The Pseudomonas aeruginosa RpoH (σ32) Regulon and Its Role in Essential Cellular Functions, Starvation Survival, and Antibiotic Tolerance
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arsene, F.; Tomoyasu, T.; Bukau, B. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 2000, 55, 3–9. [Google Scholar] [CrossRef]
- Grossman, A.D.; Erickson, J.W.; Gross, C.A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 1984, 38, 383–390. [Google Scholar] [CrossRef]
- Erickson, J.W.; Vaughn, V.; Walter, W.A.; Neidhardt, F.C.; Gross, C.A. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1987, 1, 419–432. [Google Scholar] [CrossRef]
- Guisbert, E.; Yura, T.; Rhodius, V.A.; Gross, C.A. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol. Mol. Biol. Rev. 2008, 72, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Cowing, D.W.; Bardwell, J.C.; Craig, E.A.; Woolford, C.; Hendrix, R.W.; Gross, C.A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc. Natl. Acad. Sci. USA 1985, 82, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, G.; Blankschien, M.; Herman, C.; Gross, C.A.; Rhodius, V.A. Regulon and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev. 2006, 20, 1776–1789. [Google Scholar] [CrossRef] [PubMed]
- Slamti, L.; Livny, J.; Waldor, M.K. Global gene expression and phenotypic analysis of a Vibrio cholerae rpoH deletion mutant. J. Bacteriol. 2007, 189, 351–362. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Liu, X.; Yan, T.; Wu, L.; Alm, E.; Arkin, A.; Thompson, D.K.; Zhou, J. Global transcriptome analysis of the heat shock response of Shewanella oneidensis. J. Bacteriol. 2004, 186, 7796–7803. [Google Scholar] [CrossRef] [PubMed]
- Gunesekere, I.C.; Kahler, C.M.; Powell, D.R.; Snyder, L.A.; Saunders, N.J.; Rood, J.I.; Davies, J.K. Comparison of the RpoH-dependent regulon and general stress response in Neisseria gonorrhoeae. J. Bacteriol. 2006, 188, 4769–4776. [Google Scholar] [CrossRef]
- Barnett, M.J.; Bittner, A.N.; Toman, C.J.; Oke, V.; Long, S.R. Dual RpoH sigma factors and transcriptional plasticity in a symbiotic bacterium. J. Bacteriol. 2012, 194, 4983–4994. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef]
- Malhotra, S.; Hayes, D., Jr.; Wozniak, D.J. Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef]
- Stewart, P.S.; White, B.; Boegli, L.; Hamerly, T.; Williamson, K.S.; Franklin, M.J.; Bothner, B.; James, G.A.; Fisher, S.; Vital-Lopez, F.G.; et al. Conceptual model of biofilm antibiotic tolerance that integrates phenomena of diffusion, metabolism, gene expression, and physiology. J. Bacteriol. 2019, 201, e00307-19. [Google Scholar] [CrossRef]
- Stewart, P.S.; Williamson, K.S.; Boegli, L.; Hamerly, T.; White, B.; Scott, L.; Hu, X.; Mumey, B.M.; Franklin, M.J.; Bothner, B.; et al. Search for a shared genetic or biochemical basis for biofilm tolerance to antibiotics across bacterial species. Antimicrob. Agents Chemother. 2022, 66, e0002122. [Google Scholar] [CrossRef]
- Jo, J.; Price-Whelan, A.; Dietrich, L.E.P. Gradients and consequences of heterogeneity in biofilms. Nat. Rev. Microbiol. 2022, 20, 593–607. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Williamson, K.S.; Richards, L.A.; Perez-Osorio, A.C.; Pitts, B.; McInnerney, K.; Stewart, P.S.; Franklin, M.J. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J. Bacteriol. 2012, 194, 2062–2073. [Google Scholar] [CrossRef]
- Lindner, A.B.; Madden, R.; Demarez, A.; Stewart, E.J.; Taddei, F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc. Natl. Acad. Sci. USA 2008, 105, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Zietkiewicz, S.; Liberek, K. Distinct activities of Escherichia coli small heat shock proteins IbpA and IbpB promote efficient protein disaggregation. J. Mol. Biol. 2009, 386, 178–189. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Ge Zhao, A.; Usui, M.; Underwood, R.A.; Nguyen, H.; Beyenal, H.; deLancey Pulcini, E.; Agostinho Hunt, A.; Bernstein, H.C.; Fleckman, P.; et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen. 2016, 24, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.G.; Priya, K.; Chang, C.Y.; Abdul Rahman, A.Y.; Tee, K.K.; Yin, W.F. Transcriptome analysis of Pseudomonas aeruginosa PAO1 grown at both body and elevated temperatures. PeerJ 2016, 4, e2223. [Google Scholar] [CrossRef]
- Schulz, S.; Eckweiler, D.; Bielecka, A.; Nicolai, T.; Franke, R.; Dotsch, A.; Hornischer, K.; Bruchmann, S.; Duvel, J.; Haussler, S. Elucidation of sigma factor-associated networks in Pseudomonas aeruginosa reveals a modular architecture with limited and function-specific crosstalk. PLoS Pathog. 2015, 11, e1004744. [Google Scholar] [CrossRef] [PubMed]
- Kindrachuk, K.N.; Fernandez, L.; Bains, M.; Hancock, R.E. Involvement of an ATP-dependent protease, PA0779/AsrA, in inducing heat shock in response to tobramycin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 1874–1882. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, M.; Burgess, R.R. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J. Biol. Chem. 2005, 280, 17758–17768. [Google Scholar] [CrossRef]
- Turner, K.H.; Wessel, A.K.; Palmer, G.C.; Murray, J.L.; Whiteley, M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 2015, 112, 4110–4115. [Google Scholar] [CrossRef]
- Poulsen, B.E.; Yang, R.; Clatworthy, A.E.; White, T.; Osmulski, S.J.; Li, L.; Penaranda, C.; Lander, E.S.; Shoresh, N.; Hung, D.T. Defining the core essential genome of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2019, 116, 10072–10080. [Google Scholar] [CrossRef]
