Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases
Abstract
1. Introduction
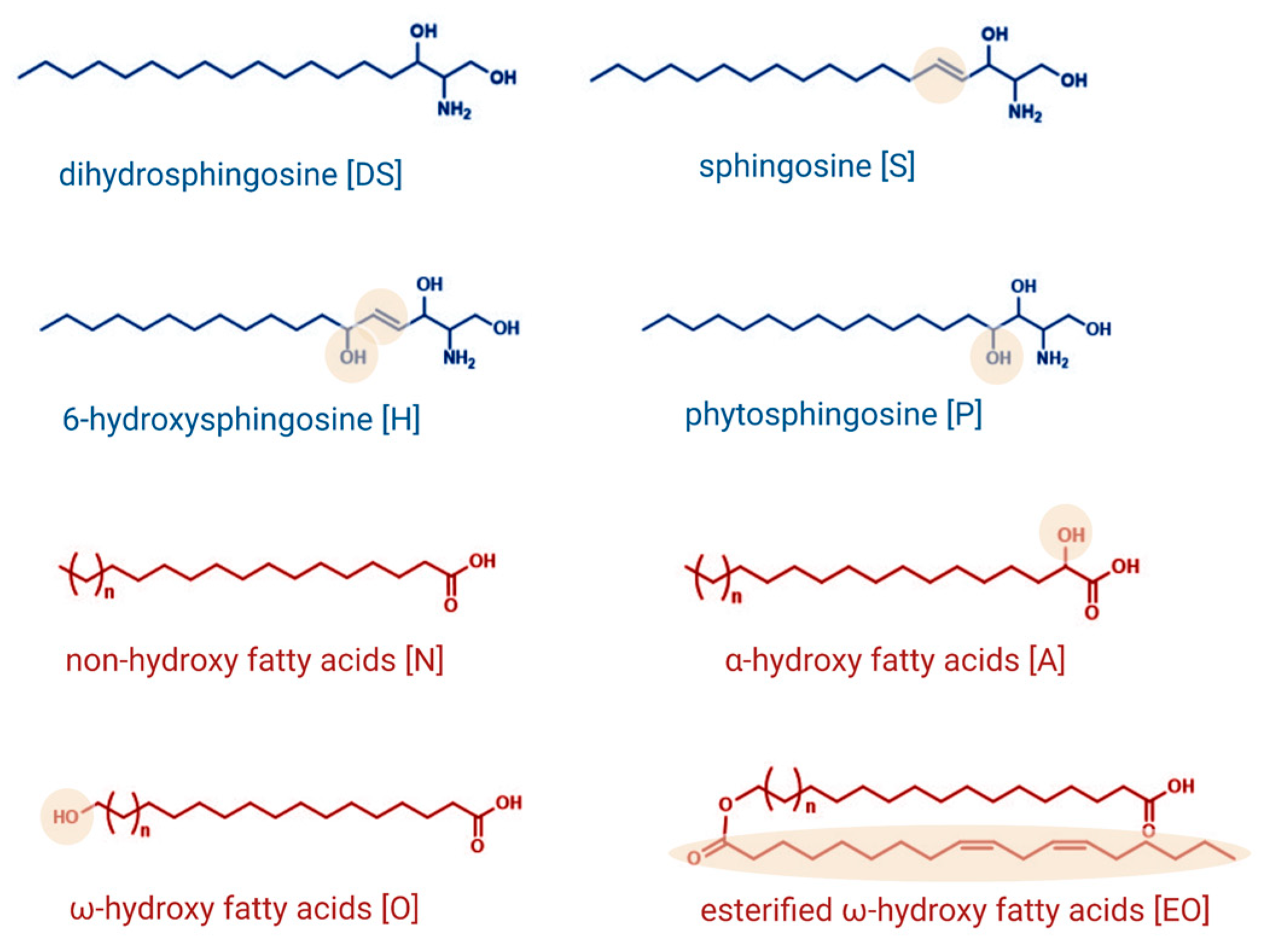
1.1. Sphingolipids as Essential Structural Components of the Skin
1.2. Sphingolipids as Signaling Molecules in the Skin
2. Sphingosine 1-Phosphate Metabolism, Signaling, and Immune Cell Migration
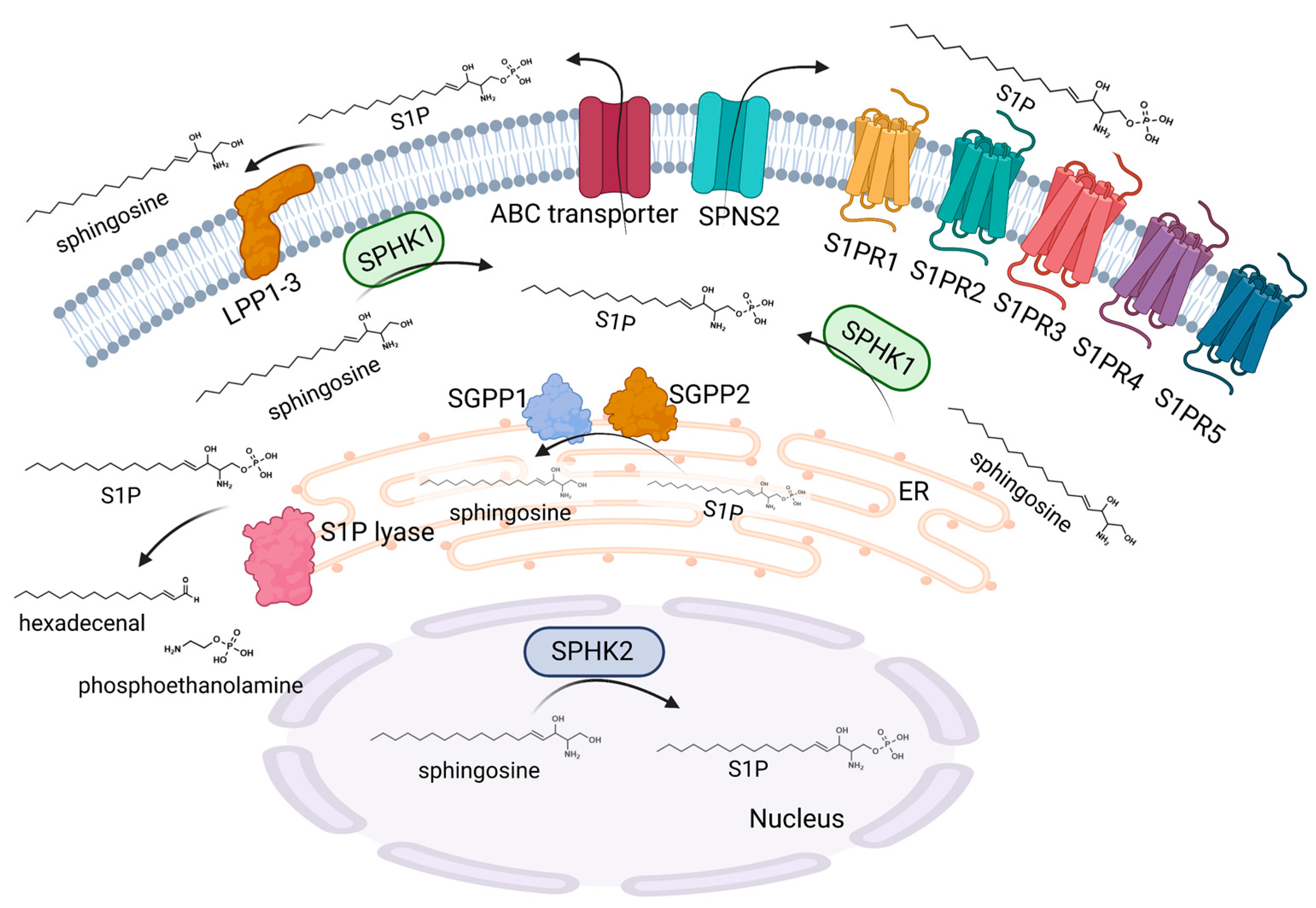
2.1. Sphingosine 1-Phosphate Metabolism
2.2. Sphingosine 1-Phosphate Signaling
2.3. Sphingosine 1-Phosphate and Immune Cell Migration
3. Sphingosine 1-Phosphate and Skin Cells
3.1. Sphingosine 1-Phosphate and Keratinocytes
3.2. Sphingosine 1-Phosphate and Dendritic Cells
3.3. Sphingosine 1-Phosphate and Macrophages
3.4. Sphingosine 1-Phosphate and Mast Cells
3.5. Sphingosine 1-Phosphate and T-Lymphocytes of the Skin
4. Sphingosine 1-Phosphate and Inflammatory Skin Diseases
4.1. Sphingosine 1-Phosphate and Psoriasis
4.2. Sphingosine 1-Phosphate and Atopic Dermatitis
4.3. Sphingosine 1-Phosphate and Itch
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Sato, H.; Naoe, A.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; et al. Comprehensive quantification of ceramide species in human stratum corneum. J. Lipid Res. 2009, 50, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Hoppel, L.; van der Heijden, R.; Hankemeier, T.; Vreeken, R.J.; Bouwstra, J.A. LC/MS analysis of stratum corneum lipids: Ceramide profiling and discovery. J. Lipid Res. 2011, 52, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Kawana, M.; Miyamoto, M.; Ohno, Y.; Kihara, A. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS[S]. J. Lipid Res. 2020, 61, 884–895. [Google Scholar] [CrossRef]
- Uchida, Y.; Park, K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin. Dermatol. 2021, 22, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444. [Google Scholar] [CrossRef]
- Vasireddy, V.; Uchida, Y.; Salem, N., Jr.; Kim, S.Y.; Mandal, M.N.; Reddy, G.B.; Bodepudi, R.; Alderson, N.L.; Brown, J.C.; Hama, H.; et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 2007, 16, 471–482. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakamichi, S.; Ohkuni, A.; Kamiyama, N.; Naoe, A.; Tsujimura, H.; Yokose, U.; Sugiura, K.; Ishikawa, J.; Akiyama, M.; et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7707–7712. [Google Scholar] [CrossRef]
- Jennemann, R.; Rabionet, M.; Gorgas, K.; Epstein, S.; Dalpke, A.; Rothermel, U.; Bayerle, A.; van der Hoeven, F.; Imgrund, S.; Kirsch, J.; et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 2012, 21, 586–608. [Google Scholar] [CrossRef]
- Grond, S.; Eichmann, T.O.; Dubrac, S.; Kolb, D.; Schmuth, M.; Fischer, J.; Crumrine, D.; Elias, P.M.; Haemmerle, G.; Zechner, R.; et al. PNPLA1 Deficiency in Mice and Humans Leads to a Defect in the Synthesis of Omega-O-Acylceramides. J. Investig. Dermatol. 2017, 137, 394–402. [Google Scholar] [CrossRef]
- Mizutani, Y.; Mitsutake, S.; Tsuji, K.; Kihara, A.; Igarashi, Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 2009, 91, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, S.; Hara, M.; Nishio, H.; Otsuka, F.; Suzuki, A.; Uchida, Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J. Investig. Dermatol. 2002, 119, 416–423. [Google Scholar] [CrossRef]
