Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal
Abstract
1. Introduction
2. Results and Discussion
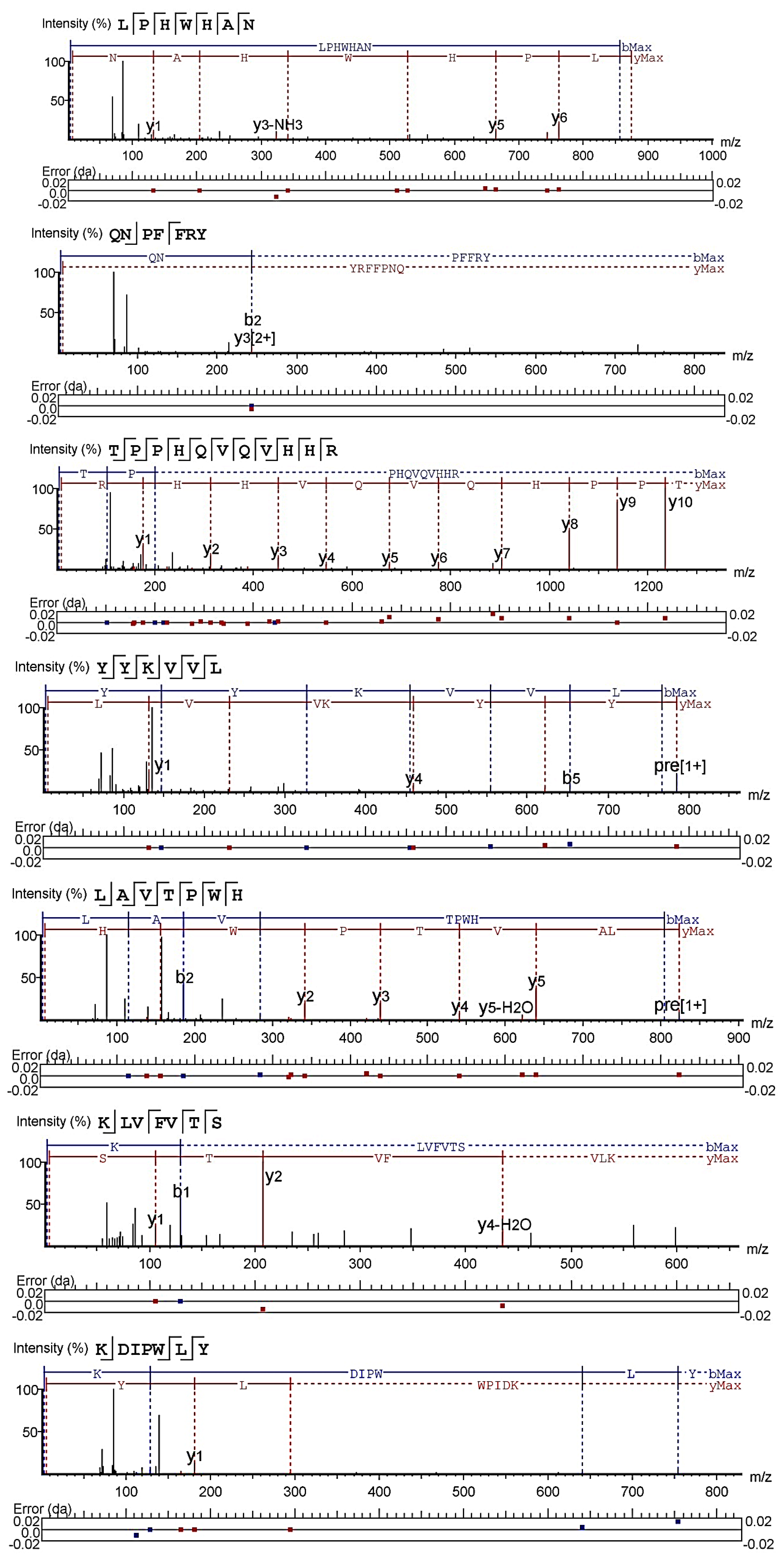
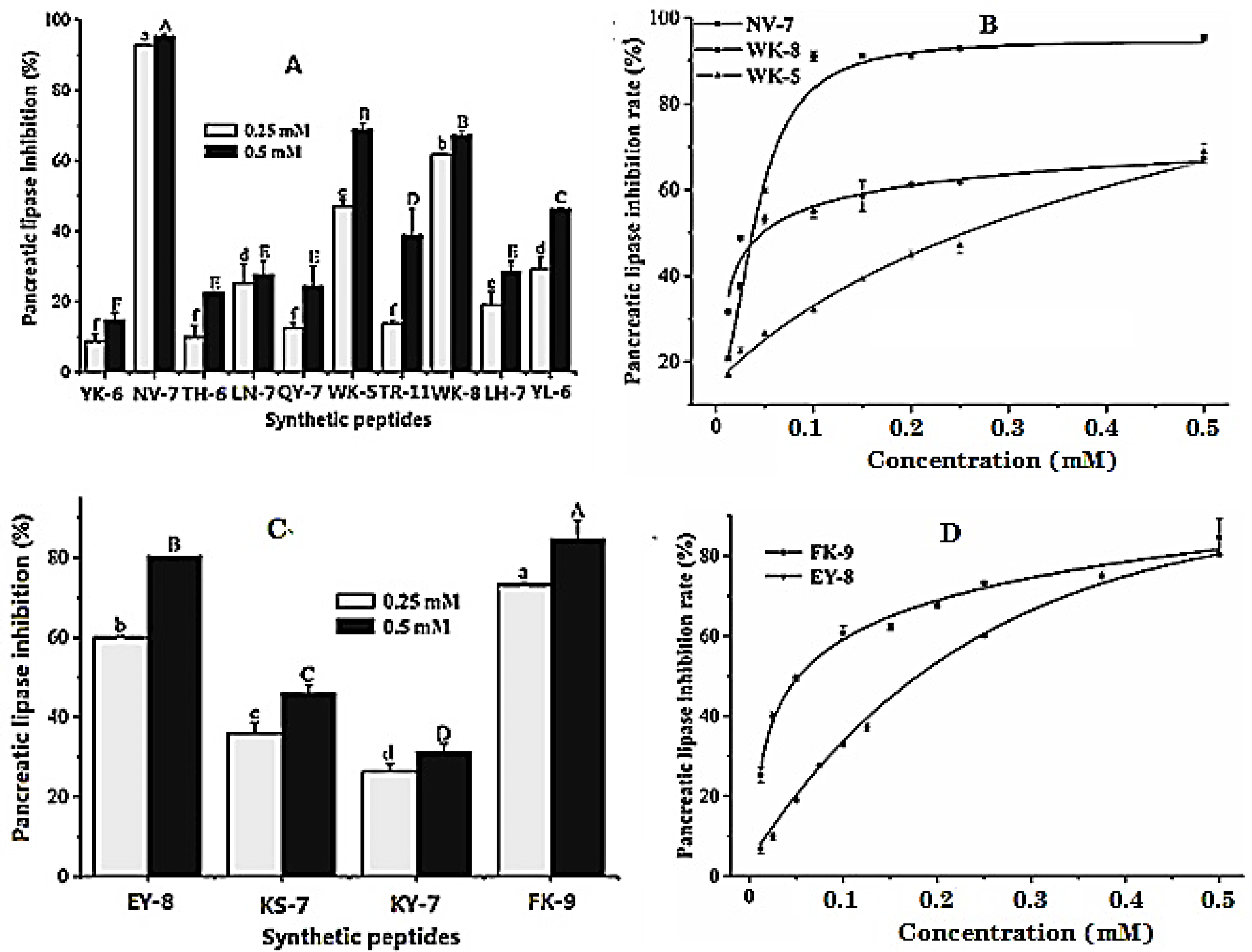
2.1. Peptides Identification and Pancreatic Lipase Inhibitory Activity
2.2. Inhibition Kinetics of Pancreatic Lipase
2.3. UV Spectroscopic Analysis of Synthetic Peptide and Pancreatic Lipase
2.4. Fluorescence Quenching Analysis of Pancreatic Lipase
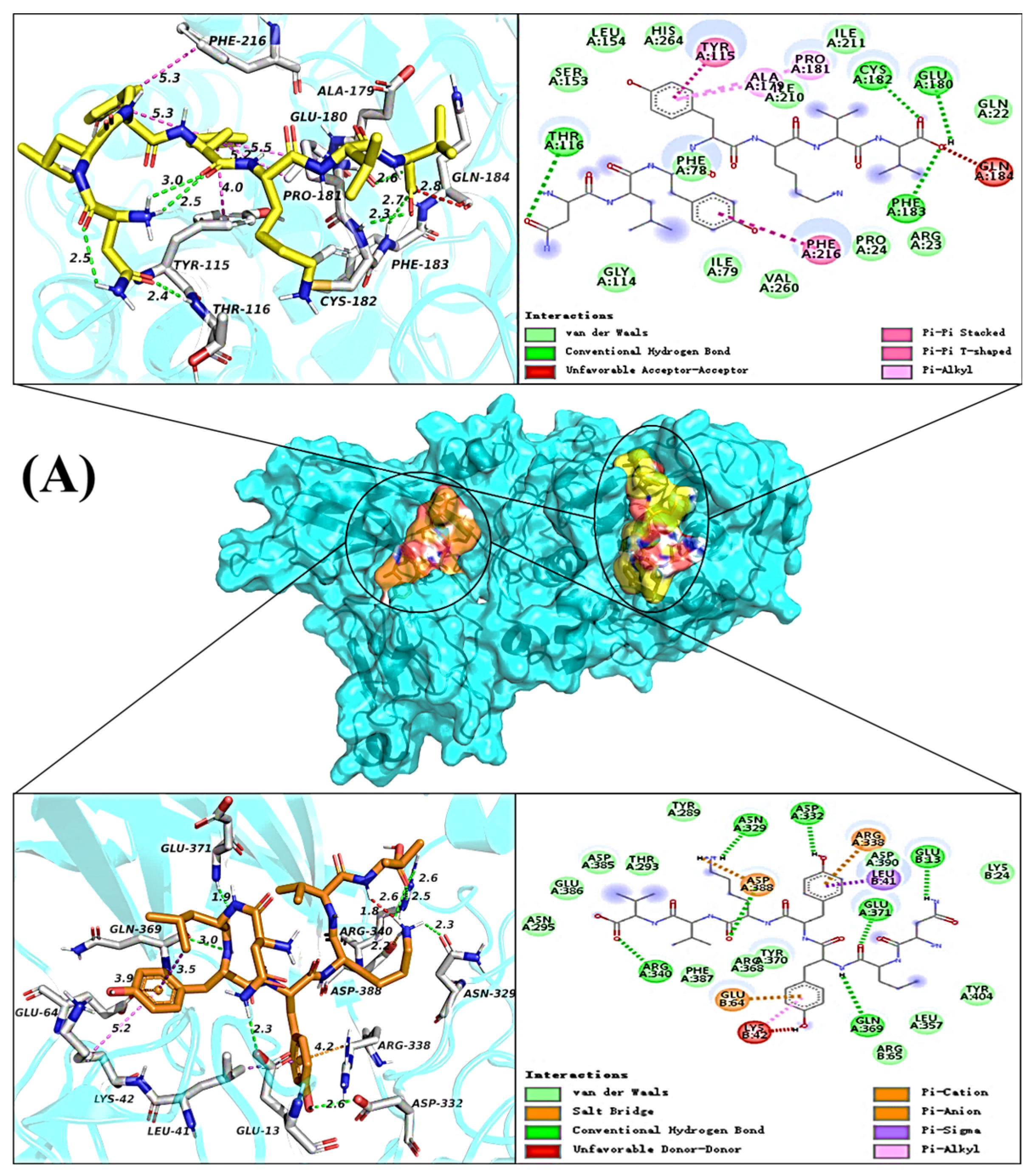
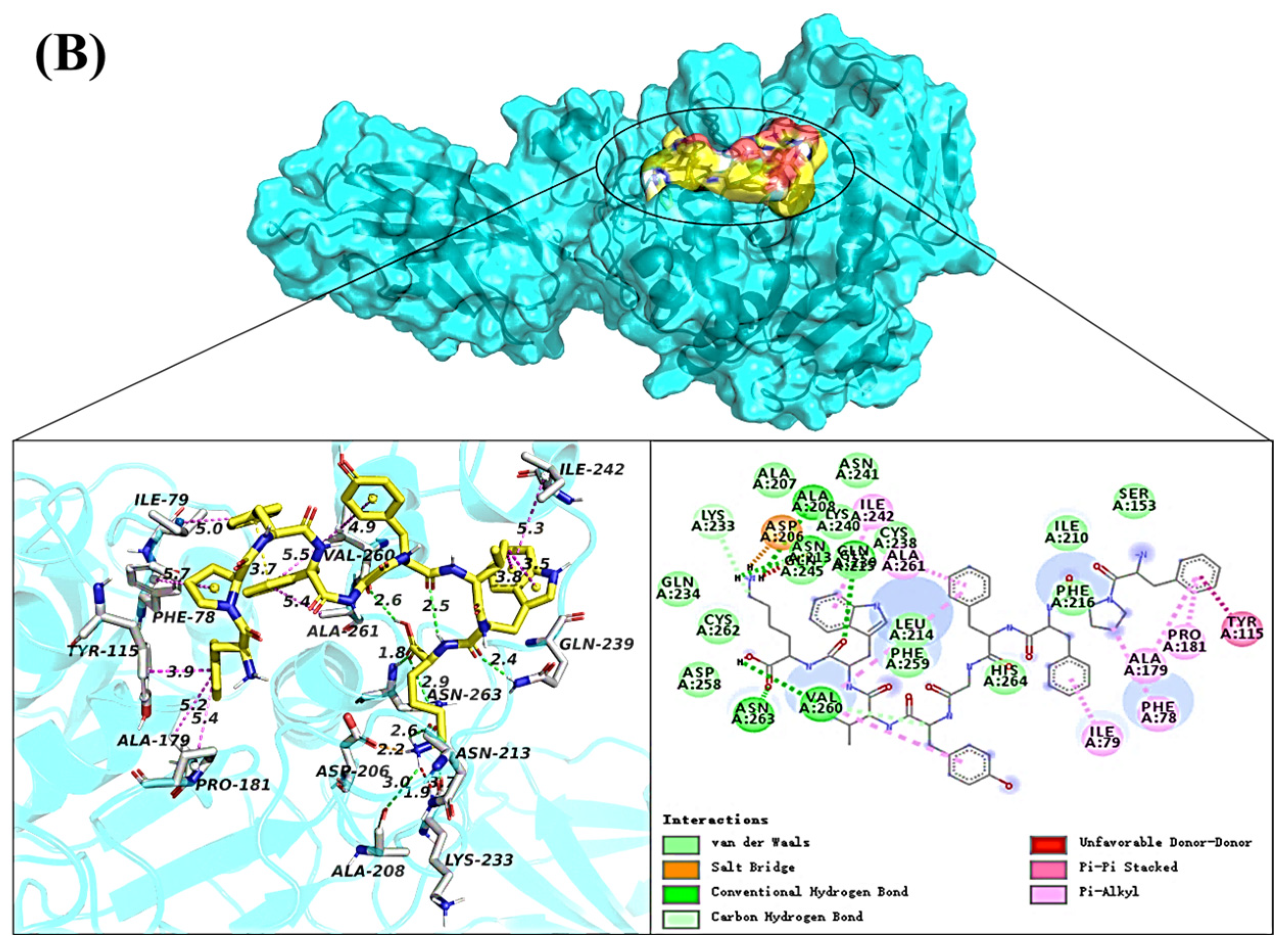
2.5. Molecular Docking
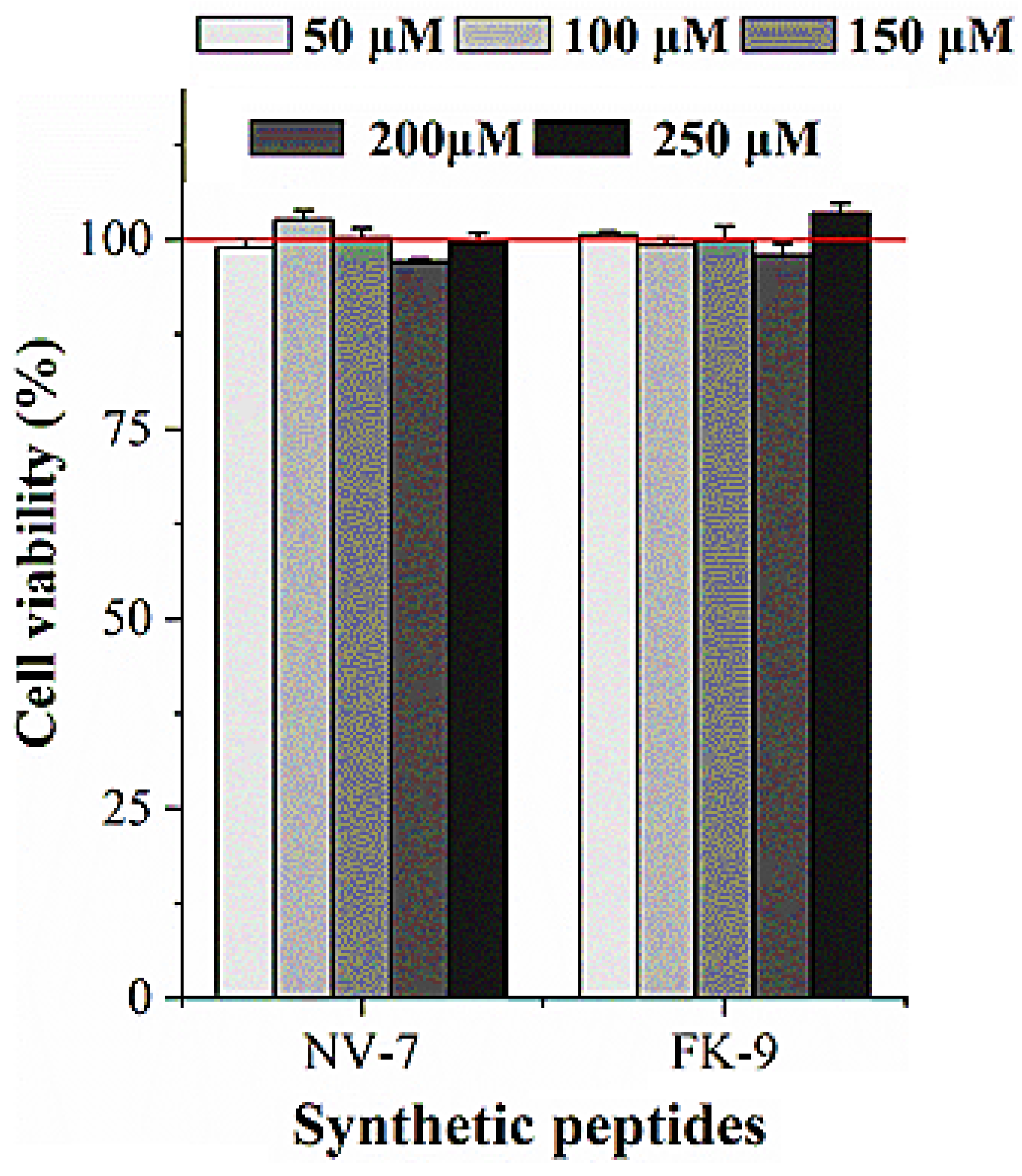
2.6. Effect of Synthetic Peptides on HepG2 Viability
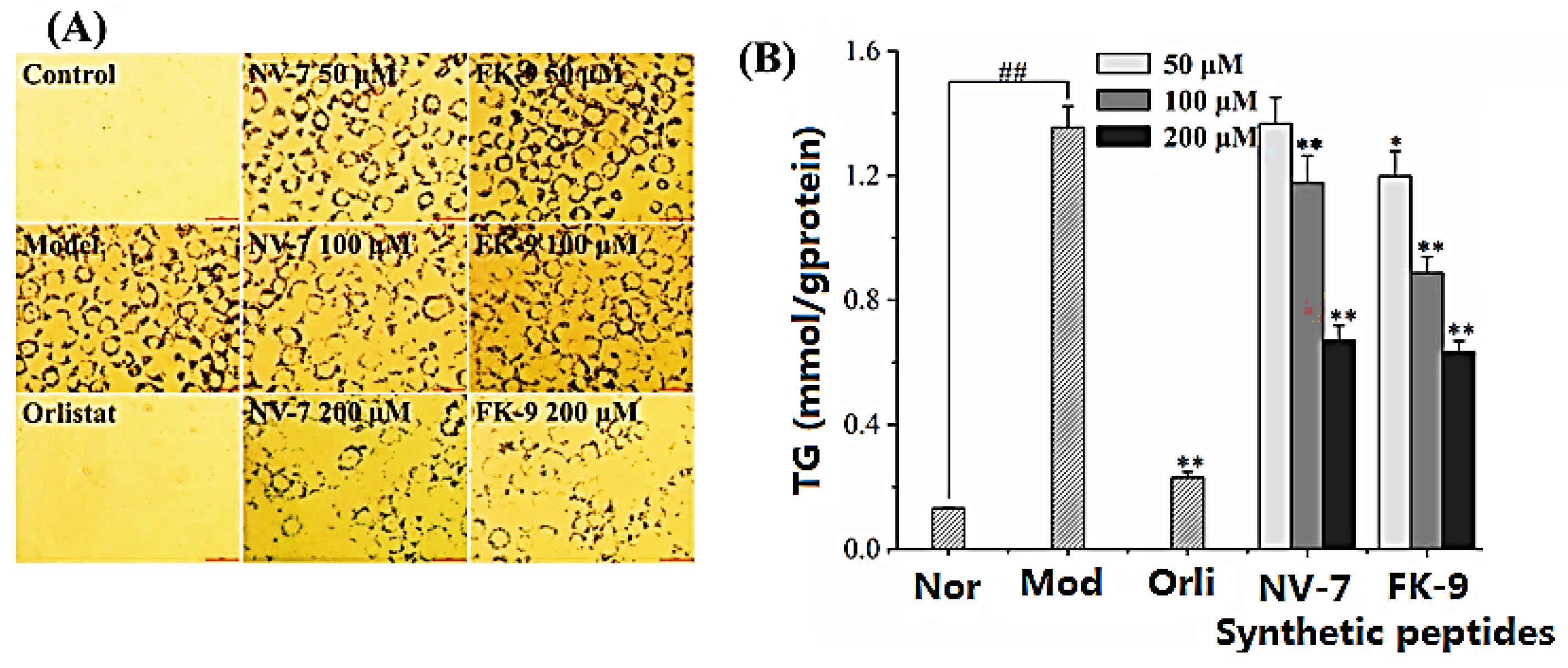
2.7. Effect of Synthetic Peptides on Fat Accumulation in HepG2 Cells
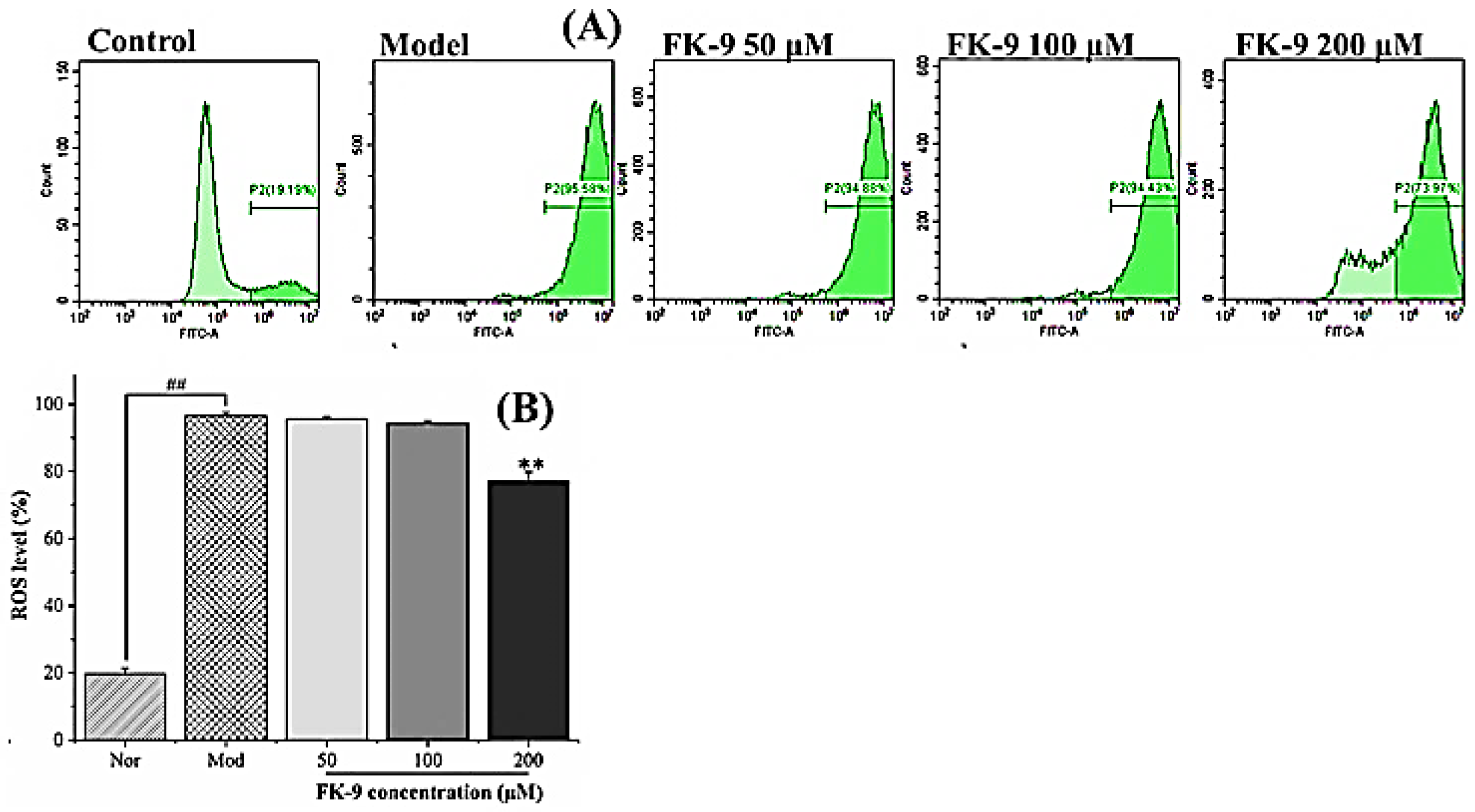
2.8. Effect of Synthetic Peptides on Reactive Oxygen Species in Oleic Acid-Induced HepG2 Cells
3. Materials and Methods
3.1. Enzymatic Hydrolysis
3.2. Separation of Ultrafiltration Affinity Chromatography