- Newman, J.R.; Fuqua, C. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 1999, 227, 197–203. [Google Scholar] [CrossRef]
- Meisner, J.; Goldberg, J.B. The Escherichia coli rhaSR-PrhaBAD inducible promoter system allows tightly controlled gene expression over a wide range in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2016, 82, 6715–6727. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Delucia, A.M.; Six, D.A.; Caughlan, R.E.; Gee, P.; Hunt, I.; Lam, J.S.; Dean, C.R. Lipopolysaccharide (LPS) inner-core phosphates are required for complete LPS synthesis and transport to the outer membrane in Pseudomonas aeruginosa PAO1. mBio 2011, 2, e00142-11. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, A.; Roche, B.; Schalk, I.J. Iron acquisition in Pseudomonas aeruginosa by the siderophore pyoverdine: An intricate interacting network including periplasmic and membrane proteins. Sci. Rep. 2020, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Breidenstein, E.B.; Khaira, B.K.; Wiegand, I.; Overhage, J.; Hancock, R.E. Complex ciprofloxacin resistome revealed by screening a Pseudomonas aeruginosa mutant library for altered susceptibility. Antimicrob. Agents Chemother. 2008, 52, 4486–4491. [Google Scholar] [CrossRef]
- Gallagher, L.A.; Shendure, J.; Manoil, C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. mBio 2011, 2, e00315-10. [Google Scholar] [CrossRef]
- Deziel, E.; Gopalan, S.; Tampakaki, A.P.; Lepine, F.; Padfield, K.E.; Saucier, M.; Xiao, G.; Rahme, L.G. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: Multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 2005, 55, 998–1014. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Wilderman, P.J.; Vasil, A.I.; Vasil, M.L. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: Identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 2002, 45, 1277–1287. [Google Scholar] [CrossRef]
- Palma, M.; Worgall, S.; Quadri, L.E. Transcriptome analysis of the Pseudomonas aeruginosa response to iron. Arch. Microbiol. 2003, 180, 374–379. [Google Scholar] [CrossRef]
- Schuster, M.; Hawkins, A.C.; Harwood, C.S.; Greenberg, E.P. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 2004, 51, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, C.; Harwood, C.S. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol. Microbiol. 2007, 65, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Lequette, Y.; Lee, J.H.; Ledgham, F.; Lazdunski, A.; Greenberg, E.P. A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J. Bacteriol. 2006, 188, 3365–3370. [Google Scholar] [CrossRef]
- Nalca, Y.; Jansch, L.; Bredenbruch, F.; Geffers, R.; Buer, J.; Haussler, S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: A global approach. Antimicrob. Agents Chemother. 2006, 50, 1680–1688. [Google Scholar] [CrossRef]
- Teitzel, G.M.; Geddie, A.; De Long, S.K.; Kirisits, M.J.; Whiteley, M.; Parsek, M.R. Survival and growth in the presence of elevated copper: Transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7242–7256. [Google Scholar] [CrossRef]
- Chang, W.; Small, D.A.; Toghrol, F.; Bentley, W.E. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genom. 2005, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Hare, N.J.; Scott, N.E.; Shin, E.H.; Connolly, A.M.; Larsen, M.R.; Palmisano, G.; Cordwell, S.J. Proteomics of the oxidative stress response induced by hydrogen peroxide and paraquat reveals a novel AhpC-like protein in Pseudomonas aeruginosa. Proteomics 2011, 11, 3056–3069. [Google Scholar] [CrossRef]
- Palma, M.; DeLuca, D.; Worgall, S.; Quadri, L.E. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 2004, 186, 248–252. [Google Scholar] [CrossRef]
- Salunkhe, P.; Topfer, T.; Buer, J.; Tummler, B. Genome-wide transcriptional profiling of the steady-state response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 2005, 187, 2565–2572. [Google Scholar] [CrossRef]
- Folsom, J.P.; Richards, L.; Pitts, B.; Roe, F.; Ehrlich, G.D.; Parker, A.; Mazurie, A.; Stewart, P.S. Physiology of Pseudomonas aeruginosa in biofilms as revealed by transcriptome analysis. BMC Microbiol. 2010, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Eberl, L.; Givskov, M. Transcriptome analysis of Pseudomonas aeruginosa biofilm development: Anaerobic respiration and iron limitation. Biofilms 2005, 2, 37–62. [Google Scholar] [CrossRef]
- Mikkelsen, H.; Bond, N.J.; Skindersoe, M.E.; Givskov, M.; Lilley, K.S.; Welch, M. Biofilms and type III secretion are not mutually exclusive in Pseudomonas aeruginosa. Microbiology 2009, 155, 687–698. [Google Scholar] [CrossRef]
- Mikkelsen, H.; Duck, Z.; Lilley, K.S.; Welch, M. Interrelationships between colonies, biofilms, and planktonic cells of Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Patrauchan, M.A.; Sarkisova, S.A.; Franklin, M.J. Strain-specific proteome responses of Pseudomonas aeruginosa to biofilm-associated growth and to calcium. Microbiology 2007, 153, 3838–3851. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef]
- Waite, R.D.; Paccanaro, A.; Papakonstantinopoulou, A.; Hurst, J.M.; Saqi, M.; Littler, E.; Curtis, M.A. Clustering of Pseudomonas aeruginosa transcriptomes from planktonic cultures, developing and mature biofilms reveals distinct expression profiles. BMC Genom. 2006, 7, 162. [Google Scholar] [CrossRef]
- Whiteley, M.; Bangera, M.G.; Bumgarner, R.E.; Parsek, M.R.; Teitzel, G.M.; Lory, S.; Greenberg, E.P. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001, 413, 860–864. [Google Scholar] [CrossRef]
