref. [28];
, ref. [28];  , ref. [29]. The horizontal solid line represents the eutectic temperature found.
, ref. [29]. The horizontal solid line represents the eutectic temperature found.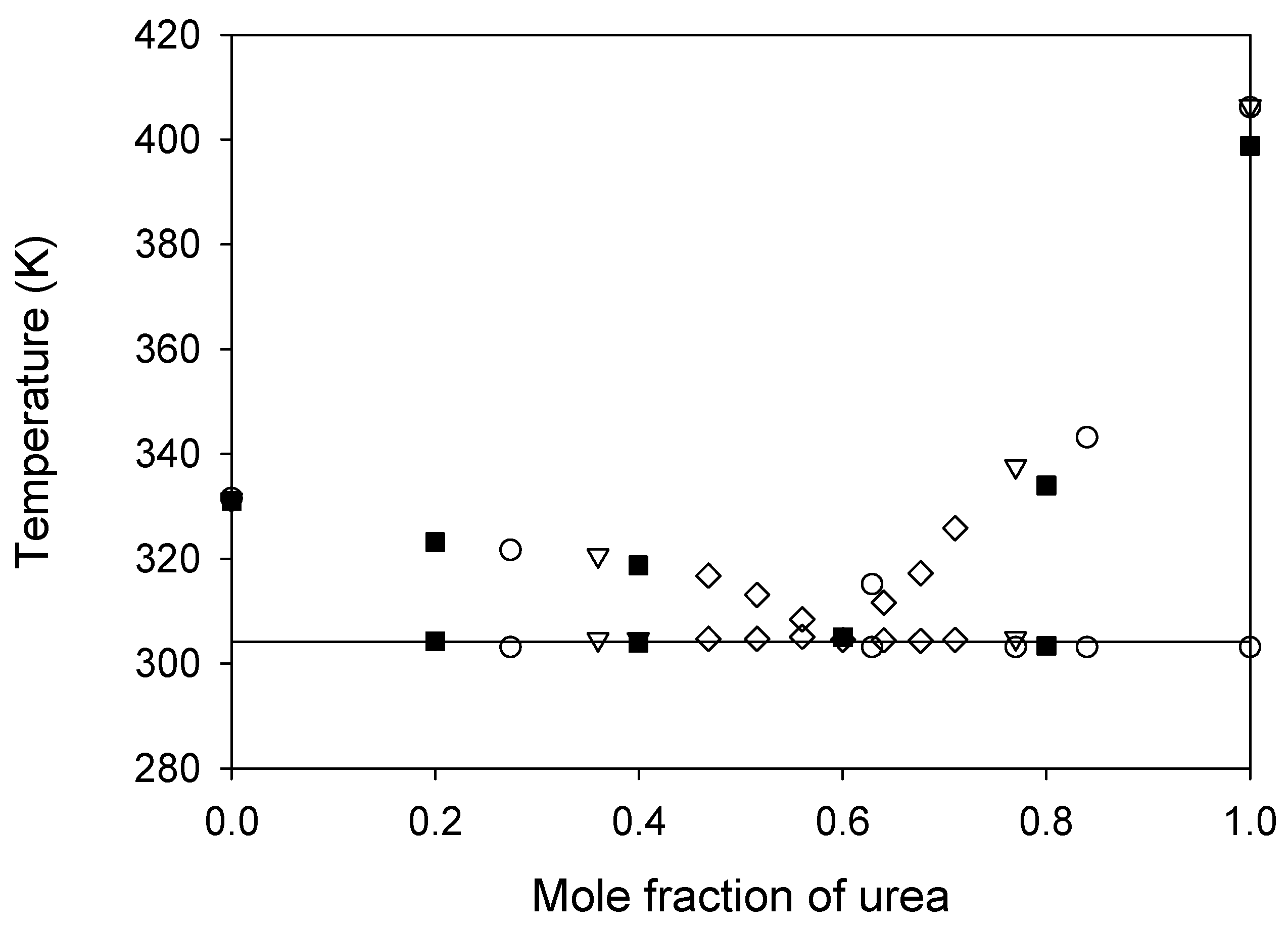
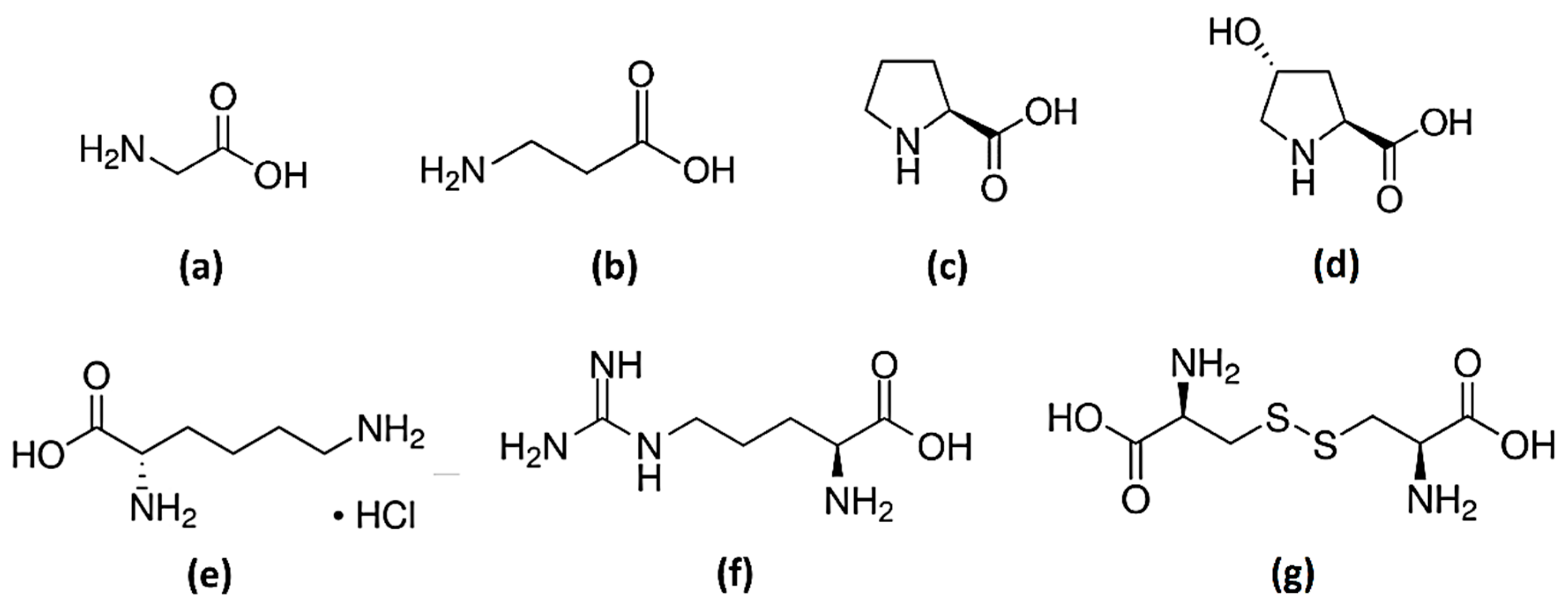
 glycine;
glycine;  β-alanine;
β-alanine;  L-proline;
L-proline;  trans-4-hydroxy-L-proline;
trans-4-hydroxy-L-proline;  L-lysine·HCl;
L-lysine·HCl;  L-arginine;
L-arginine;  L-cystine.
L-cystine.
 glycine;
glycine;  β-alanine;
β-alanine;  L-proline;
L-proline;  trans-4-hydroxy-L-proline;
trans-4-hydroxy-L-proline;  L-lysine·HCl;
L-lysine·HCl;  L-arginine;
L-arginine;  L-cystine.
