Regular Exercise in Drosophila Prevents Age-Related Cardiac Dysfunction Caused by High Fat and Heart-Specific Knockdown of skd
Abstract
1. Introduction
2. Results
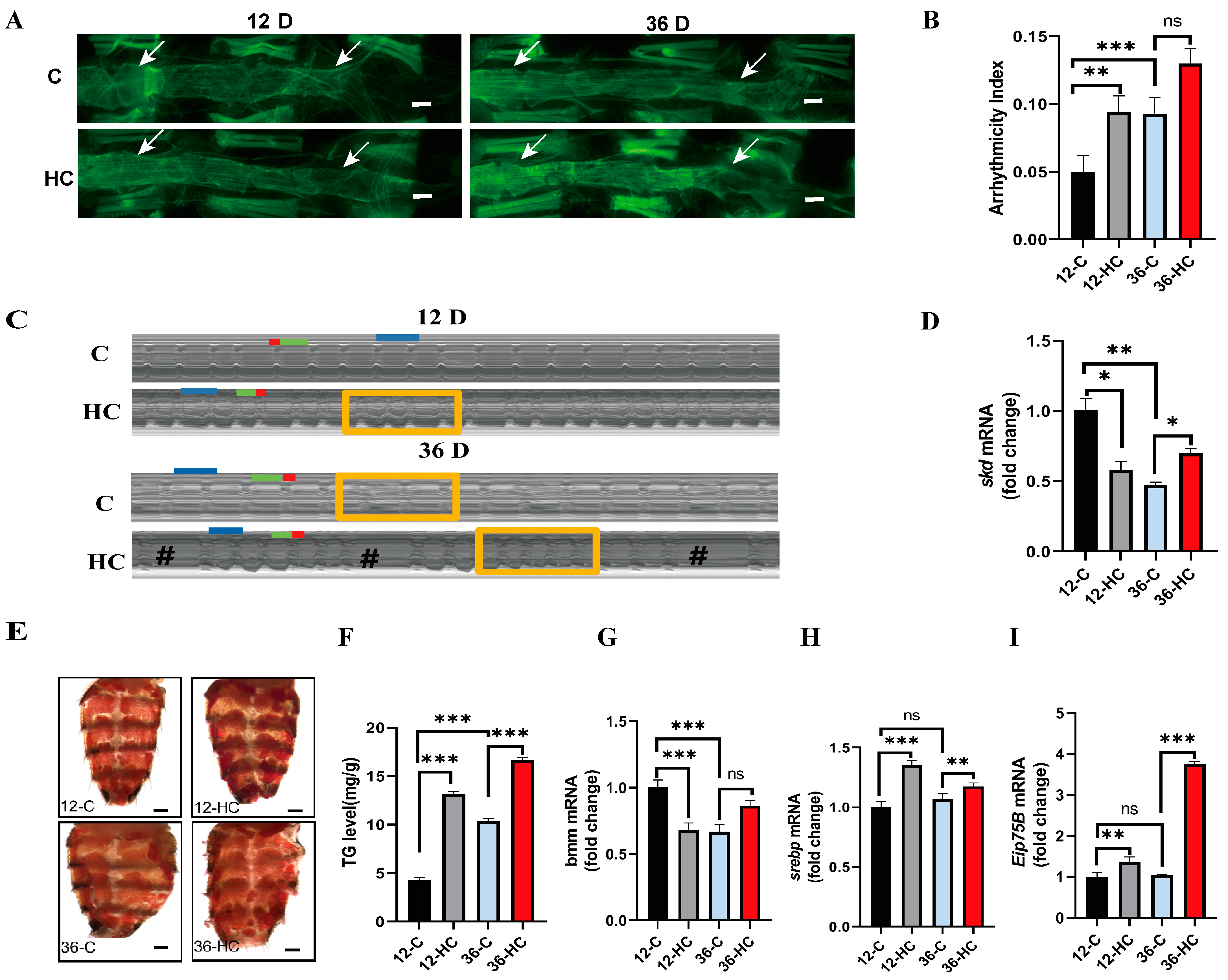
2.1. High-Fat Diet Promotes Aging-Related Cardiac Dysfunction and Systemic Lipid Accumulation
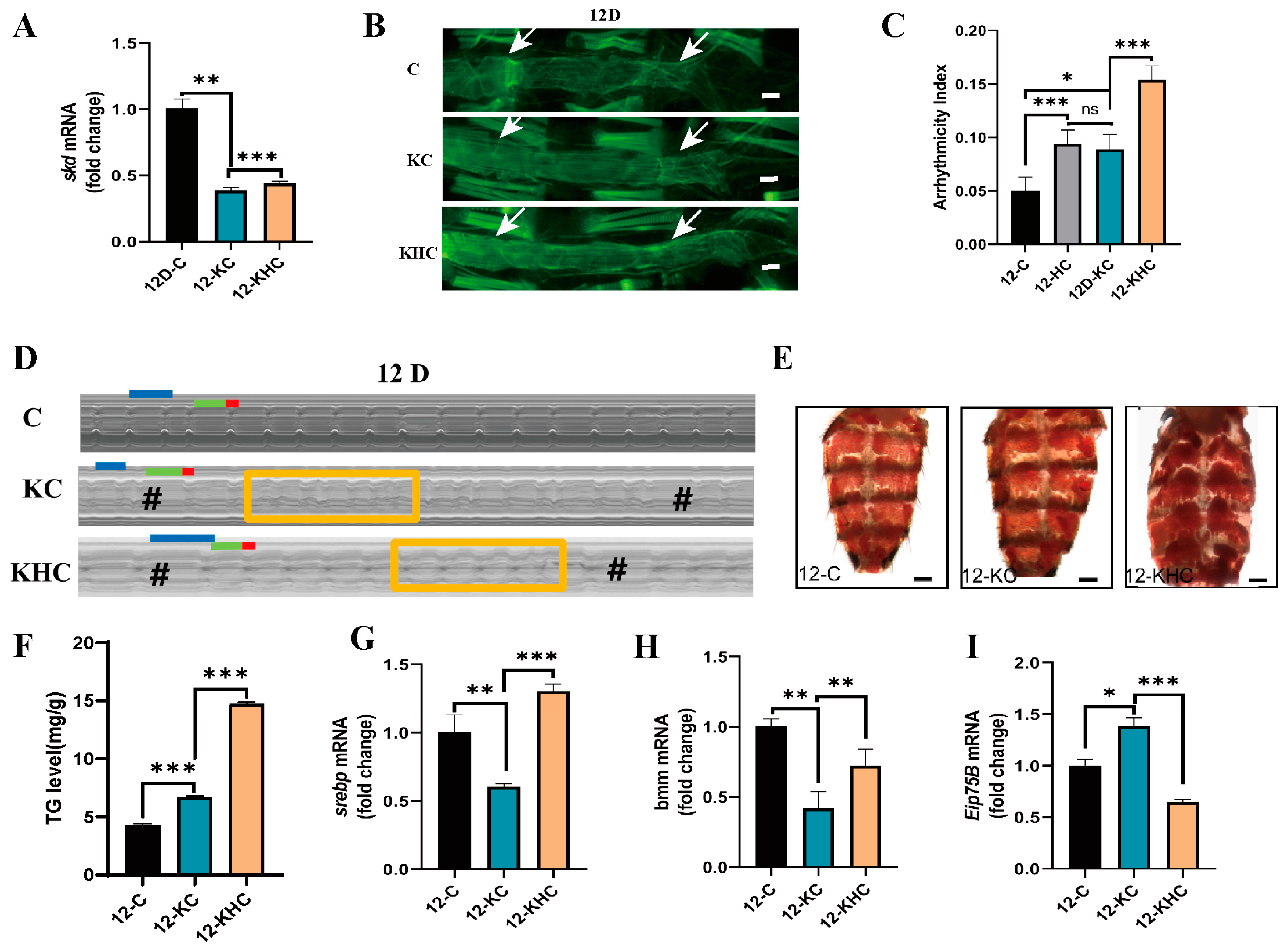
2.2. Cardiac Skd-Specific RNAi and/or HFD Promotes Age-Related Cardiac Insufficiency and Increased Systemic Lipids
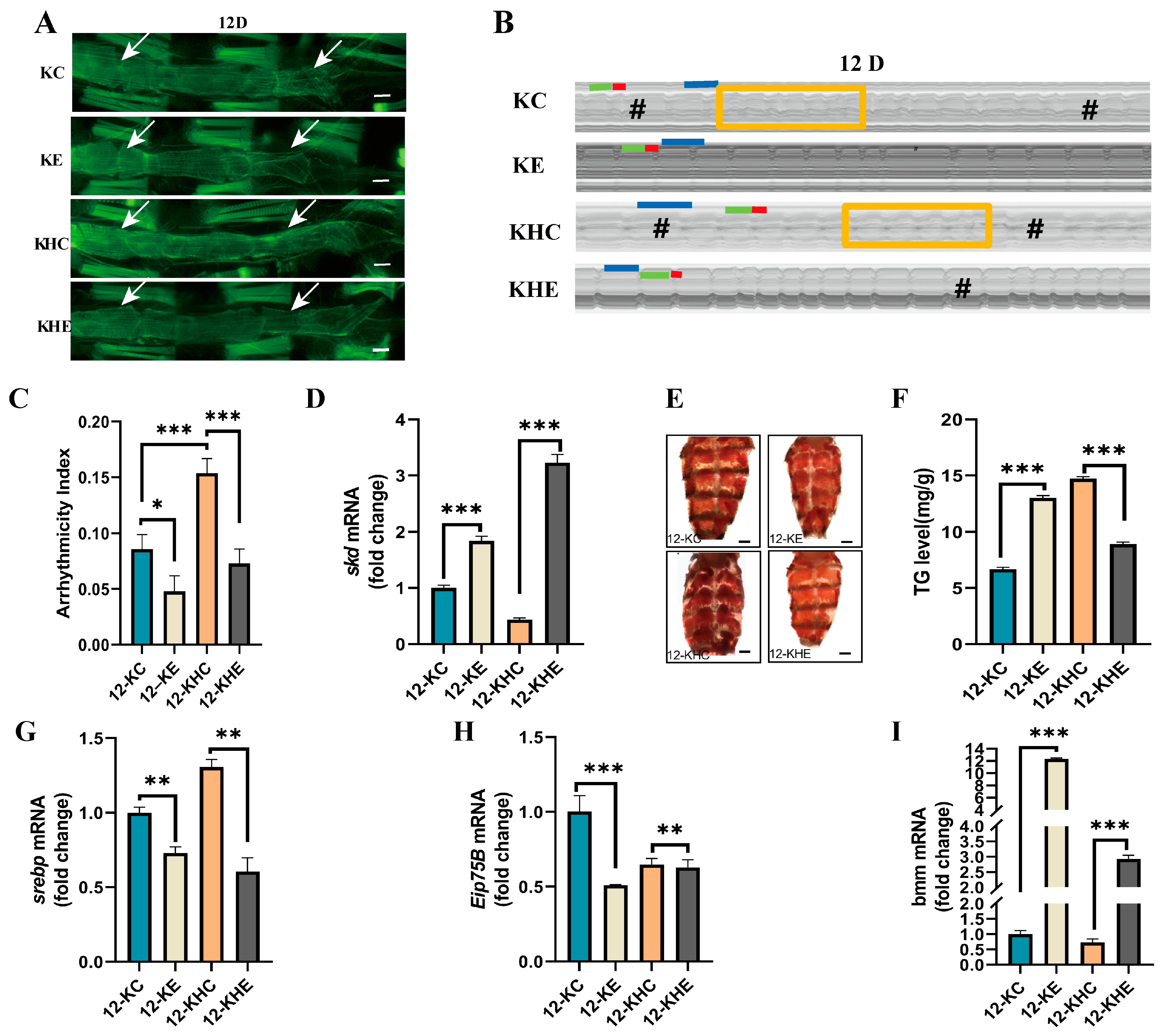
2.3. Regular Exercise Resists HFD-Induced Decline in Cardiac Skd Expression, Age-Related Cardiac Abnormalities, and Increased Systemic Lipid Accumulation
2.4. Regular Exercise Improves Age-Related Cardiac Dysfunction and Systemic Lipid Increase Caused by Cardiac Skd-Specific RNAi
3. Discussion
4. Materials and Methods
4.1. Fly Stocks and Groups
4.2. Diet Preparation
4.3. Motion Devices and Protocols
4.4. Real-Time Quantitative PCR (qPCR)
4.5. Analysis of Cardiac Function
4.6. Oil Red O (ORO)
4.7. Phalloidin
4.8. Triglyceride Determination
4.9. Statistical Analysis
4.10. Institutional Review Board Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | arrhythmia index |
| Bmm | brummer |
| DI | diastolic intervals |
| F-actin | filamentous actin |
| HP | heart period |
| HR | heart rate |
| HFD | high-fat diet |
| ORO | Oil red O |
| skd | Skuld |
| SI | systolic intervals |
| TG | triglycerides |
References
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Liao, S.; Amcoff, M.; Nassel, D.R. Impact of high-fat diet on lifespan, metabolism, fecundity and behavioral senescence in Drosophila. Insect Biochem. Mol. Biol. 2021, 133, 103495. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Birse, R.T.; Choi, J.; Reardon, K.; Rodriguez, J.; Graham, S.; Diop, S.; Ocorr, K.; Bodmer, R.; Oldham, S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010, 12, 533–544. [Google Scholar] [CrossRef]
- Grueter, C.E.; van Rooij, E.; Johnson, B.A.; DeLeon, S.M.; Sutherland, L.B.; Qi, X.; Gautron, L.; Elmquist, J.K.; Bassel-Duby, R.; Olson, E.N. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 2012, 149, 671–683. [Google Scholar] [CrossRef]
- Balaban, R.S.; Kantor, H.L.; Katz, L.A.; Briggs, R.W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 1986, 232, 1121–1123. [Google Scholar] [CrossRef]
- Baskin, K.K.; Grueter, C.E.; Kusminski, C.M.; Holland, W.L.; Bookout, A.L.; Satapati, S.; Kong, Y.M.; Burgess, S.C.; Malloy, C.R.; Scherer, P.E.; et al. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol. Med. 2014, 6, 1610–1621. [Google Scholar] [CrossRef]