- Choi, K.-H.; Schweizer, H. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 2005, 5, 30. [Google Scholar] [CrossRef]
- Akiyama, T.; Williamson, K.S.; Schaefer, R.; Pratt, S.; Chang, C.B.; Franklin, M.J. Resuscitation of Pseudomonas aeruginosa from dormancy requires hibernation promoting factor (PA4463) for ribosome preservation. Proc. Natl. Acad. Sci. USA 2017, 114, 3204–3209. [Google Scholar] [CrossRef]
- Schurr, M.J.; Deretic, V. Microbial pathogenesis in cystic fibrosis: Co-ordinate regulation of heat-shock response and conversion to mucoidy in Pseudomonas aeruginosa. Mol. Microbiol. 1997, 24, 411–420. [Google Scholar] [CrossRef]
- DeVries, C.A.; Ohman, D.E. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J. Bacteriol. 1994, 176, 6677–6687. [Google Scholar] [CrossRef]
- Martin, D.W.; Holloway, B.W.; Deretic, V. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J. Bacteriol. 1993, 175, 1153–1164. [Google Scholar] [CrossRef]
- Mathee, K.; McPherson, C.J.; Ohman, D.E. Posttranslational control of the algT (algU)-encoded sigma22 for expression of the alginate regulon in Pseudomonas aeruginosa and localization of its antagonist proteins MucA and MucB (AlgN). J. Bacteriol. 1997, 179, 3711–3720. [Google Scholar] [CrossRef]
- Filiatrault, M.J.; Picardo, K.F.; Ngai, H.; Passador, L.; Iglewski, B.H. Identification of Pseudomonas aeruginosa genes involved in virulence and anaerobic growth. Infect. Immun. 2006, 74, 4237–4245. [Google Scholar] [CrossRef]
- Schurek, K.N.; Marr, A.K.; Taylor, P.K.; Wiegand, I.; Semenec, L.; Khaira, B.K.; Hancock, R.E. Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 4213–4219. [Google Scholar] [CrossRef]
- Zambrano, M.M.; Kolter, R. Escherichia coli mutants lacking NADH dehydrogenase I have a competitive disadvantage in stationary phase. J. Bacteriol. 1993, 175, 5642–5647. [Google Scholar] [CrossRef]
- Amini, S.; Hottes, A.K.; Smith, L.E.; Tavazoie, S. Fitness landscape of antibiotic tolerance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2011, 7, e1002298. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Vergnes, A.; Banzhaf, M.; Duverger, Y.; Huguenot, A.; Brochado, A.R.; Su, S.Y.; Espinosa, L.; Loiseau, L.; Py, B.; et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 2013, 340, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, T.; Cheng, X.J.; Peng, C.T.; Li, C.C.; He, L.H.; Ju, S.M.; Wang, N.Y.; Ye, T.H.; Lian, M.; et al. Structural and functional studies on Pseudomonas aeruginosa DspI: Implications for its role in DSF biosynthesis. Sci. Rep. 2018, 8, 3928. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Marques, C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Feinbaum, R.L.; Urbach, J.M.; Liberati, N.T.; Djonovic, S.; Adonizio, A.; Carvunis, A.R.; Ausubel, F.M. Genome-wide identification of Pseudomonas aeruginosa virulence-related genes using a Caenorhabditis elegans infection model. PLoS Pathog. 2012, 8, e1002813. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.G.; Matewish, M.J.; Burrows, L.L.; Monteiro, M.A.; Perry, M.B.; Lam, J.S. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol. Microbiol. 2000, 35, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.J.; Sandvik, E.; Yanardag, S.; Williamson, K.S. Functional Characterization of the Pseudomonas aeruginosa Ribosome Hibernation-Promoting Factor. J. Bacteriol. 2020, 202, e00280-20. [Google Scholar] [CrossRef] [PubMed]
- Theng, S.; Williamson, K.S.; Franklin, M.J. Role of Hibernation Promoting Factor in Ribosomal Protein Stability during Pseudomonas aeruginosa Dormancy. Int. J. Mol. Sci. 2020, 21, 9494. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Williamson, K.S.; Franklin, M.J. Expression and regulation of the Pseudomonas aeruginosa hibernation promoting factor. Mol. Microbiol. 2018, 110, 161–175. [Google Scholar] [CrossRef]
- McMackin, E.A.W.; Corley, J.M.; Karash, S.; Marden, J.; Wolfgang, M.C.; Yahr, T.L. Cautionary Notes on the Use of Arabinose- and Rhamnose-Inducible Expression Vectors in Pseudomonas aeruginosa. J. Bacteriol. 2021, 203, e0022421. [Google Scholar] [CrossRef]
- Lindner, A.B.; Demarez, A. Protein aggregation as a paradigm of aging. Biochim. Biophys. Acta 2009, 1790, 980–996. [Google Scholar] [CrossRef]
- Dworkin, J.; Harwood, C.S. Metabolic Reprogramming and Longevity in Quiescence. Annu. Rev. Microbiol. 2022, 76, 91–111. [Google Scholar] [CrossRef]
- Sarkisova, S.; Patrauchan, M.A.; Berglund, D.; Nivens, D.E.; Franklin, M.J. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 2005, 187, 4327–4337. [Google Scholar] [CrossRef]
- Guragain, M.; King, M.M.; Williamson, K.S.; Perez-Osorio, A.C.; Akiyama, T.; Khanam, S.; Patrauchan, M.A.; Franklin, M.J. The Pseudomonas aeruginosa PAO1 two-component regulator CarSR regulates calcium homeostasis and calcium-iInduced virulence factor production through Its regulatory targets CarO and CarP. J. Bacteriol. 2016, 198, 951–963. [Google Scholar] [CrossRef]
- Held, K.; Ramage, E.; Jacobs, M.; Gallagher, L.; Manoil, C. Sequence-verified two-allele transposon mutant library for Pseudomonas aeruginosa PAO1. J. Bacteriol. 2012, 194, 6387–6389. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Blazejczyk, M.; Miron, M.; Nadon, R. FlexArray: A statistical data analysis software for gene expression microarrays. Genome Que. Montr. Can. 2007. Available online: http://genomequebecmcgillca/FlexArray (accessed on 15 September 2014).