L-cystine.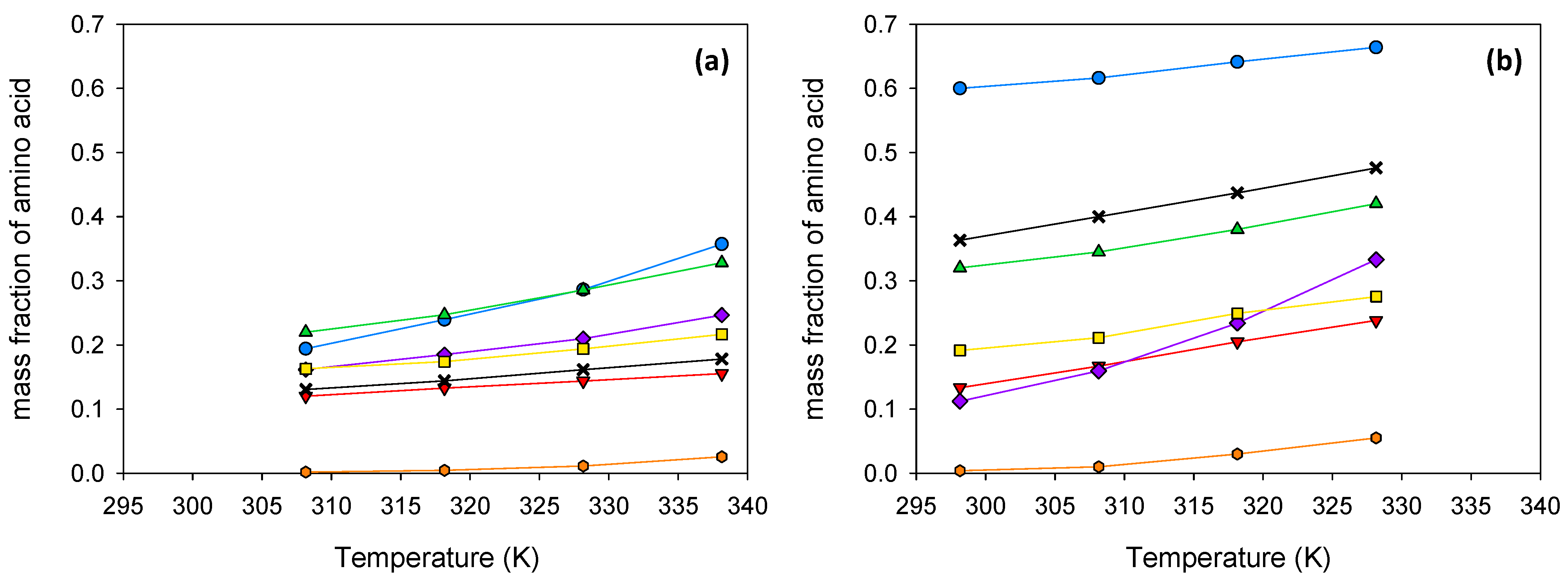
 glycine;
glycine;  β-alanine;
β-alanine;  L-proline;
L-proline;  trans-4-hydroxy-L-proline;
trans-4-hydroxy-L-proline;  L-lysine·HCl;
L-lysine·HCl;  L-arginine;
L-arginine;  L-cystine.
L-cystine.
 glycine;
glycine;  β-alanine;
β-alanine;  L-proline;
L-proline;  trans-4-hydroxy-L-proline;
trans-4-hydroxy-L-proline;  L-lysine·HCl;
L-lysine·HCl;  L-arginine;
L-arginine;  L-cystine.