- Zhou, W.; Cai, H.; Li, J.; Xu, H.; Wang, X.; Men, H.; Zheng, Y.; Cai, L. Potential roles of mediator Complex Subunit 13 in Cardiac Diseases. Int. J. Biol. Sci. 2021, 17, 328–338. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, H.; Wang, W.; Lim, H.Y. Cardiac Snail family of transcription factors directs systemic lipid metabolism in Drosophila. PLoS Genet. 2019, 15, e1008487. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Ito, M.; Yuan, C.X.; Malik, S.; Gu, W.; Fondell, J.D.; Yamamura, S.; Fu, Z.Y.; Zhang, X.; Qin, J.; Roeder, R.G. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 1999, 3, 361–370. [Google Scholar] [CrossRef]
- Janody, F.; Martirosyan, Z.; Benlali, A.; Treisman, J.E. Two subunits of the Drosophila mediator complex act together to control cell affinity. Development 2003, 130, 3691–3701. [Google Scholar] [CrossRef]
- Lee, J.H.; Bassel-Duby, R.; Olson, E.N. Heart- and muscle-derived signaling system dependent on MED13 and Wingless controls obesity in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, 9491–9496. [Google Scholar] [CrossRef]
- Lum-Naihe, K.; Toedebusch, R.; Mahmood, A.; Bajwa, J.; Carmack, T.; Kumar, S.A.; Ardhanari, S.; DeMarco, V.G.; Emter, C.A.; Pulakat, L. Cardiovascular disease progression in female Zucker Diabetic Fatty rats occurs via unique mechanisms compared to males. Sci. Rep. 2017, 7, 17823. [Google Scholar] [CrossRef]
- Yang, F.; Vought, B.W.; Satterlee, J.S.; Walker, A.K.; Jim Sun, Z.Y.; Watts, J.L.; DeBeaumont, R.; Saito, R.M.; Hyberts, S.G.; Yang, S.; et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 2006, 442, 700–704. [Google Scholar] [CrossRef]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef]
- Diop, S.B.; Bisharat-Kernizan, J.; Birse, R.T.; Oldham, S.; Ocorr, K.; Bodmer, R. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 2015, 10, 1572–1584. [Google Scholar] [CrossRef]
- Guo, S.S.; Zeller, C.; Chumlea, W.C.; Siervogel, R.M. Aging, body composition, and lifestyle: The Fels Longitudinal Study. Am. J. Clin. Nutr. 1999, 70, 405–411. [Google Scholar] [CrossRef]
- Lutz, W.; Sanderson, W.; Scherbov, S. The coming acceleration of global population ageing. Nature 2008, 451, 716–719. [Google Scholar] [CrossRef]