- Jensen, J.M.; Folster-Holst, R.; Baranowsky, A.; Schunck, M.; Winoto-Morbach, S.; Neumann, C.; Schutze, S.; Proksch, E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J. Investig. Dermatol. 2004, 122, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Lampe, M.A.; Burlingame, A.L.; Whitney, J.; Williams, M.L.; Brown, B.E.; Roitman, E.; Elias, P.M. Human stratum corneum lipids: Characterization and regional variations. J. Lipid Res. 1983, 24, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Grayson, S.; Elias, P.M. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J. Investig. Dermatol. 1986, 86, 591–597. [Google Scholar] [CrossRef]
- Bektas, M.; Dullin, Y.; Wieder, T.; Kolter, T.; Sandhoff, K.; Brossmer, R.; Ihrig, P.; Orfanos, C.E.; Geilen, C.C. Induction of apoptosis by synthetic ceramide analogues in the human keratinocyte cell line HaCaT. Exp. Dermatol. 1998, 7, 342–349. [Google Scholar] [CrossRef]
- Jung, E.M.; Griner, R.D.; Mann-Blakeney, R.; Bollag, W.B. A potential role for ceramide in the regulation of mouse epidermal keratinocyte proliferation and differentiation. J. Investig. Dermatol. 1998, 110, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef]
- Japtok, L.; Baumer, W.; Kleuser, B. Sphingosine-1-phosphate as signaling molecule in the skin: Relevance in atopic dermatitis. Allergo J. Int. 2014, 23, 54–59. [Google Scholar] [CrossRef]
- Gandy, K.A.; Obeid, L.M. Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handb. Exp. Pharmacol. 2013, 216, 275–303. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H., Jr.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef] [PubMed]
- Nishino, S.; Yamashita, H.; Tamori, M.; Mashimo, M.; Yamagata, K.; Nakamura, H.; Murayama, T. Translocation and activation of sphingosine kinase 1 by ceramide-1-phosphate. J. Cell Biochem. 2019, 120, 5396–5408. [Google Scholar] [CrossRef]
- Pulkoski-Gross, M.J.; Obeid, L.M. Molecular mechanisms of regulation of sphingosine kinase 1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Bonica, J.; Mao, C.; Obeid, L.M.; Hannun, Y.A. Transcriptional Regulation of Sphingosine Kinase 1. Cells 2020, 9, 2437. [Google Scholar] [CrossRef] [PubMed]
- Snider, A.J.; Orr Gandy, K.A.; Obeid, L.M. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie 2010, 92, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Allende, M.L.; Lee, B.G.; Chen, W.; Amar, M.J.; Remaley, A.T.; Saba, J.D.; Proia, R.L. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 2010, 285, 10880–10889. [Google Scholar] [CrossRef]
- Hagen-Euteneuer, N.; Lutjohann, D.; Park, H.; Merrill, A.H., Jr.; van Echten-Deckert, G. Sphingosine 1-phosphate (S1P) lyase deficiency increases sphingolipid formation via recycling at the expense of de novo biosynthesis in neurons. J. Biol. Chem. 2012, 287, 9128–9136. [Google Scholar] [CrossRef]
- Le Stunff, H.; Giussani, P.; Maceyka, M.; Lepine, S.; Milstien, S.; Spiegel, S. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J. Biol. Chem. 2007, 282, 34372–34380. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Kim, E.Y.; Yamada, A.; Ramachandran, S.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Milstien, S.; Takabe, K.; Spiegel, S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013, 27, 1001–1011. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Luth, A.; Chun, J.; Huwiler, A.; Pfeilschifter, J.; Schafer-Korting, M.; Kleuser, B. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P(3) in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. J. Mol. Med. 2009, 87, 645–657. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: Signaling inside and out. FEBS Lett. 2000, 476, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Choi, S.; Shin, B.; Yu, J.; Yu, J.; Hwang, J.M.; Yun, H.; Chung, Y.H.; Choi, J.S.; Choi, Y.; et al. Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction. J. Biol. Chem. 2015, 290, 9660–9673. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Panneer Selvam, S.; De Palma, R.M.; Oaks, J.J.; Oleinik, N.; Peterson, Y.K.; Stahelin, R.V.; Skordalakes, E.; Ponnusamy, S.; Garrett-Mayer, E.; Smith, C.D.; et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci. Signal. 2015, 8, ra58. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kihara, Y.; Jonnalagadda, D.; Blaho, V.A. Fingolimod: Lessons Learned and New Opportunities for Treating Multiple Sclerosis and Other Disorders. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 149–170. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Mehling, M.; Brinkmann, V.; Antel, J.; Bar-Or, A.; Goebels, N.; Vedrine, C.; Kristofic, C.; Kuhle, J.; Lindberg, R.L.; Kappos, L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008, 71, 1261–1267. [Google Scholar] [CrossRef]
- Camm, J.; Hla, T.; Bakshi, R.; Brinkmann, V. Cardiac and vascular effects of fingolimod: Mechanistic basis and clinical implications. Am. Heart J. 2014, 168, 632–644. [Google Scholar] [CrossRef]
- Oo, M.L.; Thangada, S.; Wu, M.T.; Liu, C.H.; Macdonald, T.L.; Lynch, K.R.; Lin, C.Y.; Hla, T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007, 282, 9082–9089. [Google Scholar] [CrossRef] [PubMed]
- Thangada, S.; Khanna, K.M.; Blaho, V.A.; Oo, M.L.; Im, D.S.; Guo, C.; Lefrancois, L.; Hla, T. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J. Exp. Med. 2010, 207, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Okada, T.; Matloubian, M.; Lo, C.G.; Cyster, J.G. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 2008, 28, 122–133. [Google Scholar] [CrossRef]
- Vaclavkova, A.; Chimenti, S.; Arenberger, P.; Hollo, P.; Sator, P.G.; Burcklen, M.; Stefani, M.; D’Ambrosio, D. Oral ponesimod in patients with chronic plaque psoriasis: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet 2014, 384, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, S.Y.; Kleuser, B.; Schafer-Korting, M.; Kim, K.H.; Park, K.C. Sphingosine-1-phosphate inhibits human keratinocyte proliferation via Akt/protein kinase B inactivation. Cell. Signal. 2004, 16, 89–95. [Google Scholar] [CrossRef]
- Manggau, M.; Kim, D.S.; Ruwisch, L.; Vogler, R.; Korting, H.C.; Schafer-Korting, M.; Kleuser, B. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J. Investig. Dermatol. 2001, 117, 1241–1249. [Google Scholar] [CrossRef]
- Vogler, R.; Sauer, B.; Kim, D.S.; Schafer-Korting, M.; Kleuser, B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J. Investig. Dermatol. 2003, 120, 693–700. [Google Scholar] [CrossRef]
- Lichte, K.; Rossi, R.; Danneberg, K.; ter Braak, M.; Kurschner, U.; Jakobs, K.H.; Kleuser, B.; Meyer zu Heringdorf, D. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J. Investig. Dermatol. 2008, 128, 1487–1498. [Google Scholar] [CrossRef]
- Schmitz, E.I.; Potteck, H.; Schuppel, M.; Manggau, M.; Wahydin, E.; Kleuser, B. Sphingosine 1-phosphate protects primary human keratinocytes from apoptosis via nitric oxide formation through the receptor subtype S1P(3). Mol. Cell. Biochem. 2012, 371, 165–176. [Google Scholar] [CrossRef]
