Influence of Cucurbiturils on the Production of Reactive Oxygen Species by T- and B-Lymphocytes, Platelets and Red Blood Cells
Abstract
1. Introduction
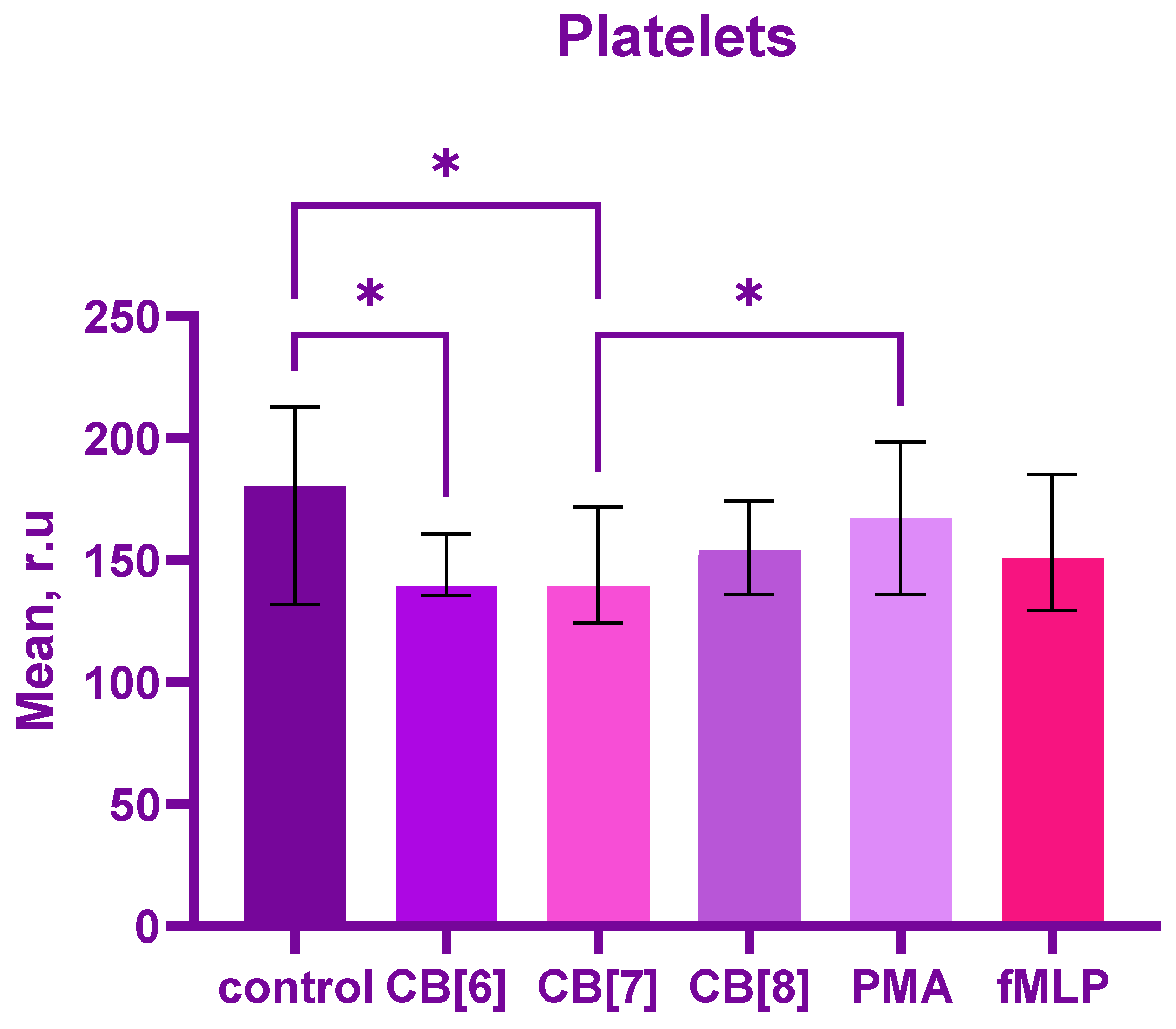
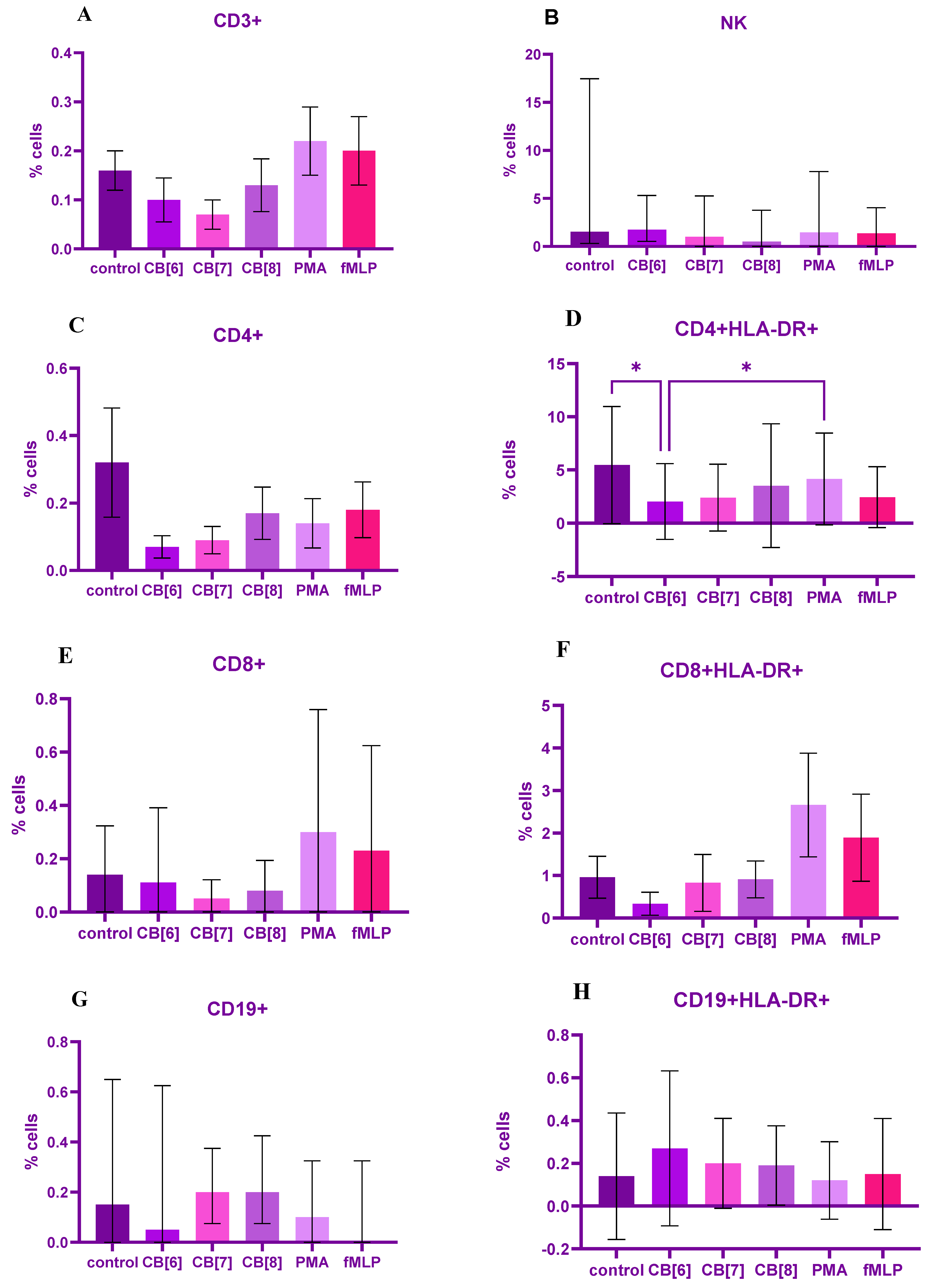
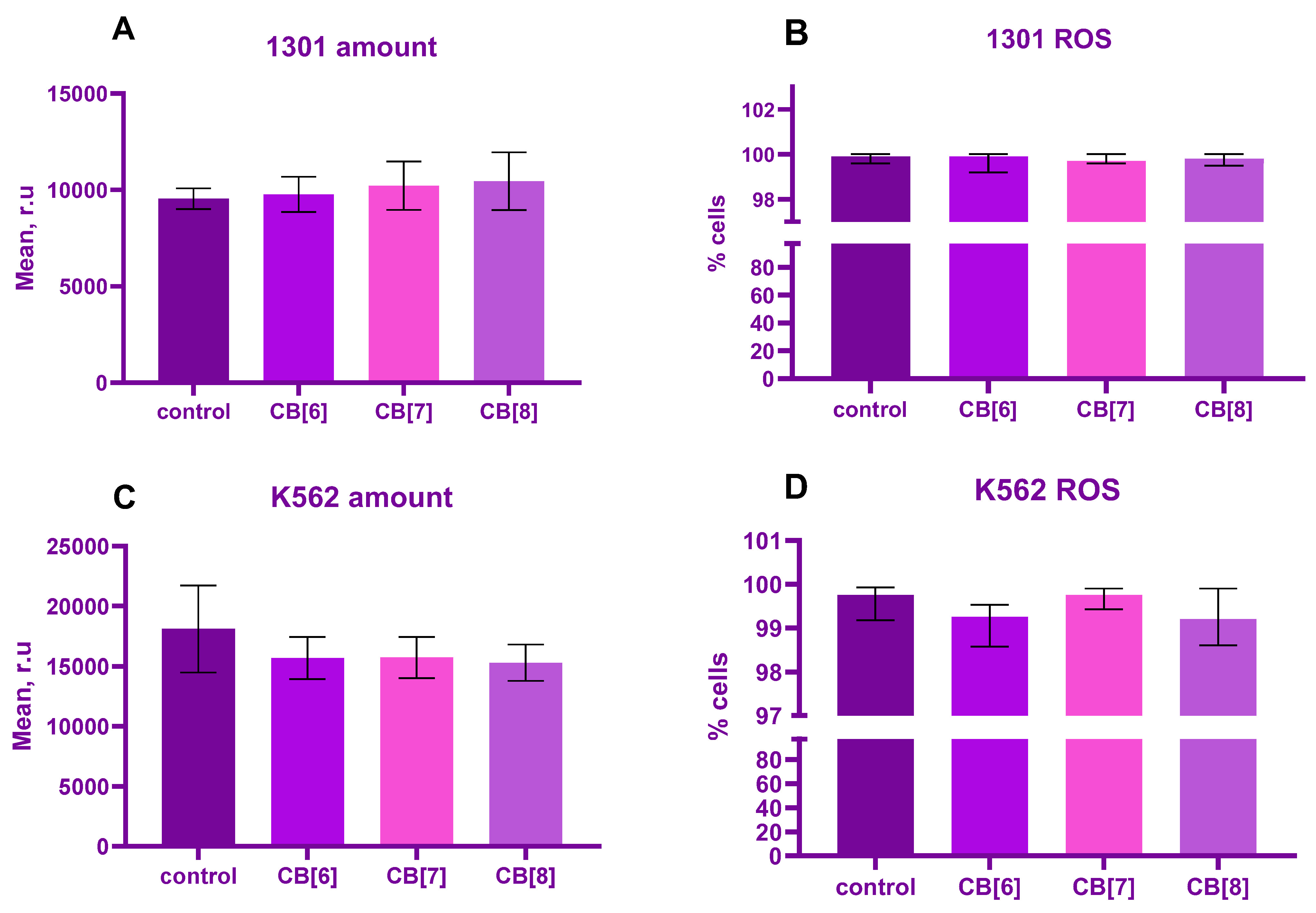
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials, Reagents and Preparation
4.2. Isolation and Cultivation of Cells
4.2.1. Isolation and Cultivation of PBMC
4.2.2. Isolation and Cultivation of RBC
4.2.3. Isolation and Cultivation of Platelets
4.2.4. Cultivation of Tumor Lines
4.3. Treatment of Cells by Cucurbit[n]urils (n = 6, 7, 8)
4.4. Measurement of Intracellular Reactive Oxygen
4.5. Phenotype of Cells
4.6. Statistical Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behrend, R.; Meyer, E.; Rusche, F. Ueber Condensationsprodukte aus Glycoluril und Formaldehyd. Justus Liebig’s Ann. Der Chem. 1905, 339, 1–37. [Google Scholar] [CrossRef]
- Mohanty, J.; Choudhury, S.D.; Barooah, N.; Pal, H.; Bhasikuttan, A. Mechanistic Aspects of Host–Guest Binding in Cucurbiturils: Physicochemical Properties. Compr. Supramol. Chem. II 2017, 435–457. [Google Scholar] [CrossRef]
- Das, D.; Assaf, K.I.; Nau, W.M. Applications of Cucurbiturils in Medicinal Chemistry and Chemical Biology. Front. Chem. 2019, 7, 619. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Floriano, R.S.; Isaacs, L.; Rowan, E.G.; Wheate, N.J. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbituril-based macrocyclic drug delivery vehicles. Toxicol. Res. 2014, 3, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Prabha, A.S.; Dorothy, R.; Jancirani, S.; Rajendran, S.; Singh, G.; Kumaran, S.S. Recent advances in the study of toxicity of polymer-based nanomaterials. Nanotoxicity 2020, 143–165. [Google Scholar] [CrossRef]