- Juppi, H.K.; Sipila, S.; Fachada, V.; Hyvarinen, M.; Cronin, N.; Aukee, P.; Karppinen, J.E.; Selanne, H.; Kujala, U.M.; Kovanen, V.; et al. Total and regional body adiposity increases during menopause-evidence from a follow-up study. Aging Cell 2022, 21, e13621. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Li, Y. Adipose Tissue Aging and Metabolic Disorder, and the Impact of Nutritional Interventions. Nutrients 2022, 14, 3134. [Google Scholar] [CrossRef]
- Barretti, D.L.; Magalhaes Fde, C.; Fernandes, T.; do Carmo, E.C.; Rosa, K.T.; Irigoyen, M.C.; Negrao, C.E.; Oliveira, E.M. Effects of aerobic exercise training on cardiac renin-angiotensin system in an obese Zucker rat strain. PLoS ONE 2012, 7, e46114. [Google Scholar] [CrossRef]
- Fernandes, T.; Barauna, V.G.; Negrao, C.E.; Phillips, M.I.; Oliveira, E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef]
- Silveira, A.C.; Fernandes, T.; Soci, U.P.R.; Gomes, J.L.P.; Barretti, D.L.; Mota, G.G.F.; Negrao, C.E.; Oliveira, E.M. Exercise Training Restores Cardiac MicroRNA-1 and MicroRNA-29c to Nonpathological Levels in Obese Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1549014. [Google Scholar] [CrossRef]
- Boulghobra, D.; Coste, F.; Geny, B.; Reboul, C. Exercise training protects the heart against ischemia-reperfusion injury: A central role for mitochondria? Free Radic. Biol. Med. 2020, 152, 395–410. [Google Scholar] [CrossRef]
- Strasser, B. Physical activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2013, 1281, 141–159. [Google Scholar] [CrossRef]
- Wen, D.T.; Zheng, L.; Yang, F.; Li, H.Z.; Hou, W.Q. Endurance exercise prevents high-fat-diet induced heart and mobility premature aging and dsir2 expression decline in aging Drosophila. Oncotarget 2018, 9, 7298–7311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, Q.F.; Ni, L.; Wang, H.; Ruan, X.C.; Wu, X.S. Lifetime regular exercise affects the incident of different arrhythmias and improves organismal health in aging female Drosophila melanogaster. Biogerontology 2017, 18, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.T.; Zheng, L.; Lu, K.; Hou, W.Q. Activation of cardiac Nmnat/NAD+/SIR2 pathways mediates endurance exercise resistance to lipotoxic cardiomyopathy in aging Drosophila. J. Exp. Biol. 2021, 224, jeb242425. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zheng, L.; Li, Q.F.; Wang, W.L.; Peng, W.D.; Zhou, M. Exercise-Training Regulates Apolipoprotein B in Drosophila to Improve HFD-Mediated Cardiac Function Damage and Low Exercise Capacity. Front. Physiol. 2021, 12, 650959. [Google Scholar] [CrossRef]