L-cystine.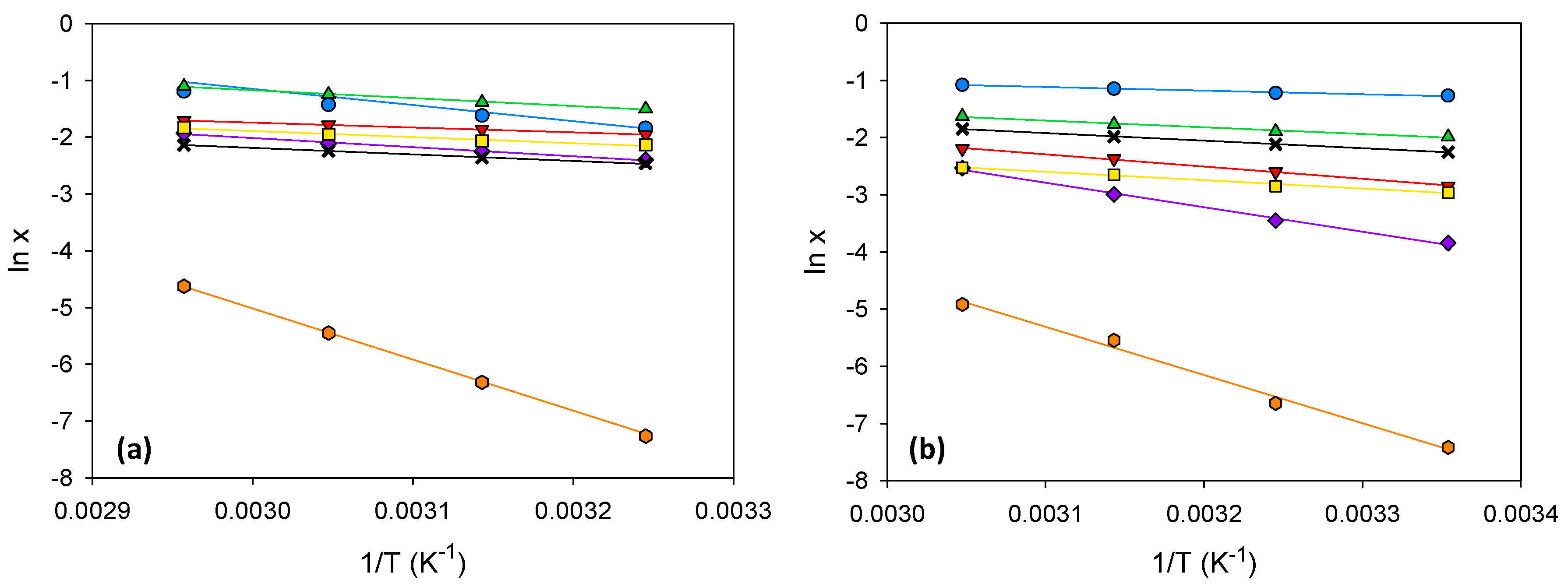

| Compound | Tm (K) | ΔHfus (kJ·mol−1) | ||
|---|---|---|---|---|
| Exp. | Lit. | Exp. | Lit. | |
| Urea | 402 | 406 [23,24] | 12.7 | 14.1 [25], 15.1 [24] |
| Sodium acetate trihydrate | 331 | 331 [26,27], 332 [24] | 37.3 | 37.0 [27], 36.2 [24] |
| Eutectic Composition (Urea Mole Fraction) | Tm (K) | Reference |
|---|---|---|
| 0.77 | 303 | [24] |
| 0.60 | 305 | [28] |
| 0.60 | 305 | [29] |
| 0.60 | 304 | This work |
| Eutectic | |||||||
| T (K) | Glycine | β-Alanine | L-Proline | trans-4-Hydroxy-L-proline | L-Lysine·HCl | L-Arginine | L-Cystine |
| 308.15 | 0.1201 | 0.2199 | 0.1941 | 0.1629 | 0.1307 | 0.1618 | 0.0019 |
| 318.15 | 0.1329 | 0.2471 | 0.2393 | 0.1739 | 0.1440 | 0.1850 | 0.0047 |
| 328.15 | 0.1439 | 0.2858 | 0.2864 | 0.1939 | 0.1615 | 0.2100 | 0.0113 |
| 338.15 | 0.1553 | 0.3282 | 0.3573 | 0.2166 | 0.1781 | 0.2467 | 0.0257 |
| Eutectic:Water 50:50 (wt:wt) | |||||||
| T (K) | Glycine | β-Alanine | L-Proline | trans-4-Hydroxy-L-proline | L-Lysine·HCl | L-Arginine | L-Cystine |
| 298.15 | 0.1334 | 0.3202 | 0.5999 | 0.1916 | 0.3633 | 0.1124 | 0.0040 |
| 308.15 | 0.1671 | 0.3448 | 0.6161 | 0.2113 | 0.4000 | 0.1596 | 0.0102 |
| 318.15 | 0.2049 | 0.3801 | 0.6412 | 0.2492 | 0.4371 | 0.2341 | 0.0300 |
| 328.15 | 0.2381 | 0.4204 | 0.6639 | 0.2753 | 0.4760 | 0.3331 | 0.0552 |
| Eutectic | Eutectic:Water 50:50 (wt:wt) | |||||
|---|---|---|---|---|---|---|
| Amino Acid | A | B (K) | % ARD | A | B (K) | % ARD |
| Glycine | −863.5 | 0.848 | 0.34 | −2133 | 4.319 | 1.12 |
| β-Alanine | −1395 | 3.014 | 0.94 | −1181 | 1.957 | 1.45 |
| L-Proline | −2219 | 5.355 | 1.12 | −629.7 | 0.835 | 0.66 |
| trans-4-Hydroxy-L-proline | −1064 | 1.300 | 1.55 | −1492 | 2.023 | 1.63 |
| L-Lysine·HCl | −1154 | 1.275 | 0.47 | −1317 | 2.162 | 0.35 |
| L-Arginine | −1604 | 2.798 | 1.35 | −4286 | 10.50 | 3.07 |
| L-Cystine | −9160 | 22.46 | 0.31 | −8959 | 22.47 | 7.32 |
| Amino Acid | |||||