3.3. Mass Spectrometry Identification
3.4. Computer Screening
3.5. Solid-Phase Synthesis of Peptides
3.6. Pancreatic Lipase Inhibitory Activity
3.7. Inhibition Kinetics of Enzyme
3.8. Ultraviolet Spectrum Scanning Determination
3.9. Fluorescence Quenching Assay of Pancreatic Lipase
3.10. Molecular Docking
3.11. Lipid-Lowing Activity Evaluation
3.12. Determination of Active Oxygen Content
3.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, M. Human digestive and metabolic lipases—A brief review. J. Mol. Catal. B Enzym. 2003, 22, 369–376. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the preparation of fish protein anti-obesity hydrolysates using response surface methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Waterhouse, D.S.; Liu, P.Z.; Waterhouse, G.I.N.; Li, J.W.; Cui, C. Pancreatic lipase-inhibiting protein hydrolysate and peptides from seabuckthorn seed meal: Preparation optimization and inhibitory mechanism. LWT 2020, 134, 109870. [Google Scholar] [CrossRef]
- Esfandi, R.; Seidu, I.; Willmore, W.; Tsopmo, A. Antioxidant, pancreatic lipase, and α-amylase inhibitory properties of oat bran hydrolyzed proteins and peptides. J. Food Biochem. 2022, 46, e13762. [Google Scholar] [CrossRef] [PubMed]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Li, S.; Kim, K.H.; Cox, A.D.; Anderson, J.M. Canary Seed (Phalaris canariensis L.) Peptides Prevent Obesity and Glucose Intolerance in Mice Fed a Western Diet. Int. J. Mol. Sci. 2022, 23, 14927. [Google Scholar] [CrossRef]
- Wang, X.; Ai, X.; Zhu, Z.; Zhang, M.; Pan, F.; Yang, Z.; Wang, O.; Zhao, L.; Zhao, L. Pancreatic lipase inhibitory effects of peptides derived from sesame proteins: In silico and in vitro analyses. Int. J. Biol. Macromol. 2022, 222, 1531–1537. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, C.; Chen, Y.; Zheng, B. Purification and Characterization of Antioxidant Peptides of Pseudosciaena crocea Protein Hydrolysates. Molecules 2017, 22, 57–68. [Google Scholar] [CrossRef]