- Huang, T.; Jian, X.; Liu, J.; Zheng, L.; Li, F.Q.; Meng, D.; Wang, T.; Zhang, S.; Liu, Y.; Guan, Z.; et al. Exercise and/or Cold Exposure Alters the Gene Expression Profile in the Fat Body and Changes the Heart Function in Drosophila. Front. Endocrinol. 2022, 13, 790414. [Google Scholar] [CrossRef]
- Piazza, N.; Gosangi, B.; Devilla, S.; Arking, R.; Wessells, R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE 2009, 4, e5886. [Google Scholar] [CrossRef]
- Sujkowski, A.; Wessells, R. Using Drosophila to Understand Biochemical and Behavioral Responses to Exercise. Exerc. Sport Sci. Rev. 2018, 46, 112–120. [Google Scholar] [CrossRef]
- Wen, D.T.; Zheng, L.; Li, J.X.; Lu, K.; Hou, W.Q. The activation of cardiac dSir2-related pathways mediates physical exercise resistance to heart aging in old Drosophila. Aging 2019, 11, 7274–7293. [Google Scholar] [CrossRef]
- Alfa, R.W.; Kim, S.K. Using Drosophila to discover mechanisms underlying type 2 diabetes. Dis. Model. Mech. 2016, 9, 365–376. [Google Scholar] [CrossRef]
- Souidi, A.; Jagla, K. Drosophila Heart as a Model for Cardiac Development and Diseases. Cells 2021, 10, 3078. [Google Scholar] [CrossRef]
- Molina, M.R.; Cripps, R.M. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech. Dev. 2001, 109, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, H.; Gutierrez Cortes, N.; Wu, D.; Wang, P.; Zhang, J.; Mattison, J.A.; Smith, E.; Bettcher, L.F.; Wang, M.; et al. Increased Drp1 Acetylation by Lipid Overload Induces Cardiomyocyte Death and Heart Dysfunction. Circ. Res. 2020, 126, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Diop, S.B.; Birse, R.T.; Bodmer, R. High Fat Diet Feeding and High Throughput Triacylglyceride Assay in Drosophila Melanogaster. J. Vis. Exp. 2017, e56029. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Schulz, R.A. Heart development in Drosophila. Semin. Cell Dev. Biol. 2007, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R.; Venkatesh, T.V. Heart development in Drosophila and vertebrates: Conservation of molecular mechanisms. Dev. Genet. 1998, 22, 181–186. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.J.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. Precise Genome Editing of Drosophila with CRISPR RNA-Guided Cas9. Methods Mol. Biol. 2015, 1311, 335–348. [Google Scholar] [PubMed]