- Weller, R.; Schwentker, A.; Billiar, T.R.; Vodovotz, Y. Autologous nitric oxide protects mouse and human keratinocytes from ultraviolet B radiation-induced apoptosis. Am. J. Physiol. Cell Physiol. 2003, 284, C1140–C1148. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Sipe, L.M.; Tuymetova, G.; Wilson-Henjum, K.L.; Chen, W.; Proia, R.L. Sphingosine-1-phosphate phosphatase 1 regulates keratinocyte differentiation and epidermal homeostasis. J. Biol. Chem. 2013, 288, 18381–18391. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Song, J.; Lee, D.; Kim, G.T.; Park, S.H.; Shin, D.Y.; Shin, K.O.; Park, K.; Shim, S.M.; Park, T.S. Inhibition of sphingosine 1-phosphate lyase activates human keratinocyte differentiation and attenuates psoriasis in mice. J. Lipid Res. 2020, 61, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Youm, J.K.; Kwon, M.J.; Park, B.D.; Lee, Y.M.; Lee, S.I.; Shin, D.M.; Lee, S.H. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J. Investig. Dermatol. 2008, 128, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Cho, K.A.; Hahn, S.; Lee, Y.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H.; Park, W.J.; Park, J.W. Inhibiting Sphingosine Kinase 2 Derived-sphingosine-1-phosphate Ameliorates Psoriasis-like Skin Disease via Blocking Th17 Differentiation of Naive CD4 T Lymphocytes in Mice. Acta Derm. Venereol. 2019, 99, 594–601. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, H.Y.; Yoon, H.S.; Park, W.J.; Adams, D.R.; Pyne, N.J.; Pyne, S.; Park, J.W. A Novel Selective Sphingosine Kinase 2 Inhibitor, HWG-35D, Ameliorates the Severity of Imiquimod-Induced Psoriasis Model by Blocking Th17 Differentiation of Naive CD4 T Lymphocytes. Int. J. Mol. Sci. 2020, 21, 8371. [Google Scholar] [CrossRef]
- Alsanafi, M.; Kelly, S.L.; Jubair, K.; McNaughton, M.; Tate, R.J.; Merrill, A.H., Jr.; Pyne, S.; Pyne, N.J. Native and Polyubiquitinated Forms of Dihydroceramide Desaturase Are Differentially Linked to Human Embryonic Kidney Cell Survival. Mol. Cell. Biol. 2018, 38, e00222-18. [Google Scholar] [CrossRef]
- McNaughton, M.; Pitman, M.; Pitson, S.M.; Pyne, N.J.; Pyne, S. Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKi or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget 2016, 7, 16663–16675. [Google Scholar] [CrossRef]
- Schuppel, M.; Kurschner, U.; Kleuser, U.; Schafer-Korting, M.; Kleuser, B. Sphingosine 1-phosphate restrains insulin-mediated keratinocyte proliferation via inhibition of Akt through the S1P2 receptor subtype. J. Investig. Dermatol. 2008, 128, 1747–1756. [Google Scholar] [CrossRef]
- Igawa, S.; Ohzono, A.; Pham, P.; Wang, Z.; Nakatsuji, T.; Dokoshi, T.; Di Nardo, A. Sphingosine 1-Phosphate Receptor 2 Is Central to Maintaining Epidermal Barrier Homeostasis. J. Investig. Dermatol. 2021, 141, 1188–1197.e5. [Google Scholar] [CrossRef]
- Christensen, A.D.; Haase, C. Immunological mechanisms of contact hypersensitivity in mice. APMIS 2012, 120, 1–27. [Google Scholar] [CrossRef]
- Kaplan, D.H. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010, 31, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Japtok, L.; Schaper, K.; Baumer, W.; Radeke, H.H.; Jeong, S.K.; Kleuser, B. Sphingosine 1-phosphate modulates antigen capture by murine Langerhans cells via the S1P2 receptor subtype. PLoS ONE 2012, 7, e49427. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Im, D.S. Blockage of sphingosine-1-phosphate receptor 2 attenuates 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice. Acta Pharmacol. Sin. 2020, 41, 1487–1496. [Google Scholar] [CrossRef]