- Chen, G.L.; Xu, Y.B.; Wu, J.L.; Li, N.; Guo, M.Q. Hypoglycemic and hypolipidemic effects of Moringa oleifera leaves and their functional chemical constituents. Food Chem. 2020, 333, 127478. [Google Scholar] [CrossRef]
- Fang, Y.-X.; Song, H.-P.; Liang, J.-X.; Li, P.; Yang, H. Rapid screening of pancreatic lipase inhibitors from Monascus-fermented rice by ultrafiltration liquid chromatography-mass spectrometry. Anal. Methods 2017, 9, 3422–3429. [Google Scholar] [CrossRef]
- Zhao, B.; Su, K.; Mao, X.; Zhang, X. Separation and identification of enzyme inhibition peptides from dark tea protein. Bioorganic Chem. 2020, 99, 103772. [Google Scholar] [CrossRef]
- Bueno-Borges, L.B.; Sartim, M.A.; Gil, C.C.; Sampaio, S.V.; Rodrigues, P.H.V.; Regitano-d’Arce, M.A.B. Sacha inchi seeds from sub-tropical cultivation: Effects of roasting on antinutrients, antioxidant capacity and oxidative stability. J. Food Sci. Technol. 2018, 55, 4159–4166. [Google Scholar] [CrossRef] [PubMed]