- Blice-Baum, A.C.; Guida, M.C.; Hartley, P.S.; Adams, P.D.; Bodmer, R.; Cammarato, A. As time flies by: Investigating cardiac aging in the short-lived Drosophila model. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1831–1844. [Google Scholar] [CrossRef]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends Endocrinol. Metab. 2011, 22, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.C.; Hwang, S.J.; Larson, M.G.; Lichtman, J.H.; Parikh, N.I.; Vasan, R.S.; Levy, D.; Fox, C.S. Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am. J. Kidney Dis. 2008, 52, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hirose, H.; Ohneda, M.; Johnson, J.H.; McGarry, J.D.; Unger, R.H. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: Impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA 1994, 91, 10878–10882. [Google Scholar] [CrossRef]
- Borradaile, N.M.; Schaffer, J.E. Lipotoxicity in the heart. Curr. Hypertens. Rep. 2005, 7, 412–417. [Google Scholar] [CrossRef]
- Unger, R.H.; Scherer, P.E. Gluttony, sloth and the metabolic syndrome: A roadmap to lipotoxicity. Trends Endocrinol. Metab. 2010, 21, 345–352. [Google Scholar] [CrossRef]
- Wiersma, M.; van Marion, D.M.S.; Wust, R.C.I.; Houtkooper, R.H.; Zhang, D.; Groot, N.M.S.; Henning, R.H.; Brundel, B. Mitochondrial Dysfunction Underlies Cardiomyocyte Remodeling in Experimental and Clinical Atrial Fibrillation. Cells 2019, 8, 1202. [Google Scholar] [CrossRef]
- Vaquero, M.; Calvo, D.; Jalife, J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm 2008, 5, 872–879. [Google Scholar] [CrossRef]
- Emelyanova, L.; Boukatina, A.; Myers, C.; Oyarzo, J.; Lustgarten, J.; Shi, Y.; Jahangir, A. High calories but not fat content of lard-based diet contribute to impaired mitochondrial oxidative phosphorylation in C57BL/6J mice heart. PLoS ONE 2019, 14, e0217045. [Google Scholar] [CrossRef]
- Jurado-Ruiz, E.; Alvarez-Amor, L.; Varela, L.M.; Berna, G.; Parra-Camacho, M.S.; Oliveras-Lopez, M.J.; Martinez-Force, E.; Rojas, A.; Hmadcha, A.; Soria, B.; et al. Extra virgin olive oil diet intervention improves insulin resistance and islet performance in diet-induced diabetes in mice. Sci. Rep. 2019, 9, 11311. [Google Scholar] [CrossRef]
- Perez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Galvez, B.G. ‘Adipaging’: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Gower, B.A.; Kane, B.L. Age Related Shift in Visceral Fat. Int. J. Body Compos. Res. 2010, 8, 103–108. [Google Scholar] [PubMed]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef]