- Schaper, K.; Kietzmann, M.; Baumer, W. Sphingosine-1-phosphate differently regulates the cytokine production of IL-12, IL-23 and IL-27 in activated murine bone marrow derived dendritic cells. Mol. Immunol. 2014, 59, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Panther, E.; Corinti, S.; Morelli, A.; Ferrari, D.; Herouy, Y.; Dichmann, S.; Mockenhaupt, M.; Gebicke-Haerter, P.; Di Virgilio, F.; et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002, 16, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M.; Richter, C.; Juan, M.H.; Maltusch, K.; Giegold, O.; Quintini, G.; Pfeilschifter, J.M.; Huwiler, A.; Radeke, H.H. The sphingosine kinase 1 and S1P1 axis specifically counteracts LPS-induced IL-12p70 production in immune cells of the spleen. Mol. Immunol. 2011, 48, 1139–1148. [Google Scholar] [CrossRef]
- Dillmann, C.; Ringel, C.; Ringleb, J.; Mora, J.; Olesch, C.; Fink, A.F.; Roberts, E.; Brune, B.; Weigert, A. S1PR4 Signaling Attenuates ILT 7 Internalization To Limit IFN-alpha Production by Human Plasmacytoid Dendritic Cells. J. Immunol. 2016, 196, 1579–1590. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Studer, S.; Leaf, N.; Kiosses, W.B.; Nguyen, N.; Matsuki, K.; Negishi, H.; Taniguchi, T.; Oldstone, M.B.; Rosen, H. S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proc. Natl. Acad. Sci. USA 2016, 113, 1351–1356. [Google Scholar] [CrossRef]
- Kim, J.; Moreno, A.; Krueger, J.G. The imbalance between Type 17 T-cells and regulatory immune cell subsets in psoriasis vulgaris. Front. Immunol. 2022, 13, 1005115. [Google Scholar] [CrossRef]
- Mohammed, S.; Vineetha, N.S.; James, S.; Aparna, J.S.; Babu Lankadasari, M.; Maeda, T.; Ghosh, A.; Saha, S.; Li, Q.Z.; Spiegel, S.; et al. Regulatory role of SphK1 in TLR7/9-dependent type I interferon response and autoimmunity. FASEB J. 2020, 34, 4329–4347. [Google Scholar] [CrossRef]
- Duong, C.Q.; Bared, S.M.; Abu-Khader, A.; Buechler, C.; Schmitz, A.; Schmitz, G. Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim. Biophys. Acta 2004, 1682, 112–119. [Google Scholar] [CrossRef]
- Michaud, J.; Im, D.S.; Hla, T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J. Immunol. 2010, 184, 1475–1483. [Google Scholar] [CrossRef]
- Weigert, A.; Weichand, B.; Brune, B. S1P regulation of macrophage functions in the context of cancer. Anticancer Agents Med. Chem. 2011, 11, 818–829. [Google Scholar] [CrossRef]
- Keul, P.; Lucke, S.; von Wnuck Lipinski, K.; Bode, C.; Graler, M.; Heusch, G.; Levkau, B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 2011, 108, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Weichand, B.; Weis, N.; Weigert, A.; Grossmann, N.; Levkau, B.; Brune, B. Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur. J. Immunol. 2013, 43, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.; Huard, A.; Sirait-Fischer, E.; Dillmann, C.; Brune, B.; Weigert, A. S1PR4-dependent CCL2 production promotes macrophage recruitment in a murine psoriasis model. Eur. J. Immunol. 2020, 50, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.E.; Srinivasan, S.; Lynch, K.R.; Proia, R.L.; Ferdek, P.; Hedrick, C.C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008, 102, 950–958. [Google Scholar] [CrossRef]