- Aktanova, A.; Abramova, T.; Pashkina, E.; Boeva, O.; Grishina, L.; Kovalenko, E.; Kozlov, V. Assessment of the Biocompatibility of Cucurbiturils in Blood Cells. Nanomaterials 2021, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Montes-Navajas, P.; González-Béjar, M.; Scaiano, J.C.; García, H. Cucurbituril complexes cross the cell membrane. Photochem. Photobiol. Sci. 2009, 8, 1743. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Paardekooper, L.M.; Vos, W.; van den Bogaart, G. Oxygen in the tumor microenvironment: Effects on dendritic cell function. Oncotarget 2019, 10, 883–896. [Google Scholar] [CrossRef]
- Kraaij, M.D.; Savage, N.D.L.; van der Kooij, S.W.; Koekkoek, K.; Wang, J.; van den Bers, J.M.; Ottenhoff, T.H.M.; Kujipers, T.W.; Holmdahl, R.; van Kooten, C.; et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 17686–17691. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Gluschko, A.; Schramm, M. Reactive Oxygen Species: Not Omnipresent but Important in Many Locations. Front. Cell Dev. Biol. 2021, 9, 716406. [Google Scholar] [CrossRef] [PubMed]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Gibboney, J.J.; Haak, R.A.; Kleinhans, F.W.; Brahmi, Z. Electron spin resonance spectroscopy does not reveal hydroxyl radical production in activated natural killer lymphocytes. J. Leukoc. Biol. 1988, 44, 545–550. [Google Scholar] [CrossRef]
- Duwe, A.K.; Werkmeister, J.; Roder, J.C.; Lauzon, R.; Payne, U. Natural killer cell-mediated lysis involves an hydroxyl radical-dependent step. J. Immunol. 1985, 134, 2637–2644. [Google Scholar] [CrossRef]
- Franchina, D.G.; Dostert, C.; Brenner, D. Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism. Trends Immunol. 2018, 39, 489–502. [Google Scholar] [CrossRef]
- Rashida Gnanaprakasam, J.N.; Wu, R.; Wang, R. Metabolic Reprogramming in Modulating T Cell Reactive Oxygen Species Generation and Antioxidant Capacity. Front. Immunol. 2018, 9, 1075. [Google Scholar] [CrossRef]
- Hildeman, D.A.; Mitchell, T.; Teague, T.K.; Henson, P.; Day, B.J.; Kappler, J.; Marrack, P.C. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 1999, 10, 735–744. [Google Scholar] [CrossRef]