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Remmert, M.; Biegert, A.; Hauser, A.; Soding, J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat. Methods 2011, 9, 173–175. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- van Kempen, M.; Kim, S.; Tumescheit, C.; Mirdita, M.; Gilchrist, C.L.M.; Söding, J.; Steinegger, M. Foldseek: Fast and accurate protein structure search. bioRxiv 2022. [Google Scholar] [CrossRef]
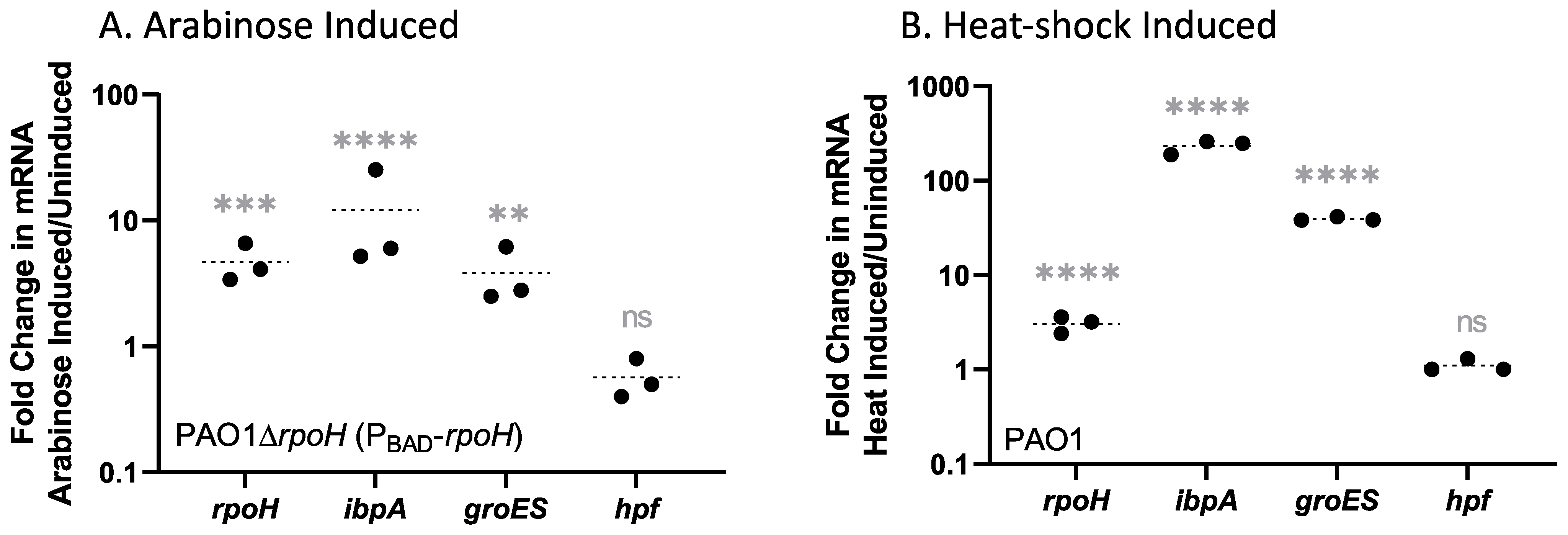


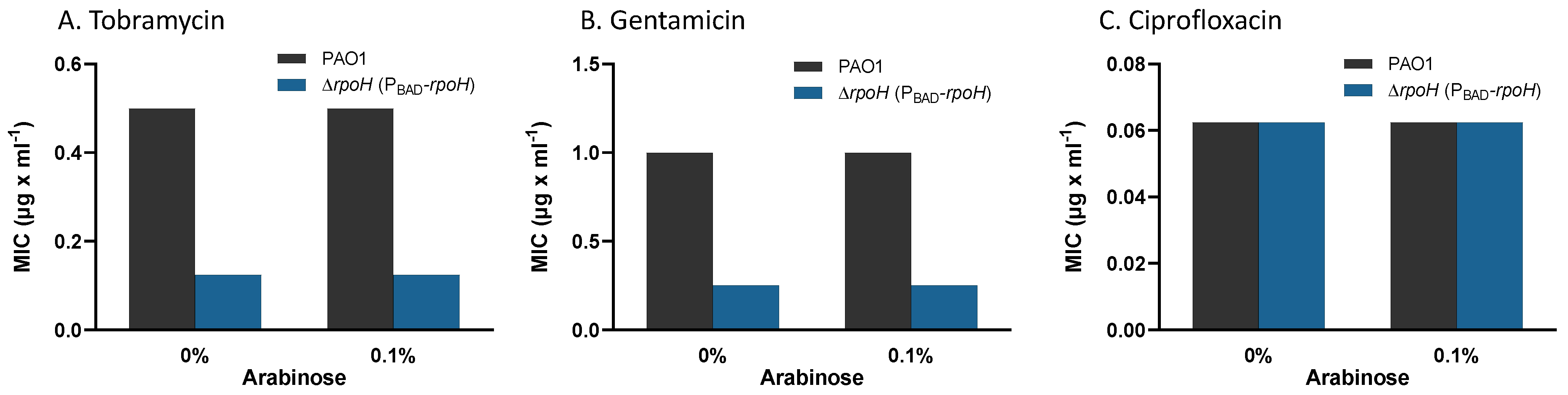
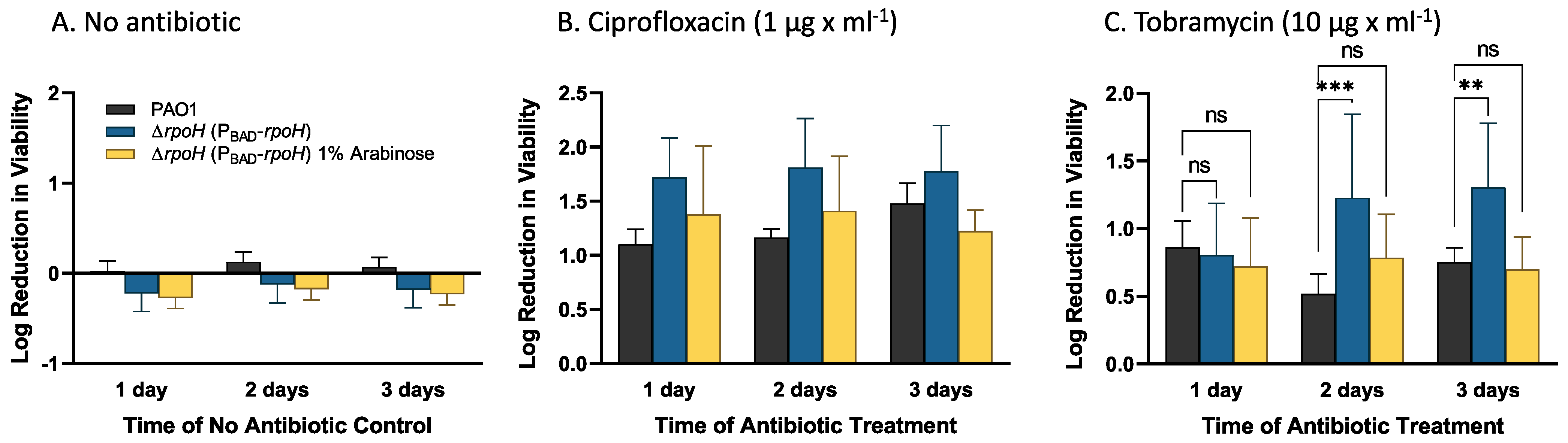

| Upregulated Gene Clusters * | p-Value |
|---|---|
| Oxidative phosphorylation | 1.7 × 10−9 |
| Stress response | 3.9 × 10−4 |
| Aerobic respiration | 5.0 × 10−3 |
| Chaperones | 7.1 × 10−3 |
| Downregulated Gene Clusters * | p-Value |
| FecR | 1.1 × 10−5 |
| AMP-binding enzymes | 1.5 × 10−3 |
| TonB-dependent receptor | 8.9 × 10−3 |
| ID 1 | Gene | Function | Fold Change | p-Value | Essential 2 |
|---|---|---|---|---|---|
| PA0376 | rpoH | Heat shock sigma factor | 16.6 | 1.4 × 10−3 | +++ |
| PA0778 | icp | Inhibitor of cysteine peptidases | 8.6 | 3.8 × 10−6 | |
| PA0779 | asrA | Stress response ATP-dependent protease | 8.1 | 3.7 × 10−4 | |
| PA1596 | htpG | Heat shock protein—molecular chaperone | 6.5 | 5.5 × 10−7 | |
| PA1597 | Dienelactone hydrolase/oligo peptidase | 8.2 | 1.6 × 10−4 | ||
| PA1802 | clpX | Protease | 2.2 | 5.9 × 10−3 | ++ |
| PA1803 | lon | Protease | 3.6 | 8.7 × 10−3 | ++ |
| PA1805 | ppiD | Peptidyl-prolyl cis-trans isomerase D | 3.9 | 2.9 × 10−4 | |
| PA2830 | htpX | Zn-dependent protease/chaperone | 3.1 | 6.2 × 10−3 | |
| PA3126 | ibpA | Chaperone | 3.8 | 9.1 × 10−4 | |
| PA3257 | algO | Periplasmic protease | 3.0 | 1.3 × 10−3 | |
| PA3602 | Glutamate synthase | 2.4 | 3.7 × 10−3 | ||
| PA4472 | pmbA | Zn-dependent protease | 15.5 | 5.2 × 10−5 | |
| PA4474 | Zn-dependent protease | 16.4 | 1.1 × 10−3 | ||
| PA4475 | Amidohydrolase | 5.0 | 4.0 × 10−4 | ||
| PA4542 | clpB | Protease | 10.8 | 4.8 × 10−4 | |
| PA4751 | ftsH | Membrane-bound, ATP-dependent protease | 3.0 | 1.1 × 10−4 | +++ |
| PA4756 | carB | Carbamoylphosphate synthase large subunit | 2.2 | 1.7 × 10−2 | ++ |
| PA4757 | Amino acid efflux permease | 2.9 | 1.1 × 10−2 | ||
| PA4759 | dapB | Dihydropicolinate reductase | 7.8 | 4.4 × 10−3 | +++ |
| PA4760 | dnaJ | Chaperone | 5.5 | 7.9 × 10−4 | ++ |
| PA4761 | dnaK | Chaperone | 4.5 | 5.4 × 10−3 | +++ |
| PA4762 | grpE | Chaperone | 3.9 | 1.2 × 10−3 | + |
| PA4858 | Solute-binding sensor protein | 2.1 | 4.0 × 10−2 | ||
| PA4870 | DnaK suppressor; c4-type zinc finger protein | 13.9 | 7.2 × 10−4 | ||
| PA4942 | hflK | Inhibitor of protease activity | 2.3 | 4.5 × 10−2 | + |
| PA4943 | GTPase | 2.8 | 3.4 × 10−3 | ||
| PA5053 | hslV | Protease | 9.4 | 2.4 × 10−4 | |