- Kulinski, J.M.; Munoz-Cano, R.; Olivera, A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur. J. Pharmacol. 2016, 778, 56–67. [Google Scholar] [CrossRef]
- Jolly, P.S.; Bektas, M.; Olivera, A.; Gonzalez-Espinosa, C.; Proia, R.L.; Rivera, J.; Milstien, S.; Spiegel, S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 2004, 199, 959–970. [Google Scholar] [CrossRef]
- Olivera, A.; Dillahunt, S.E.; Rivera, J. Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset. Immunol. Lett. 2013, 150, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Oskeritzian, C.A.; Price, M.M.; Hait, N.C.; Kapitonov, D.; Falanga, Y.T.; Morales, J.K.; Ryan, J.J.; Milstien, S.; Spiegel, S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J. Exp. Med. 2010, 207, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Eisner, C.; Kitamura, Y.; Dillahunt, S.; Allende, L.; Tuymetova, G.; Watford, W.; Meylan, F.; Diesner, S.C.; Li, L.; et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J. Clin. Investig. 2010, 120, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.G.; Wang, T.; Zheng, J.; et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef]
- Gray, E.E.; Suzuki, K.; Cyster, J.G. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 2011, 186, 6091–6095. [Google Scholar] [CrossRef]
- Sumaria, N.; Roediger, B.; Ng, L.G.; Qin, J.; Pinto, R.; Cavanagh, L.L.; Shklovskaya, E.; Fazekas de St Groth, B.; Triccas, J.A.; Weninger, W. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 2011, 208, 505–518. [Google Scholar] [CrossRef]
- Nakamizo, S.; Egawa, G.; Tomura, M.; Sakai, S.; Tsuchiya, S.; Kitoh, A.; Honda, T.; Otsuka, A.; Nakajima, S.; Dainichi, T.; et al. Dermal Vgamma4(+) gammadelta T cells possess a migratory potency to the draining lymph nodes and modulate CD8(+) T-cell activity through TNF-alpha production. J. Investig. Dermatol. 2015, 135, 1007–1015. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Gray, E.E.; Zhang, Y.; Ramirez-Valle, F.; Cyster, J.G. Sphingosine-1-phosphate receptor 2 restrains egress of gammadelta T cells from the skin. J. Exp. Med. 2019, 216, 1487–1496. [Google Scholar] [CrossRef]
- Sandrock, I.; Reinhardt, A.; Ravens, S.; Binz, C.; Wilharm, A.; Martins, J.; Oberdorfer, L.; Tan, L.; Lienenklaus, S.; Zhang, B.; et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing gammadelta T cells. J. Exp. Med. 2018, 215, 3006–3018. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Morizane, S.; Akagi, T.; Hiramatsu-Asano, S.; Tachibana, K.; Yahagi, A.; Iseki, M.; Kaneto, H.; Wada, J.; Ishihara, K.; et al. Obesity and Dyslipidemia Synergistically Exacerbate Psoriatic Skin Inflammation. Int. J. Mol. Sci. 2022, 23, 4312. [Google Scholar] [CrossRef]
- Zhou, S.; Yao, Z. Roles of Infection in Psoriasis. Int. J. Mol. Sci. 2022, 23, 6955. [Google Scholar] [CrossRef]