- Hildeman, D.A. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1496–1504. [Google Scholar] [CrossRef]
- Kiselevsky, D.B. Granzymes and Mitochondria. Biochemistry 2020, 85, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, G.; Margiotta, D.; Kasahara, A.; Bassoy, E.Y.; Walch, M.; Thiery, J.; Lieberman, J.; Martinvalet, D. Granzyme B-induced mitochondrial ROS are required for apoptosis. Cell Death Differ. 2015, 22, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Y.; Tang, M.; Suzuki, M.; Gunasekara, C.; Anbe, Y.; Hiraoka, Y.; Liu, J.; Grasberger, H.; Ohkita, M.; Matsumura, Y.; et al. Essential Role of NADPH Oxidase–Dependent Production of Reactive Oxygen Species in Maintenance of Sustained B Cell Receptor Signaling and B Cell Proliferation. J. Immunol. 2019, 202, 2546–2557. [Google Scholar] [CrossRef]
- Bertolotti, M.; Yim, S.H.; Garcia-Manteiga, J.M.; Masciarelli, S.; Kim, Y.-J.; Kang, M.-H.; Sitia, R. B- to Plasma-Cell Terminal Differentiation Entails Oxidative Stress and Profound Reshaping of the Antioxidant Responses. Antioxid. Redox Signal. 2010, 13, 1133–1144. [Google Scholar] [CrossRef]
- Khoory, J.; Estanislau, J.; Elkhal, A.; Lazaar, A.; Melhorn, M.I.; Brodsky, A.; Ghiran, I.C. Ligation of Glycophorin A Generates Reactive Oxygen Species Leading to Decreased Red Blood Cell Function. PLoS ONE 2016, 11, e0141206. [Google Scholar] [CrossRef]
- Diederich, L.; Suvorava, T.; Sansone, R.; Keller, T.C.S.; Barbarino, F.; Sutton, T.R.; Cortese-Krott, M.M. On the Effects of Reactive Oxygen Species and Nitric Oxide on Red Blood Cell Deformability. Front. Physiol. 2018, 9, 332. [Google Scholar] [CrossRef]
- Atamna, H.; Ginsburg, H. Origin of reactive oxygen species in erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol. 1993, 61, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Hosseini, E.; Shahbaz Ghasabeh, A.; Mousavi Hosseini, K. Reactive Oxygen Species Generated by CD45- Cells Distinct from Leukocyte Population in Platelet Concentrates Is Correlated with the Expression and Release of Platelet Activation Markers during Storage. Transfus. Med. Hemother. 2018, 45, 33–41. [Google Scholar] [CrossRef]