| PA5054 | hslU | Protease | 5.7 | 2.2 × 10−4 | |
| PA5055 | Similar to gamma-butyrobetaine dioxygenase | 8.0 | 4.5 × 10−4 |
| ID | Gene | Function | Fold Change | p-Value | Essential 1 |
|---|---|---|---|---|---|
| PA0392 | Membrane protein | 5.7 | 3.0 × 10−4 | ||
| PA0393 | proC | Pyrroline-5-carboxylate reductase | 7.5 | 5.2 × 10−5 | + |
| PA0394 | Tm barrel protein | 2.3 | 1.5 × 10−2 | ||
| PA1742 | pauD2 | Glutamine amidotransferase | 4.8 | 6.9 × 10−4 | |
| PA1779 | Assimilatory nitrate reductase | 3.8 | 1.9 × 10−2 | ||
| PA1780 | nirD | Assimilatory nitrite reductase small subunit | 5.6 | 6.0 × 10−3 | |
| PA1781 | nirB | Assimilatory nitrite reductase large subunit | 3.6 | 4.6 × 10−2 | |
| PA1783 | nasA | Nitrate transporter | 4.0 | 3.0 × 10−2 | |
| PA2776 | pauB3 | Arginine proline metabolism | 4.8 | 1.0 × 10−5 |
| ID 1 | Gene | Function | Fold Change | p-Value | Essential 2 |
|---|---|---|---|---|---|
| PA3010 | Nucleotide binding domain | 3.0 | 2.7 × 10−3 | ||
| PA3011 | topA | Topoisomerase | 5.4 | 6.7 × 10−4 | +++ |
| PA3272 | ATP-dependent helicase | 17.7 | 8.5 × 10−5 | ||
| PA4944 | hfq | RNA binding | 2.1 | 3.4 × 10−3 | |
| PA4969 | cpdA | 3′,5′-cyclic-AMP phosphodiesterase | 8.6 | 3.7 × 10−4 | +++ |
| PA4970 | Conserved hypothetical protein | 7.0 | 1.4 × 10−3 | ||
| PA4971 | aspP | NTP pyrophosphohydrolase | 11.9 | 6.1 × 10−4 |
| ID 1 | Gene | Function | Fold Change | p-Value | Essential 2 |
|---|---|---|---|---|---|
| PA0042 | Hypothetical protein | 2.1 | 3.3 × 10−3 | ||
| PA0576 | rpoD | RNA polymerase sigma factor RpoD | 2.7 | 2.0 × 10−3 | +++ |
| PA0744 | Enoyl-CoA hydratase/isomerase | 8.3 | 3.0 × 10−4 | ||
| PA0745 | dspI | Enoyl-CoA hydratase/isomerase | 10.7 | 6.2 × 10−4 | |
| PA0746 | Acyl-CoA dehydrogenase | 10.5 | 1.8 × 10−3 | ||
| PA0747 | NAD-dependent aldehyde dehydrogenase | 14.2 | 6.3 × 10−4 | ||
| PA0763 | mucA | Anti-sigma factor | 2.1 | 2.5 × 10−3 | + |
| PA0764 | mucB | Negative regulator for alginate biosynthesis | 2.9 | 1.9 × 10−3 | + |
| PA0905 | rsmA | CsrA carbon storage regulator | 2.8 | 4.4 × 10−2 | + |
| PA0916 | Ribosomal protein S12 methylthiotransferase | 2.0 | 3.2 × 10−3 | ||
| PA1211 | α/β -hydrolase | 2.1 | 1.3 × 10−2 | ||
| PA1212 | Major facilitator superfamily; transporter | 3.4 | 1.2 × 10−2 | ||
| PA1213 | Gamma-butyrobetaine dioxygenase | 4.5 | 4.5 × 10−2 | ||
| PA1214 | Asparagine synthetase B | 4.2 | 1.2 × 10−2 | ||
| PA1215 | Acetyl-CoA synthetase; non-ribosomal peptide synthase | 14.7 | 5.0 × 10−3 | ||
| PA1216 | Isopropylmalate/homocitrate/citramalate synthase + methyltransferase | 79.6 | 6.1 × 10−5 | ||
| PA1217 | 2-isopropylmalate or homocitrate synthase | 47.9 | 3.1 × 10−5 | ||
| PA1218 | 2OG oxygenases that catalyze hydroxylation reactions | 10.5 | 1.1 × 10−4 | ||