- Harmancey, R.; Wilson, C.R.; Taegtmeyer, H. Adaptation and maladaptation of the heart in obesity. Hypertension 2008, 52, 181–187. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, H.; Zheng, L.; Yang, F.; Li, H.Z.; Li, J.X.; Cheng, D.; Lu, K.; Liu, Y. Effects of Modest Hypoxia and Exercise on Cardiac Function, Sleep-Activity, Negative Geotaxis Behavior of Aged Female Drosophila. Front. Physiol. 2019, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Amoasii, L.; Holland, W.; Sanchez-Ortiz, E.; Baskin, K.K.; Pearson, M.; Burgess, S.C.; Nelson, B.R.; Bassel-Duby, R.; Olson, E.N. A MED13-dependent skeletal muscle gene program controls systemic glucose homeostasis and hepatic metabolism. Genes Dev. 2016, 30, 434–446. [Google Scholar] [CrossRef]
- Minerath, R.A.; Dewey, C.M.; Hall, D.D.; Grueter, C.E. Regulation of cardiac transcription by thyroid hormone and Med13. J. Mol. Cell. Cardiol. 2019, 129, 27–38. [Google Scholar] [CrossRef]
- Lompre, A.M.; Lambert, F.; Lakatta, E.G.; Schwartz, K. Expression of sarcoplasmic reticulum Ca(2+)-ATPase and calsequestrin genes in rat heart during ontogenic development and aging. Circ. Res. 1991, 69, 1380–1388. [Google Scholar] [CrossRef]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Baris, O.R.; Ederer, S.; Neuhaus, J.F.; von Kleist-Retzow, J.C.; Wunderlich, C.M.; Pal, M.; Wunderlich, F.T.; Peeva, V.; Zsurka, G.; Kunz, W.S.; et al. Mosaic Deficiency in Mitochondrial Oxidative Metabolism Promotes Cardiac Arrhythmia during Aging. Cell Metab. 2015, 21, 667–677. [Google Scholar] [CrossRef]
- Joseph, L.C.; Avula, U.M.R.; Wan, E.Y.; Reyes, M.V.; Lakkadi, K.R.; Subramanyam, P.; Nakanishi, K.; Homma, S.; Muchir, A.; Pajvani, U.B.; et al. Dietary Saturated Fat Promotes Arrhythmia by Activating NOX2 (NADPH Oxidase 2). Circ. Arrhythmia Electrophysiol. 2019, 12, e007573. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.C.; Reyes, M.V.; Homan, E.A.; Gowen, B.; Avula, U.M.R.; Goulbourne, C.N.; Wan, E.Y.; Elrod, J.W.; Morrow, J.P. The mitochondrial calcium uniporter promotes arrhythmias caused by high-fat diet. Sci. Rep. 2021, 11, 17808. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef]
- Abed, H.S.; Samuel, C.S.; Lau, D.H.; Kelly, D.J.; Royce, S.G.; Alasady, M.; Mahajan, R.; Kuklik, P.; Zhang, Y.; Brooks, A.G.; et al. Obesity results in progressive atrial structural and electrical remodeling: Implications for atrial fibrillation. Heart Rhythm 2013, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zheng, J.; Liu, X.F.; Feng, Z.L.; Zhang, X.P.; Cao, L.L.; Zhou, Z.P. Exercise improved lipid metabolism and insulin sensitivity in rats fed a high-fat diet by regulating glucose transporter 4 (GLUT4) and musclin expression. Braz. J. Med. Biol. Res. 2016, 49, e5129. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Li, Q.F.; Yin, G.; Liu, J.L.; Jan, X.Y.; Huang, T.; Li, A.C.; Zheng, L. Effects of Drosophila melanogaster regular exercise and apolipoprotein B knockdown on abnormal heart rhythm induced by a high-fat diet. PLoS ONE 2022, 17, e0262471. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Elliott, A.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Hendriks, J.M.; Twomey, D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J. Am. Coll. Cardiol. 2015, 66, 985–996. [Google Scholar] [CrossRef]
- Fernandes, T.; Barretti, D.L.; Phillips, M.I.; Menezes Oliveira, E. Exercise training prevents obesity-associated disorders: Role of miRNA-208a and MED13. Mol. Cell. Endocrinol. 2018, 476, 148–154. [Google Scholar] [CrossRef]
- Gronke, S.; Mildner, A.; Fellert, S.; Tennagels, N.; Petry, S.; Muller, G.; Jackle, H.; Kuhnlein, R.P. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005, 1, 323–330. [Google Scholar] [CrossRef]
- Xiao, J.; Xiong, Y.; Yang, L.T.; Wang, J.Q.; Zhou, Z.M.; Dong, L.W.; Shi, X.J.; Zhao, X.; Luo, J.; Song, B.L. POST1/C12ORF49 regulates the SREBP pathway by promoting site-1 protease maturation. Protein Cell 2021, 12, 279–296. [Google Scholar]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a Small Molecule, Betulin, Improves Hyperlipidemia and Insulin Resistance and Reduces Atherosclerotic Plaques. Cell Metab. 2021, 33, 222. [Google Scholar] [CrossRef]
- Khan, D.; Ara, T.; Ravi, V.; Rajagopal, R.; Tandon, H.; Parvathy, J.; Gonzalez, E.A.; Asirvatham-Jeyaraj, N.; Krishna, S.; Mishra, S.; et al. SIRT6 transcriptionally regulates fatty acid transport by suppressing PPARgamma. Cell Rep. 2021, 35, 109190. [Google Scholar] [CrossRef]
- Kadowaki, T. PPAR gamma agonist and antagonist. Nihon Yakurigaku Zasshi 2001, 118, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Riddle, N.C. Drosophila melanogaster, a new model for exercise research. Acta Physiol. 2019, 227, e13352. [Google Scholar] [CrossRef]
- Choma, M.A.; Suter, M.J.; Vakoc, B.J.; Bouma, B.E.; Tearney, G.J. Physiological homology between Drosophila melanogaster and vertebrate cardiovascular systems. Dis. Model. Mech. 2011, 4, 411–420. [Google Scholar] [CrossRef]
