The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance
Abstract
1. Introduction
2. The Role of Membrane Lipids in Colorectal Cancer
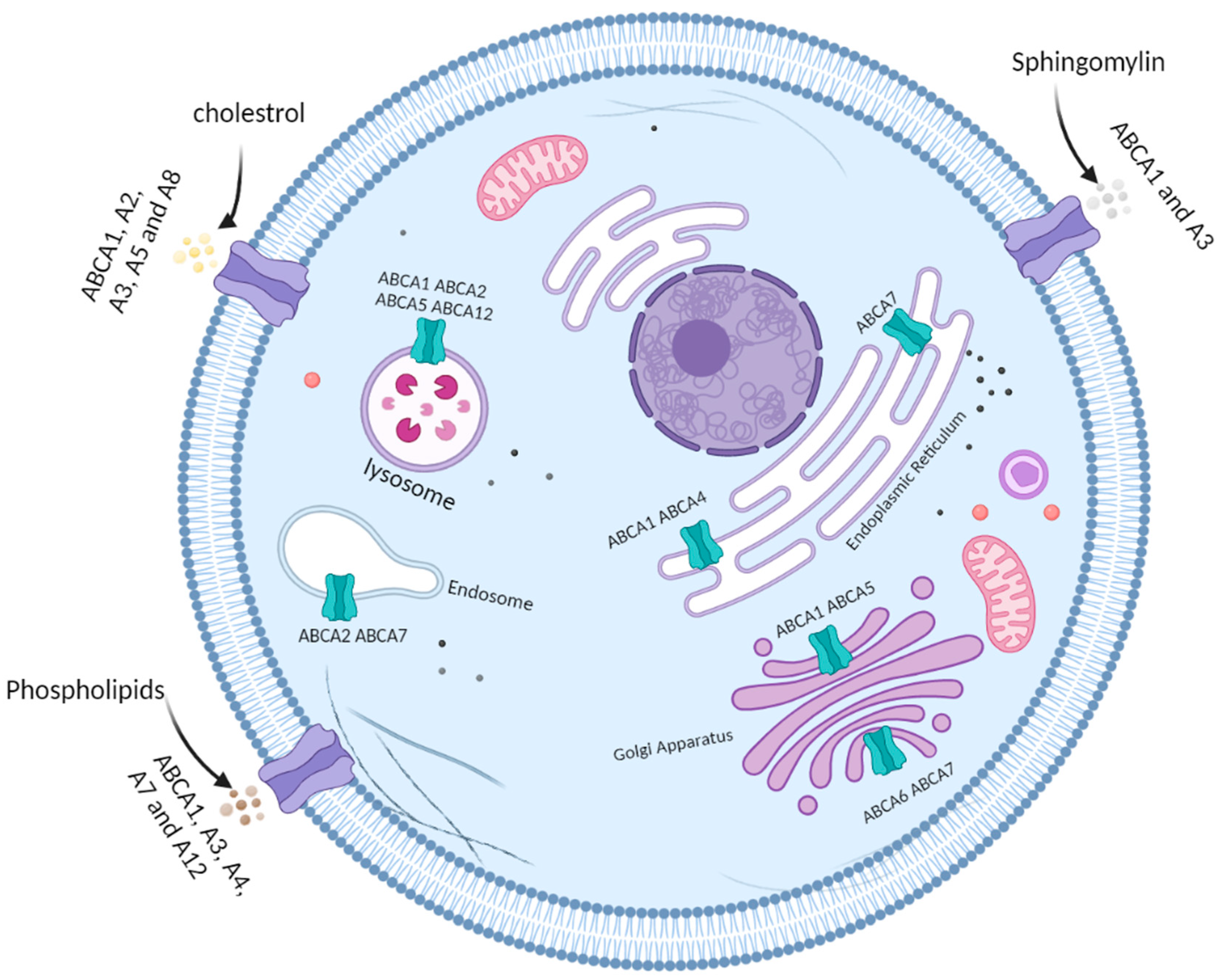
3. ATP-Binding Cassette Subfamily A
4. The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer
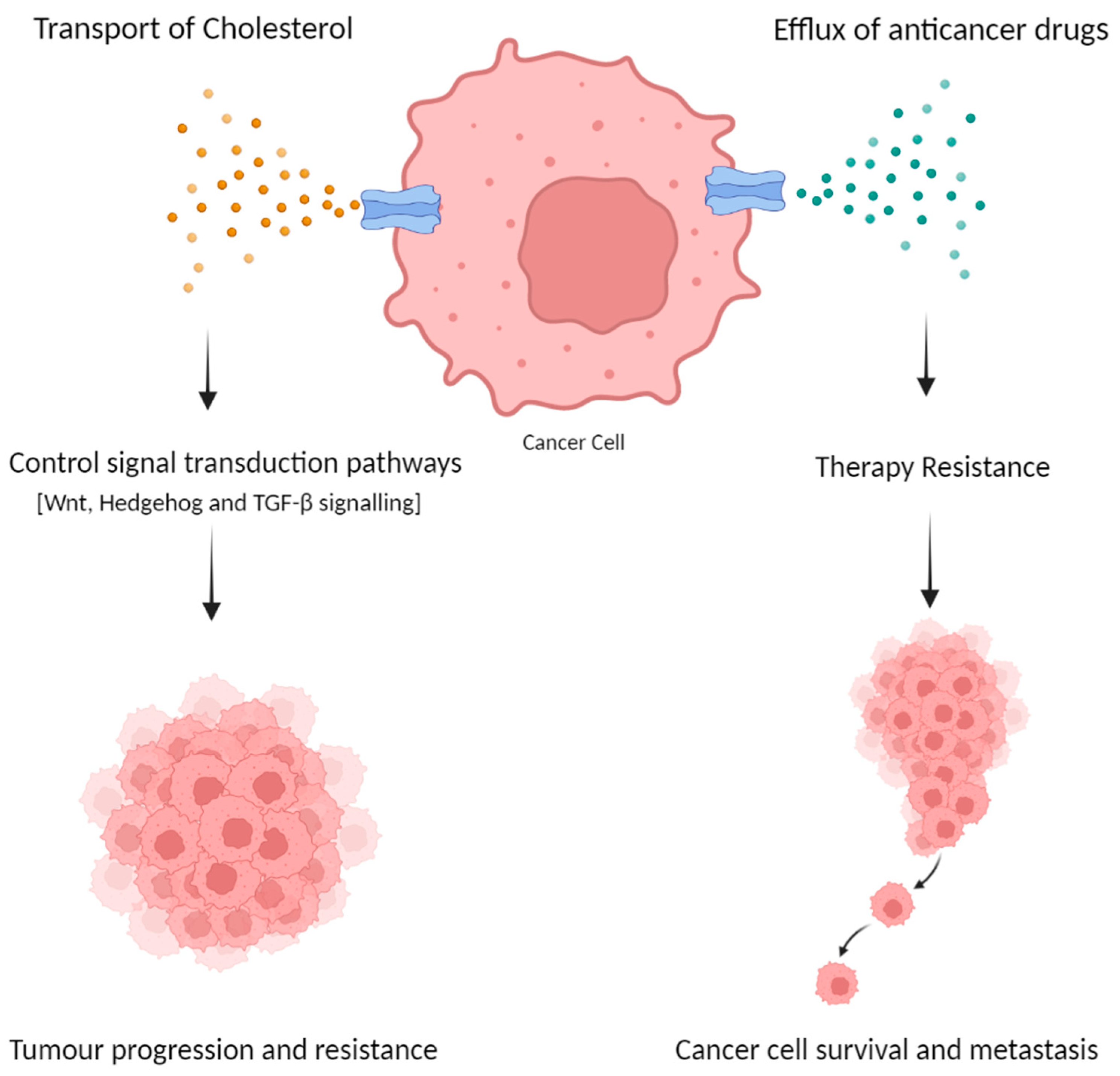
4.1. The Role of ABCA Subfamily in Colorectal Cancer Progression
4.2. Therapy Resistance
| Transporter | Cellular Localization (*) | Lipophilic Substrate (**) | Possible Role in CRC |
|---|---|---|---|
| ABCA1 | Plasma membrane, lysosome, endoplasmic reticulum, Golgi apparatus | Cholesterol Phospholipids Sphingomyelin | Therapy resistance [49] Tumour progression [47] |
| ABCA2 | Plasma membrane, lysosome, endosome | Cholesterol | Therapy resistance [49] |
| ABCA3 | Plasma membrane | Cholesterol Phospholipids Sphingomyelin | - |
| ABCA4 | Plasma membrane, endoplasmic reticulum | Phospholipids | - |
| ABCA5 | Plasma membrane, lysosome, Golgi apparatus | Cholesterol | Therapy resistance [49] Tumour progression [43] |
| ABCA6 | Golgi apparatus | - | - |
| ABCA7 | Plasma membrane, endosome, lysosome, endoplasmic reticulum, Golgi apparatus | Phospholipids | - |
| ABCA8 | Plasma membrane | Cholesterol | - |
| ABCA9 | Plasma membrane | - | - |
| ABCA10 | Plasma membrane | - | - |
| ABCA12 | Plasma membrane, lysosome | Phospholipids | - |
| ABCA13 | Plasma membrane | - | - |
5. Clinical Implications and Future Directions
6. Conclusions
Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sun, W.; Zhou, Y.; Li, P.; Chen, F.; Chen, H.; Xia, D.; Xu, E.; Lai, M.; Wu, Y.; et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018, 37, 173–187. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef]
- Buccafusca, G.; Proserpio, I.; Tralongo, A.C.; Giuliano, S.R.; Tralongo, P. Early colorectal cancer: Diagnosis, treatment and survivorship care. Crit. Rev. Oncol. 2019, 136, 20–30. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef]
- Rizzo, A.; Nannini, M.; Novelli, M.; Ricci, A.D.; Di Scioscio, V.; Pantaleo, M.A. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936932. [Google Scholar] [CrossRef]