|---|---|---|---|---|---|
| Eutectic | |||||
| Glycine | 7.18 | 2.28 | 4.90 | 0.76 | 0.24 |
| β-Alanine | 11.60 | 8.09 | 3.51 | 0.59 | 0.41 |
| L-Proline | 18.45 | 14.37 | 4.08 | 0.56 | 0.44 |
| trans-4-Hydroxy-L-proline | 8.85 | 3.49 | 5.36 | 0.72 | 0.28 |
| L-Lysine·HCl | 9.60 | 3.42 | 6.18 | 0.74 | 0.26 |
| L-Arginine | 13.34 | 7.51 | 5.83 | 0.64 | 0.36 |
| L-Cystine | 76.16 | 60.28 | 15.87 | 0.56 | 0.44 |
| Eutectic:Water 50:50 (wt:wt) | |||||
| Glycine | 17.74 | 11.23 | 6.51 | 0.61 | 0.39 |
| β-Alanine | 9.82 | 5.09 | 4.73 | 0.66 | 0.34 |
| L-Proline | 5.24 | 2.17 | 3.06 | 0.71 | 0.29 |
| trans-4-Hydroxy-L-proline | 12.40 | 5.26 | 7.15 | 0.71 | 0.29 |
| L-Lysine·HCl | 10.95 | 5.62 | 5.33 | 0.66 | 0.34 |
| L-Arginine | 35.63 | 27.30 | 8.34 | 0.57 | 0.43 |
| L-Cystine | 74.49 | 58.42 | 16.07 | 0.56 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, C.; Rodríguez, H.; Soto, A. Solubility of Amino Acids in the Eutectic Solvent Constituted by Sodium Acetate Trihydrate and Urea and in Its Mixture with Water. Int. J. Mol. Sci. 2023, 24, 1550. https://doi.org/10.3390/ijms24021550
Gallego C, Rodríguez H, Soto A. Solubility of Amino Acids in the Eutectic Solvent Constituted by Sodium Acetate Trihydrate and Urea and in Its Mixture with Water. International Journal of Molecular Sciences. 2023; 24(2):1550. https://doi.org/10.3390/ijms24021550
Chicago/Turabian StyleGallego, Cristina, Héctor Rodríguez, and Ana Soto. 2023. "Solubility of Amino Acids in the Eutectic Solvent Constituted by Sodium Acetate Trihydrate and Urea and in Its Mixture with Water" International Journal of Molecular Sciences 24, no. 2: 1550. https://doi.org/10.3390/ijms24021550
APA StyleGallego, C., Rodríguez, H., & Soto, A. (2023). Solubility of Amino Acids in the Eutectic Solvent Constituted by Sodium Acetate Trihydrate and Urea and in Its Mixture with Water. International Journal of Molecular Sciences, 24(2), 1550. https://doi.org/10.3390/ijms24021550