- Rawdkuen, S.; Murdayanti, D.; Ketnawa, S.; Phongthai, S. Chemical properties and nutritional factors of pressed-cake from tea and sacha inchi seeds. Food Biosci. 2016, 15, 64–71. [Google Scholar] [CrossRef]
- Neethu, C.; Rahiman, M.; Rosmine, E.; Saramma, A.; Hatha, M. Utilization of agroindustrial wastes for the production of lipase from Stenotrophomonas maltophilia isolated from Arctic and optimization of physical parameters. Biocatal. Agric. Biotechnol. 2015, 4, 703–709. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Rodzi, N.; Pinijsuwan, S. Characterization of sacha inchi protein hydrolysates produced by crude papain and Calotropis proteases. LWT Food Sci. Technol. 2018, 98, 18–24. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Campos, D. Enzyme-assisted hydrolysates from sacha inchi (Plukenetia volubilis) protein with in vitro antioxidant and antihypertensive properties. J. Food Proc. Preserv. 2020, 44, e14969. [Google Scholar] [CrossRef]
- Suwanangul, S.; Sangsawad, P.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Chittrakorn, S.; Ruttarattanamongkol, K. Antioxidant activities of sacha inchi (Plukenetia volubilis L.) protein isolate and its hydrolysates produced with different proteases. MAEJO Int. J. Sci. Technol. 2021, 15, 48–60. [Google Scholar]
- Zhan, Q.; Wang, Q.; Liu, Q.; Guo, Y.; Gong, F.; Hao, L.; Wu, H.; Dong, Z. The antioxidant activity of protein fractions from Sacha inchi seeds after a simulated gastrointestinal digestion. LWT Food Sci. Technol. 2021, 145, 111356. [Google Scholar] [CrossRef]
- Wang, K. Hypoglycemic and Hypolipidemic Activity of Peptides from the Meal of Plukenetia volubilis. Master Dissertation, South China University of Technology, Guangzhou, China, 2021. [Google Scholar]
- Ying, M.; Meti, M.D.; Xu, H.; Wang, Y.; Lin, J.; Wu, Z.; Han, Q.; Xu, X.; He, Z.; Hong, W.; et al. Binding mechanism of lipase to Ligupurpuroside B extracted from Ku-Ding tea as studied by multi-spectroscopic and molecular docking methods. Int. J. Biol. Macromol. 2018, 120, 1345–1352. [Google Scholar] [CrossRef]