- Carrim, N.; Arthur, J.F.; Hamilton, J.R.; Gardiner, E.E.; Andrews, R.K.; Moran, N.; Metharom, P. Thrombin-induced reactive oxygen species generation in platelets: A novel role for protease-activated receptor 4 and GPIbα. Redox Biol. 2015, 6, 640–647. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Bao, S.; Lu, Z.; Li, Q.; Li, C.M. Plastic protein microarray to investigate the molecular pathways of magnetic nanoparticle-induced nanotoxicity. Nanotechnology 2013, 24, 175501. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.; Købler, C.; Mølhave, K.; Samuelson, L.; Tegenfeldt, J.O.; Oredsson, S.; Prinz, C.N. Fibroblasts cultured on nanowires exhibit low motility, impaired cell division, and DNA damage. Small 2013, 9, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhao, J.; Meng, H.; Hu, D.; Zhou, Y.; Zhang, X.; Wang, C.; Li, J.; Yuan, J.; Wei, Y. Carnosine-Modified Fullerene as a Highly Enhanced ROS Scavenger for Mitigating Acute Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 16104–16113. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, R.; Xu, J.; Wang, L.; Sun, J.; Jiang, X.; Wang, T.; Li, X.; Luo, Q.; Liu, J. Cucurbit[8]uril-based supramolecular nanocapsules with a multienzyme-cascade antioxidative effect. Chem. Commun. 2019, 55, 13820–13823. [Google Scholar] [CrossRef]
- Gonzalez, L.; Lison, D.; Kirsch-Volders, M. Genotoxicity of engineered nanomaterials: A critical review. Nanotoxicology 2008, 2, 252–273. [Google Scholar] [CrossRef]
- Michlewska, S.; Ionov, M.; Szwed, A.; Rogalska, A.; Sanz Del Olmo, N.; Ortega, P.; Denel, M.; Jacenik, D.; Shcharbin, D.; De La Mata, F.J.; et al. Ruthenium dendrimers against human lymphoblastic leukemia 1301 cells. Int. J. Mol. Sci. 2020, 21, 4119. [Google Scholar] [CrossRef]