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 52–60. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2020, 218, e20201606. [Google Scholar] [CrossRef] [PubMed]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Nedelcu, D.; Watanabe, M.; Jao, C.; Kim, Y.; Liu, J.; Salic, A. Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell 2016, 166, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Trouillas, P.; Gašparović, A.; Gazzano, E.; Assaraf, Y.G.; Riganti, C. Phospholipids and cholesterol: Inducers of cancer multidrug resistance and therapeutic targets. Drug Resist. Updat. 2020, 49, 100670. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, L.; Zhu, B.; Li, Y. Lipidome in colorectal cancer. Oncotarget 2016, 7, 33429–33439. [Google Scholar] [CrossRef]
- Bayerdörffer, E.; Mannes, G.A.; Richter, W.O.; Ochsenkühn, T.; Seeholzer, G.; Köpcke, W.; Wiebecke, B.; Paumgartner, G. Decreased High-Density Lipoprotein Cholesterol and Increased Low-Density Cholesterol Levels in Patients with Colorectal Adenomas. Ann. Intern. Med. 1993, 118, 481. [Google Scholar] [CrossRef]
- Van Duijnhoven, F.J.B.; Bueno-De-Mesquita, H.B.; Calligaro, M.; Jenab, M.; Pischon, T.; Jansen, E.H.J.M.; Frohlich, J.; Ayyobi, A.; Overvad, K.; Toft-Petersen, A.P.; et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011, 60, 1094–1102. [Google Scholar] [CrossRef]
- Kurabe, N.; Hayasaka, T.; Ogawa, M.; Masaki, N.; Ide, Y.; Waki, M.; Nakamura, T.; Kurachi, K.; Kahyo, T.; Shinmura, K.; et al. Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 2013, 104, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Mjabri, B.; Boucrot, P.; Aubry, J. The 1-0-octadecyl-2-0-methyl-sn-glycero-3-phosphocholine causes a differential incorporation of hexadecanol into neutral ether ester glycerolipids of 2 variant cell lines of rat colon carcinoma. Arch. Int. Physiol. Biochim. Biophys. 1992, 100, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Pakiet, A.; Czumaj, A.; Kaczynski, Z.; Liakh, I.; Kobiela, J.; Perdyan, A.; Adrych, K.; Makarewicz, W.; Sledzinski, T. Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study. J. Clin. Med. 2020, 9, 1095. [Google Scholar] [CrossRef]