- Checa, A.; Xu, N.; Sar, D.G.; Haeggstrom, J.Z.; Stahle, M.; Wheelock, C.E. Circulating levels of sphingosine-1-phosphate are elevated in severe, but not mild psoriasis and are unresponsive to anti-TNF-alpha treatment. Sci. Rep. 2015, 5, 12017. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, D.; Harasim-Symbor, E.; Mysliwiec, H.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Serum sphingolipid level in psoriatic patients with obesity. Postepy Dermatol. Alergol. 2019, 36, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, H.; Baran, A.; Harasim-Symbor, E.; Choromanska, B.; Mysliwiec, P.; Milewska, A.J.; Chabowski, A.; Flisiak, I. Increase in circulating sphingosine-1-phosphate and decrease in ceramide levels in psoriatic patients. Arch. Dermatol. Res. 2017, 309, 79–86. [Google Scholar] [CrossRef]
- Nada, H.A.; Elshabrawy, M.M.; Ismail, N.I.; Hassan, E.T.; Jafferany, M.; Elsaie, M.L. Therapeutic implications and role of serum sphingolipids on psoriasis severity after narrow band ultraviolet B treatment: A cross sectional controlled study. Dermatol. Ther. 2020, 33, e13988. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, B.; Liu, Q.; Liu, T.; Wang, H.; Zhang, P.; Lu, L.; Zhang, L.; Zhang, F.; Huang, R.; et al. A Murine Point Mutation of Sgpl1 Skin Is Enriched With Vgamma6 IL17-Producing Cell and Revealed With Hyperpigmentation After Imiquimod Treatment. Front. Immunol. 2022, 13, 728455. [Google Scholar] [CrossRef]
- Schaper, K.; Dickhaut, J.; Japtok, L.; Kietzmann, M.; Mischke, R.; Kleuser, B.; Baumer, W. Sphingosine-1-phosphate exhibits anti-proliferative and anti-inflammatory effects in mouse models of psoriasis. J. Dermatol. Sci. 2013, 71, 29–36. [Google Scholar] [CrossRef]
- Liao, J.J.; Huang, M.C.; Goetzl, E.J. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J. Immunol. 2007, 178, 5425–5428. [Google Scholar] [CrossRef]
- Xue, Y.; Bao, W.; Zhou, J.; Zhao, Q.L.; Hong, S.Z.; Ren, J.; Yang, B.C.; Wang, P.; Yin, B.; Chu, C.C.; et al. Global Burden, Incidence and Disability-Adjusted Life-Years for Dermatitis: A Systematic Analysis Combined With Socioeconomic Development Status, 1990–2019. Front. Cell. Infect. Microbiol. 2022, 12, 861053. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef]
- Elias, P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp. Dermatol. 2018, 27, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y. Atopic dermatitis: Is innate or adaptive immunity in control? A clinical perspective. Front. Immunol. 2022, 13, 943640. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Herrmann, N.; Maintz, L.; Numm, T.J.; Welchowski, T.; Claus, R.A.; Graler, M.H.; Bieber, T. Serum sphingosine-1-phosphate is elevated in atopic dermatitis and associated with severity. Allergy 2021, 76, 2592–2595. [Google Scholar] [CrossRef]
- Baumer, W.; Rossbach, K.; Mischke, R.; Reines, I.; Langbein-Detsch, I.; Luth, A.; Kleuser, B. Decreased concentration and enhanced metabolism of sphingosine-1-phosphate in lesional skin of dogs with atopic dermatitis: Disturbed sphingosine-1-phosphate homeostasis in atopic dermatitis. J. Investig. Dermatol. 2011, 131, 266–268. [Google Scholar] [CrossRef]
- Seo, E.Y.; Park, G.T.; Lee, K.M.; Kim, J.A.; Lee, J.H.; Yang, J.M. Identification of the target genes of atopic dermatitis by real-time PCR. J. Investig. Dermatol. 2006, 126, 1187–1189. [Google Scholar] [CrossRef]
- Wood, S.H.; Clements, D.N.; Ollier, W.E.; Nuttall, T.; McEwan, N.A.; Carter, S.D. Gene expression in canine atopic dermatitis and correlation with clinical severity scores. J. Dermatol. Sci. 2009, 55, 27–33. [Google Scholar] [CrossRef]