- Das, A.; Konyak, P.M.; Das, A.; Dey, S.K.; Saha, C. Physicochemical characterization of dual action liposomal formulations: Anticancer and antimicrobial. Heliyon 2019, 5, e02372. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Zhang, H.; Li, F.; Zhang, S. Theoretical study of complexation of resveratrol with cyclodextrins and cucurbiturils: Structure and antioxidative activity. RSC Adv. 2015, 5, 14114–14122. [Google Scholar] [CrossRef]
- Lee, J.S.; Song, I.H.; Shinde, P.B.; Nimse, S.B. Macrocycles and Supramolecules as Antioxidants: Excellent Scaffolds for Development of Potential Therapeutic Agents. Antioxidants 2020, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Sun, H.; Wang, L.; Zhao, L.; Li, J.; Dong, Z.; Luo, Q.; Xu, J.; Liu, J. Construction of a smart temperature-responsive GPx mimic based on the self-assembly of supra-amphiphiles. Soft Matter 2016, 12, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yuan, X.; Shi, R.; Wei, X.; Ren, S.; Yan, C.; Ding, Y.; Lin, Y.; Fan, D.; Yang, M.; et al. Phii-7 inhibits cell growth and induces apoptosis in leukemia cell line K562 as well as its mdr- counterpart K562/A02 through producing reactive oxygen species. Eur. J. Pharmacol. 2013, 718, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.T.; Zhang, D.M.; Qin, Q.; Lu, L.; Luo, M.; Guo, F.C.; Shi, H.S.; Jiang, L.; Shao, B.; Li, M.; et al. The promotion of erythropoiesis via the regulation of reactive oxygen species by lactic acid. Sci. Rep. 2017, 7, 38105. [Google Scholar] [CrossRef]
- Wang, S.X.; Wen, X.; Bell, C.; Appiah, S. liposome delivered baicalein induction of myeloid leukemia K562 cell death via reactive oxygen species generation. Mol. Med. Rep. 2018, 17, 4524–4530. [Google Scholar] [CrossRef]
- Hoshiko, T.; Kubota, Y.; Onodera, R.; Higashi, T.; Yokoo, M.; Motoyama, K.; Kimura, S. Folic acid-appended hydroxypropyl-β-cyclodextrin exhibits potent antitumor activity in chronic myeloid leukemia cells via autophagic cell death. Cancers 2021, 13, 5413. [Google Scholar] [CrossRef]