- Hofmanová, J.; Slavík, J.; Ovesná, P.; Tylichová, Z.; Dušek, L.; Straková, N.; Vaculová, A.H.; Ciganek, M.; Kala, Z.; Jíra, M.; et al. Phospholipid profiling enables to discriminate tumor- and non-tumor-derived human colon epithelial cells: Phospholipidome similarities and differences in colon cancer cell lines and in patient-derived cell samples. PLoS ONE 2020, 15, e0228010. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Viturro, E. The ABCA subfamily—Gene and protein structures, functions and associated hereditary diseases. Pflügers Arch.-Eur. J. Physiol. 2006, 453, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Beers, M.F.; Hawkins, A.; Shuman, H.; Zhao, M.; Newitt, J.L.; Maguire, J.A.; Ding, W.; Mulugeta, S. A novel conserved targeting motif found in ABCA transporters mediates trafficking to early post-Golgi compartments. J. Lipid Res. 2011, 52, 1471–1482. [Google Scholar] [CrossRef]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef]
- Gabbi, C.; Warner, M.; Gustafsson, J. Action mechanisms of Liver X Receptors. Biochem. Biophys. Res. Commun. 2014, 446, 647–650. [Google Scholar] [CrossRef]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein Sensors for Membrane Sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef]
- Martinez, L.O.; Agerholm-Larsen, B.; Wang, N.; Chen, W.; Tall, A.R. Phosphorylation of a Pest Sequence in ABCA1 Promotes Calpain Degradation and Is Reversed by ApoA-I. J. Biol. Chem. 2003, 278, 37368–37374. [Google Scholar] [CrossRef]
- Lu, R.; Tsuboi, T.; Okumura-Noji, K.; Iwamoto, N.; Yokoyama, S. Caveolin-1 facilitates internalization and degradation of ABCA1 and probucol oxidative products interfere with this reaction to increase HDL biogenesis. Atherosclerosis 2016, 253, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.uniprot.org (accessed on 1 September 2022).
- Neumann, J.; Rose-Sperling, D.; Hellmich, U.A. Diverse relations between ABC transporters and lipids: An overview. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Quazi, F.; Molday, R.S. Differential Phospholipid Substrates and Directional Transport by ATP-binding Cassette Proteins ABCA1, ABCA7, and ABCA4 and Disease-causing Mutants. J. Biol. Chem. 2013, 288, 34414–34426. [Google Scholar] [CrossRef] [PubMed]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e4. [Google Scholar] [CrossRef]