- Wu, X.; Ding, H.; Hu, X.; Pan, J.; Liao, Y.; Gong, D.; Zhang, G. Exploring inhibitory mechanism of gallocatechin gallate on a-amylase and a-glucosidase relevant to postprandial hyperglycemia. J. Funct. Foods 2018, 48, 200–209. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; McClements, D.J.; Li, T.; Liu, C. Investigation the interaction between procyanidin dimer and alpha-glucosidase: Spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2019, 130, 315–322. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, K.; Matsutaka, H.; Fukuhama, C.; Watanabe, Y.; Fujino, H. Globin digest, acidic protease hydrolysate, inhibits dietary hypertriglyceridemia and Val-Val-Try-Pro, one of its constituents, possesses most superior effect. Life Sci. 1996, 58, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.D.; Cui, Y.J.; Zhang, R.L.; Zhang, X.W. Purification and identification of anti-obesity peptides derived from Spirulina platensis. J. Funct. Foods 2018, 47, 350–360. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Mao, X.; Qi, P.; Zhang, X. Separation and Lipid Inhibition Effects of a Novel Decapeptide from Chlorella pyenoidose. Molecules 2019, 24, 3527. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal. Int. J. Mol. Sci. 2023, 24, 1529. https://doi.org/10.3390/ijms24021529
Wang K, Liu X, Zhang X. Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal. International Journal of Molecular Sciences. 2023; 24(2):1529. https://doi.org/10.3390/ijms24021529
Chicago/Turabian StyleWang, Kai, Xiaofei Liu, and Xuewu Zhang. 2023. "Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal" International Journal of Molecular Sciences 24, no. 2: 1529. https://doi.org/10.3390/ijms24021529
APA StyleWang, K., Liu, X., & Zhang, X. (2023). Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal. International Journal of Molecular Sciences, 24(2), 1529. https://doi.org/10.3390/ijms24021529