- Neagu, M.; Ion, R.M.; Manda, G.; Constantin, C.; Radu, E.; Cristu, Z. Antitumoral effect of calixarenes on experimental photodynamic therapy with K562 tumor cell line. Rom. J. Biochem. 2010, 47, 17–35. [Google Scholar]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ahmad, R.; Vaali-Mohammed, M.A.; Elwatidy, M.; Al-Obeed, O.; Al-Khayal, K.; Eldehna, W.M.; Abdel-Aziz, H.A.; Alafeefy, A.; Abdulla, M. Induction of ROS mediated cell death and activation of the Jnk pathway by a sulfonamide derivative. Int. J. Mol. Med. 2019, 44, 1552–1562. [Google Scholar] [CrossRef]
- Shafagh, M.; Rahmani, F.; Delirezh, N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and p53. Iran J. Basic Med. Sci. 2015, 18, 993–1000. [Google Scholar]
- Cao, D.X.; Qiao, B.; Ge, Z.Q.; Yuan, Y.J. comparison of burst of reactive oxygen species and activation of caspase-3 in apoptosis of K562 and Hl-60 cells induced by docetaxel. Cancer Lett. 2004, 214, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Pashkina, E.; Aktanova, A.; Blinova, E.; Mirzaeva, I.; Kovalenko, E.; Knauer, N.; Ermakov, A.; Kozlov, V. Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro. Molecules 2020, 25, 3388. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 2008, 10, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, K.; Pieniazek, A.; Gwozdzinski, L. Reactive Oxygen Species and Their Involvement in Red Blood Cell Damage in Chronic Kidney Disease. Oxid. Med. Cell Longev. 2021, 2021, 6639199. [Google Scholar] [CrossRef] [PubMed]
- Rajashekaraiah, V.; Pallavi, M.; Choudhary, A.; Bhat, C.; Banerjee, P.; Ranjithvishal; Laavanyaa, S.; Nithindran, S. Reactive Oxygen Species and Antioxidant Interactions in Erythrocytes. In The Erythrocyte—A Unique Cell [Working Title]; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Kesarwani, P.; Murali, A.K.; Al-Khami, A.A.; Mehrotra, S. Redox regulation of T-cell function: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2013, 18, 1497–1534. [Google Scholar] [CrossRef] [PubMed]