| PA1219 | α/β-hydrolase | 10.9 | 3.8 × 10−3 | ||
| PA1220 | Condensation domain which makes peptide antibiotics | 4.9 | 1.1 × 10−2 | ||
| PA1221 | Non-ribosomal peptide synthase | 5.3 | 2.0 × 10−2 | ||
| PA1318 | cyoB | Cytochrome o ubiquinol oxidase, subunit I | 2.2 | 9.2 × 10−3 | |
| PA1319 | cyoC | Cytochrome o ubiquinol oxidase, subunit III | 2.1 | 6.3 × 10−3 | |
| PA1555 | ccoP2 | Cytochrome c oxidase cbb3-type | 4.0 | 7.0 × 10−3 | |
| PA1557 | ccoN2 | Cytochrome c oxidase cbb3-type | 3.4 | 4.5 × 10−2 | |
| PA1550 | Ig-like domain; also in FixH protein involved in cation pumping | 2.1 | 5.1 × 10−3 | ||
| PA1826 | LysR type regulator | 2.3 | 1.4 × 10−2 | ||
| PA2101 | Permease of the drug/metabolite transporters | 4.3 | 5.9 × 10−3 | ||
| PA2102 | Metal-dependent protease of the PAD1/JAB1 superfamily | 3.8 | 2.2 × 10−3 | ||
| PA2103 | MoeB | Molybdopterin biosynthesis MoeB protein | 3.3 | 2.7 × 10−3 | |
| PA2104 | Cysteine synthase | 3.2 | 1.9 × 10−4 | ||
| PA2106 | Metal-dependent hydrolase, contains AlaS domain; alanyl-tRNA synthetase | 2.2 | 2.2 × 10−3 | ||
| PA2275 | Alcohol dehydrogenase | 7.2 | 2.0 × 10−4 | ||
| PA2481 | Cytochrome c oxidase, cbb3-type, subunit p | 2.1 | 1.2 × 10−2 | ||
| PA2559 | 23 kda subunit of oxygen evolving system of photosystem II of plants | 2.6 | 5.9 × 10−3 | ||
| PA2637 | nuoA | NADH dehydrogenase I chain A | 2.7 | 3.4 × 10−3 | |
| PA2638 | nuoB | NADH dehydrogenase I chain B | 2.1 | 2.9 × 10−2 | |
| PA2639 | nuoD | NADH dehydrogenase I chain C,D | 3.2 | 1.1 × 10−2 | |
| PA2640 | nuoE | NADH dehydrogenase I chain E | 3.1 | 1.5 × 10−2 | |
| PA2641 | nuoF | NADH dehydrogenase I chain F | 2.9 | 2.8 × 10−3 | |
| PA2642 | nuoG | NADH dehydrogenase I chain G | 3.7 | 3.5 × 10−3 | |
| PA2643 | nuoH | NADH dehydrogenase I chain H | 6.3 | 2.4 × 10−3 | |
| PA2644 | nuoI | NADH Dehydrogenase I chain I | 2.9 | 9.6 × 10−4 | |
| PA2645 | nuoJ | NADH dehydrogenase I chain J | 5.2 | 3.6 × 10−4 | + |
| PA2646 | nuoK | NADH dehydrogenase I chain K | 3.9 | 1.8 × 10−4 | |
| PA2647 | nuoL | NADH dehydrogenase I chain L | 4.4 | 2.5 × 10−3 | |
| PA2648 | nuoM | NADH dehydrogenase I chain M | 3.6 | 1.5 × 10−2 | |
| PA2649 | nuoN | NADH dehydrogenase I chain N | 5.4 | 1.3 × 10−3 | |
| PA2811 | ABC transporter, permease component | 6.2 | 1.6 × 10−3 | ||
| PA2812 | ABC transporter, ATP-binding component | 5.7 | 5.1 × 10−5 | ||
| PA3136 | Involved in substrate efflux | 2.0 | 1.1 × 10−2 | ||
| PA3472 | Invasion protein; cell wall-associated hydrolase degrades peptidoglycans | 2.4 | 4.2 × 10−2 | ||
| PA3531 | bfrB | Bacterioferritin | 6.6 | 7.1 × 10−4 | |
| PA3950 | ATP-dependent helicase | 2.6 | 5.5 × 10−4 | ||
| PA3951 | PIN-domain nuclease | 13.7 | 2.8 × 10−3 | ||
| PA3952 | Hypothetical protein | 16.2 | 7.9 × 10−4 | ||