- Olivetti, G.; Melissari, M.; Capasso, J.M.; Anversa, P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 1991, 68, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Li, H.; Zheng, L. Drosophila exercise, an emerging model bridging the fields of exercise and aging in human. Front. Cell Dev. Biol. 2022, 10, 966531. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Z.; Sun, C.; Yang, D.; Zhou, Z.; Peng, X.; Zheng, L.; Tang, C. Exercise Intervention Mitigates Pathological Liver Changes in NAFLD Zebrafish by Activating SIRT1/AMPK/NRF2 Signaling. Int. J. Mol. Sci. 2021, 22, 10940. [Google Scholar] [CrossRef] [PubMed]
- Vogler, G.; Ocorr, K. Visualizing the beating heart in Drosophila. J. Vis. Exp. 2009, e1425. [Google Scholar] [CrossRef]
- Fink, M.; Callol-Massot, C.; Chu, A.; Ruiz-Lozano, P.; Izpisua Belmonte, J.C.; Giles, W.; Bodmer, R.; Ocorr, K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 2009, 46, 101–113. [Google Scholar] [CrossRef]

| Dependent Variable | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Heart rate | 7.183 | 1 | 7.183 | 38.747 | 0.000 |
| Heart period | 0.736 | 1 | 0.736 | 56.233 | 0.000 |
| Arrhythmicity index | 0.004 | 1 | 0.004 | 0.856 | 0.357 |
| Diastolic intervals | 0.607 | 1 | 0.607 | 55.378 | 0.000 |
| Systolic interval | 0.006 | 1 | 0.006 | 7.252 | 0.008 |
| TG level | 0.528 | 1 | 0.528 | 4.425 | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; He, S.; Ding, M.; Gu, W.; Wang, T.; Zhang, S.; Feng, J.; Li, Q.; Zheng, L. Regular Exercise in Drosophila Prevents Age-Related Cardiac Dysfunction Caused by High Fat and Heart-Specific Knockdown of skd. Int. J. Mol. Sci. 2023, 24, 1216. https://doi.org/10.3390/ijms24021216
Cao Y, He S, Ding M, Gu W, Wang T, Zhang S, Feng J, Li Q, Zheng L. Regular Exercise in Drosophila Prevents Age-Related Cardiac Dysfunction Caused by High Fat and Heart-Specific Knockdown of skd. International Journal of Molecular Sciences. 2023; 24(2):1216. https://doi.org/10.3390/ijms24021216
Chicago/Turabian StyleCao, Yurou, Shiyi He, Meng Ding, Wenzhi Gu, Tongquan Wang, Shihu Zhang, Jiadong Feng, Qiufang Li, and Lan Zheng. 2023. "Regular Exercise in Drosophila Prevents Age-Related Cardiac Dysfunction Caused by High Fat and Heart-Specific Knockdown of skd" International Journal of Molecular Sciences 24, no. 2: 1216. https://doi.org/10.3390/ijms24021216
APA StyleCao, Y., He, S., Ding, M., Gu, W., Wang, T., Zhang, S., Feng, J., Li, Q., & Zheng, L. (2023). Regular Exercise in Drosophila Prevents Age-Related Cardiac Dysfunction Caused by High Fat and Heart-Specific Knockdown of skd. International Journal of Molecular Sciences, 24(2), 1216. https://doi.org/10.3390/ijms24021216