- Reines, I.; Kietzmann, M.; Mischke, R.; Tschernig, T.; Luth, A.; Kleuser, B.; Baumer, W. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J. Investig. Dermatol. 2009, 129, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, J.H.; Im, D.S. Topical Application of S1P2 Antagonist JTE-013 Attenuates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice. Biomol. Ther. 2020, 28, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.; Pfalzgraff, A.; Weindl, G. Sphingosine 1-phospate differentially modulates maturation and function of human Langerhans-like cells. J. Dermatol. Sci. 2016, 82, 9–17. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Hait, N.C.; Wedman, P.; Chumanevich, A.; Kolawole, E.M.; Price, M.M.; Falanga, Y.T.; Harikumar, K.B.; Ryan, J.J.; Milstien, S.; et al. The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway T-cell infiltration in murine mast cell-dependent acute allergic responses. J. Allergy Clin. Immunol. 2015, 135, 1008–1018.e1. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Im, D.S. Blockage of sphingosine-1-phosphate receptor 2 attenuates allergic asthma in mice. Br. J. Pharmacol. 2019, 176, 938–949. [Google Scholar] [CrossRef]
- Szollosi, A.G.; Olah, A.; Lisztes, E.; Griger, Z.; Toth, B.I. Pruritus: A Sensory Symptom Generated in Cutaneous Immuno-Neuronal Crosstalk. Front. Pharmacol. 2022, 13, 745658. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Nguyen, M.Q.; von Buchholtz, L.J.; Reker, A.N.; Ryba, N.J.; Davidson, S. Single-nucleus transcriptomic analysis of human dorsal root ganglion neurons. Elife 2021, 10, e71752. [Google Scholar] [CrossRef]
- Ehling, S.; Butler, A.; Thi, S.; Ghashghaei, H.T.; Baumer, W. To scratch an itch: Establishing a mouse model to determine active brain areas involved in acute histaminergic itch. IBRO Rep. 2018, 5, 67–73. [Google Scholar] [CrossRef]
- Andoh, T.; Saito, A.; Kuraishi, Y. Leukotriene B(4) mediates sphingosylphosphorylcholine-induced itch-associated responses in mouse skin. J. Investig. Dermatol. 2009, 129, 2854–2860. [Google Scholar] [CrossRef]
- Hill, R.Z.; Morita, T.; Brem, R.B.; Bautista, D.M. S1PR3 Mediates Itch and Pain via Distinct TRP Channel-Dependent Pathways. J. Neurosci. 2018, 38, 7833–7843. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, H.; DeBrecht, J.; Mishra, S.K. Differential contribution of sensory transient receptor potential channels in response to the bioactive lipid sphingosine-1-phosphate. Mol. Pain. 2020, 16, 1744806920903515. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.Z.; Rifi, Z.; Vuong, C.; Bautista, D.M. Loss of S1PR3 attenuates scratching behaviors in mice in the imiquimod model of psoriasis, but not in the MC903 model of atopic dermatitis. Itch 2020, 5, e35. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleuser, B.; Bäumer, W. Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023, 24, 1456. https://doi.org/10.3390/ijms24021456
Kleuser B, Bäumer W. Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. International Journal of Molecular Sciences. 2023; 24(2):1456. https://doi.org/10.3390/ijms24021456
Chicago/Turabian StyleKleuser, Burkhard, and Wolfgang Bäumer. 2023. "Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases" International Journal of Molecular Sciences 24, no. 2: 1456. https://doi.org/10.3390/ijms24021456
APA StyleKleuser, B., & Bäumer, W. (2023). Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. International Journal of Molecular Sciences, 24(2), 1456. https://doi.org/10.3390/ijms24021456