| PA3979 | Similarity to stringent starvation protein | 2.0 | 5.6 × 10−3 | ||
| PA4061 | ybbN | Thioredoxin | 7.8 | 1.2 × 10−4 | |
| PA4333 | Fumarase | 2.0 | 4.1 × 10−3 | +++ | |
| PA4387 | FxsA protein affecting phage T7 exclusion by the F plasmid | 10.4 | 6.7 × 10−6 | ||
| PA4428 | sspA | Stringent response protein | 3.0 | 3.2 × 10−2 | |
| PA4429 | Cytochrome c1 precursor | 2.3 | 5.4 × 10−3 | +++ | |
| PA4430 | Cytochrome b | 2.7 | 2.7 × 10−3 | + | |
| PA4503 | dppB | ABC transporter, permease component; nickel, sulfate or small peptides | 2.0 | 3.5 × 10−2 | |
| PA4504 | dppC | ABC transporter, permease component | 2.2 | 3.2 × 10−2 | |
| PA4749 | glmM | Phosphoglucosamine mutase | 2.3 | 6.3 × 10−4 | +++ |
| PA4750 | folP | Dihydropteroate synthase | 3.8 | 3.2 × 10−3 | +++ |
| PA4812 | fdnG | Formate dehydrogenase-O, major subunit | 2.1 | 3.4 × 10−2 | |
| PA4839 | speA | Arginine decarboxylase | 3.0 | 3.8 × 10−4 | |
| PA4840 | Putative translation initiation factor SUI1 | 3.9 | 1.4 × 10−5 | ||
| PA4841 | Nudix hydrolase | 15.6 | 1.2 × 10−4 | ||
| PA4842 | Hypothetical protein | 7.2 | 2.4 × 10−3 | ||
| PA4862 | ABC transporter, ATP-binding subunit | 3.1 | 3.0 × 10−2 | ||
| PA4865 | ureA | Urease gamma subunit | 2.7 | 1.5 × 10−2 | |
| PA4866 | Urease complex protein | 2.1 | 3.1 × 10−2 | ||
| PA4888 | desB | Acyl-CoA delta-9-desaturasE | 2.9 | 1.6 × 10−2 | |
| PA4889 | Dehydrogenase/reductase | 2.2 | 2.9 × 10−2 | ||
| PA4909 | ABC transporter ATP-binding subunit | 2.4 | 2.1 × 10−2 | ||
| PA4910 | ABC transporter ATP-binding subunit | 3.2 | 1.9 × 10−2 | ||
| PA4912 | Branched-chain amino acid ABC transporter, permease subunit | 2.4 | 2.5 × 10−2 | ||
| PA4913 | Periplasmic; branched-chain amino acid ABC transporter | 2.8 | 1.5 × 10−2 | ||
| PA5001 | ssg | Cell surface-sugar biosynthetic glycosyltransferase | 3.0 | 3.9 × 10−3 | + |
| PA5002 | dnpA | De-N-acetylase involved in persistence | 2.5 | 1.8 × 10−2 | |
| PA5003 | Mig-14-like protein | 3.3 | 3.3 × 10−3 | + | |
| PA5004 | wapH | Glycosyltransferase, group 1 | 4.1 | 9.3 × 10−3 | |
| PA5005 | Carbamoyl transferase | 3.9 | 1.7 × 10−3 | ||
| PA5006 | Serine/threonine-protein kinase | 7.6 | 1.2 × 10−3 | +++ | |
| PA5007 | wapG | Serine/threonine-protein kinase | 10.8 | 1.3 × 10−4 | |
| PA5008 | wapP | Serine/threonine-protein kinase | 8.9 | 1.3 × 10−5 | +++ |
| PA5009 | waaP | Lipopolysaccharide kinase | 11.7 | 9.5 × 10−5 | +++ |
| PA5010 | waaG | UDP-glucose:(heptosyl) LPS alpha 1,3-glucosyltransferase | 8.7 | 1.9 × 10−5 | +++ |
| PA5124PA5124 | ntrB | Two-component sensor | 2.2 | 1.8 × 10−2 | |
| PA5159 | Multidrug resistance efflux pump | 2.7 | 2.3 × 10−2 | ||
| PA5160 | Drug efflux transporter | 2.0 | 4.1 × 10−2 | ||
| PA5203 | gshA | Glutamate--cysteine ligase | 3.7 | 5.2 × 10−3 | + |