- Byamba, D.; Kim, T.G.; Kim, D.H.; Je, J.H.; Lee, M.G. The Roles of Reactive Oxygen Species Produced by Contact Allergens and Irritants in Monocyte-derived Dendritic Cells. Ann. Dermatol. 2010, 22, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Uzunova, V.D.; Cullinane, C.; Brix, K.; Nau, W.M.; Day, A.I. Toxicity of cucurbit[7]uril and cucurbit[8]uril: An exploratory in vitro and in vivo study. Org. Biomol. Chem. 2010, 8, 2037–2042. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktanova, A.A.; Boeva, O.S.; Barkovskaya, M.S.; Kovalenko, E.A.; Pashkina, E.A. Influence of Cucurbiturils on the Production of Reactive Oxygen Species by T- and B-Lymphocytes, Platelets and Red Blood Cells. Int. J. Mol. Sci. 2023, 24, 1441. https://doi.org/10.3390/ijms24021441
Aktanova AA, Boeva OS, Barkovskaya MS, Kovalenko EA, Pashkina EA. Influence of Cucurbiturils on the Production of Reactive Oxygen Species by T- and B-Lymphocytes, Platelets and Red Blood Cells. International Journal of Molecular Sciences. 2023; 24(2):1441. https://doi.org/10.3390/ijms24021441
Chicago/Turabian StyleAktanova, Alina A., Olga S. Boeva, Margarita Sh. Barkovskaya, Ekaterina A. Kovalenko, and Ekaterina A. Pashkina. 2023. "Influence of Cucurbiturils on the Production of Reactive Oxygen Species by T- and B-Lymphocytes, Platelets and Red Blood Cells" International Journal of Molecular Sciences 24, no. 2: 1441. https://doi.org/10.3390/ijms24021441
APA StyleAktanova, A. A., Boeva, O. S., Barkovskaya, M. S., Kovalenko, E. A., & Pashkina, E. A. (2023). Influence of Cucurbiturils on the Production of Reactive Oxygen Species by T- and B-Lymphocytes, Platelets and Red Blood Cells. International Journal of Molecular Sciences, 24(2), 1441. https://doi.org/10.3390/ijms24021441