- Kim, B.-H.; Oh, H.-K.; Kim, D.-W.; Kang, S.-B.; Choi, Y.; Shin, E. Clinical Implications of Cancer Stem Cell Markers and ABC Transporters as a Predictor of Prognosis in Colorectal Cancer Patients. Anticancer Res. 2020, 40, 4481–4489. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.-X.; Xu, Y.-M.; Zhang, J.-Z.; Rong, S.-D.; Qin, Y.-Q.; Fang, J. IRE1α-targeting downregulates ABC transporters and overcomes drug resistance of colon cancer cells. Cancer Lett. 2020, 476, 67–74. [Google Scholar] [CrossRef]
- Hu, H.; Wang, M.; Guan, X.; Yuan, Z.; Liu, Z.; Zou, C.; Wang, G.; Gao, X.; Wang, X. Loss of ABCB4 attenuates the caspase-dependent apoptosis regulating resistance to 5-Fu in colorectal cancer. Biosci. Rep. 2018, 38, BSR20171428. [Google Scholar] [CrossRef]
- Katoh, S.-Y.; Ueno, M.; Takakura, N. Involvement of MDR1 Function in Proliferation of Tumour Cells. J. Biochem. 2007, 143, 517–524. [Google Scholar] [CrossRef]
- Smyth, M.J.; Krasovskis, E.; Sutton, V.R.; Johnstone, R.W. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 7024–7029. [Google Scholar] [CrossRef]
- Muriithi, W.; Macharia, L.W.; Heming, E.A.C.P.; Echevarria, J.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC transporters and the hallmarks of cancer: Roles in cancer aggressiveness beyond multidrug resistance. Cancer Biol. Med. 2020, 17, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Kamoi, M.; Watanabe, Y.; Suzuki, H.; Hori, S.; Terasaki, A. Correlation of Induction of ATP Binding Cassette Transporter A5 (ABCA5) and ABCB1 mRNAs with Differentiation State of Human Colon Tumor. Biol. Pharm. Bull. 2007, 30, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Sekiya, S.; Ohigashi, M.; Takenaka, C.; Tamura, K.; Nada, S.; Nishi, T.; Yamamoto, A.; Yamaguchi, A. ABCA5 Resides in Lysosomes, and ABCA5 Knockout Mice Develop Lysosomal Disease-Like Symptoms. Mol. Cell. Biol. 2005, 25, 4138–4149. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Okuda, S.; Taniguchi, H. Enhancement by thyroxine of experimental carcinogenesis induced in rat colon by azoxymethane. Int. J. Cancer 1992, 50, 974–976. [Google Scholar] [CrossRef]
- Ray, A.G.; Choudhury, K.R.; Chakraborty, S.; Chakravarty, D.; Chander, V.; Jana, B.; Siddiqui, K.N.; Bandyopadhyay, A. Novel Mechanism of Cholesterol Transport by ABCA5 in Macrophages and Its Role in Dyslipidemia. J. Mol. Biol. 2020, 432, 4922–4941. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Portolés, C.; Feliu, J.; Reglero, G.; de Molina, A.R. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BETinhibitor apabetalone. Mol. Oncol. 2018, 12, 1735–1752. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Farabaugh, S.M.; Ford, H.L. Epithelial-Mesenchymal Transition in Cancer: Parallels Between Normal Development and Tumor Progression. J. Mammary Gland. Biol. Neoplasia 2010, 15, 117–134. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Goel, A. Oligomeric proanthocyanidins (OPCs) from grape seed extract suppress the activity of ABC transporters in overcoming chemoresistance in colorectal cancer cells. Carcinogenesis 2019, 40, 412–421. [Google Scholar] [CrossRef]
- Duz, M.B.; Karatas, O.F. Expression profile of stem cell markers and ABC transporters in 5-fluorouracil resistant Hep-2 cells. Mol. Biol. Rep. 2020, 47, 5431–5438. [Google Scholar] [CrossRef]
- Abdulla, N.; Vincent, C.T.; Kaur, M. Mechanistic Insights Delineating the Role of Cholesterol in Epithelial Mesenchymal Transition and Drug Resistance in Cancer. Front. Cell Dev. Biol. 2021, 9, 728325. [Google Scholar] [CrossRef]
- Dharmapuri, G.; Doneti, R.; Philip, G.H.; Kalle, A.M. Celecoxib sensitizes imatinib-resistant K562 cells to imatinib by inhibiting MRP1–5, ABCA2 and ABCG2 transporters via Wnt and Ras signaling pathways. Leuk. Res. 2015, 39, 696–701. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alketbi, L.; Al-Ali, A.; Talaat, I.M.; Hamid, Q.; Bajbouj, K. The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance. Int. J. Mol. Sci. 2023, 24, 1344. https://doi.org/10.3390/ijms24021344
Alketbi L, Al-Ali A, Talaat IM, Hamid Q, Bajbouj K. The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance. International Journal of Molecular Sciences. 2023; 24(2):1344. https://doi.org/10.3390/ijms24021344
Chicago/Turabian StyleAlketbi, Latifa, Abeer Al-Ali, Iman M. Talaat, Qutayba Hamid, and Khuloud Bajbouj. 2023. "The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance" International Journal of Molecular Sciences 24, no. 2: 1344. https://doi.org/10.3390/ijms24021344
APA StyleAlketbi, L., Al-Ali, A., Talaat, I. M., Hamid, Q., & Bajbouj, K. (2023). The Role of ATP-Binding Cassette Subfamily A in Colorectal Cancer Progression and Resistance. International Journal of Molecular Sciences, 24(2), 1344. https://doi.org/10.3390/ijms24021344





_Talaat.jpg)