| PA5380 | gbdR | Transcriptional regulator containing an amidase domain and an AraC-type DNA-binding HTH domain | 2.0 | 4.0 × 10−2 | |
| PA5479 | gltP | Proton-glutamate symporter | 2.0 | 7.6 × 10−4 |
| p Value for Overlap with Genes Significantly: | ||
|---|---|---|
| Activity 1 | Upregulated by rpoH Induction | Downregulated by rpoH Induction |
| Heat shock (ref. [25]) | p < 1 × 10−15 | p > 0.999 |
| Heat shock (pseudomonas.com) | p = 7.7 × 10−6 | p > 0.999 |
| Planktonic ciproflaxacin tolerance | p = 0.001 | p = 0.510 |
| MvfR+ | p = 0.009 | p = 0.215 |
| Mouse wound | p = 0.031 | p = 0.033 |
| Planktonic tobramycin sensitivity | p = 0.034 | p = 0.445 |
| Iron limitation | p = 0.789 | p< 1 × 10−15 |
| Stationary phase | p = 0.893 | p< 1 × 10−15 |
| RpoS+ | p = 0.127 | p = 2.3 × 10−15 |
| Virulence | p = 0.993 | p = 7.3 × 10−8 |
| Peroxide stress | p = 0.352 | p = 0.003 |
| Biofilm | p = 0.062 | p = 0.007 |
| HLS QS | p > 0.999 | p = 0.442 |
| Mg limitation | p = 0.443 | p > 0.999 |
| Phenazine biosynthesis | p > 0.999 | p > 0.999 |
| Efflux pumps | p > 0.999 | p > 0.999 |
| Nitrosative stress | p > 0.999 | p > 0.999 |
| Planktonic ciproflaxacin sensitivity | p = 0.228 | p > 0.999 |
| Up in biofilm treated with ciprofloxacin | p > 0.999 | p = 0.807 |
| Up in biofilm treated with tobramycin | p = 0.551 | p = 0.675 |
| SOS response | p > 0.999 | p > 0.999 |
| Zn limitation | p > 0.999 | p > 0.999 |
| Anr+ | p = 0.732 | p = 0.536 |
| Crc+ | p > 0.999 | p > 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williamson, K.S.; Dlakić, M.; Akiyama, T.; Franklin, M.J. The Pseudomonas aeruginosa RpoH (σ32) Regulon and Its Role in Essential Cellular Functions, Starvation Survival, and Antibiotic Tolerance. Int. J. Mol. Sci. 2023, 24, 1513. https://doi.org/10.3390/ijms24021513
Williamson KS, Dlakić M, Akiyama T, Franklin MJ. The Pseudomonas aeruginosa RpoH (σ32) Regulon and Its Role in Essential Cellular Functions, Starvation Survival, and Antibiotic Tolerance. International Journal of Molecular Sciences. 2023; 24(2):1513. https://doi.org/10.3390/ijms24021513
Chicago/Turabian StyleWilliamson, Kerry S., Mensur Dlakić, Tatsuya Akiyama, and Michael J. Franklin. 2023. "The Pseudomonas aeruginosa RpoH (σ32) Regulon and Its Role in Essential Cellular Functions, Starvation Survival, and Antibiotic Tolerance" International Journal of Molecular Sciences 24, no. 2: 1513. https://doi.org/10.3390/ijms24021513
APA StyleWilliamson, K. S., Dlakić, M., Akiyama, T., & Franklin, M. J. (2023). The Pseudomonas aeruginosa RpoH (σ32) Regulon and Its Role in Essential Cellular Functions, Starvation Survival, and Antibiotic Tolerance. International Journal of Molecular Sciences, 24(2), 1513. https://doi.org/10.3390/ijms24021513







