Transcriptomic Analysis of Glycosylation and Neuroregulatory Pathways in Rodent Models in Response to Psychedelic Molecules
Abstract
1. Introduction
2. Results
2.1. Differentially Expressed Glycogene Transcripts
2.2. Differentially Expressed Neuro-Regulatory Transcripts
3. Discussion
4. Materials and Methods
4.1. Transcriptomic Data Selection, Processing and Differentially Expressed Genes (DEGs)
4.2. Gene Set Refinement
4.3. Functional Enrichment Analysis, Cellular Process Mapping and Network Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Johnson, M.W.; Hendricks, P.S.; Barrett, F.S.; Griffiths, R.R. Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 2019, 197, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Vizeli, P.; Liechti, M.E. Safety pharmacology of acute MDMA administration in healthy subjects. J. Psychopharmacol. 2017, 31, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Bouso, J.C.; Alcazar-Corcoles, M.A.; Hallak, J.E.C. Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: A systematic review of systematic reviews. Expert. Rev. Clin. Pharmacol. 2018, 11, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Doblin, R.E.; Christiansen, M.; Jerome, L.; Burge, B. The Past and Future of Psychedelic Science: An Introduction to This Issue. J. Psychoact. Drugs 2019, 51, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X. Brain mechanisms of hallucinogens and entactogens. Dialogues Clin. Neurosci. 2001, 3, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Conio, B.; Martino, M.; Magioncalda, P.; Escelsior, A.; Inglese, M.; Amore, M.; Northoff, G. Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Mol. Psychiatry 2020, 25, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Wadsak, W.; Windischberger, C.; Baldinger, P.; Hoflich, A.S.; Losak, J.; Nics, L.; Philippe, C.; Kranz, G.S.; Kraus, C.; et al. Differential modulation of the default mode network via serotonin-1A receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-dimethyltryptamine. Brain Res. Bull. 2016, 126, 74–88. [Google Scholar] [CrossRef]
- De Gregorio, D.; Comai, S.; Posa, L.; Gobbi, G. d-Lysergic Acid Diethylamide (LSD) as a Model of Psychosis: Mechanism of Action and Pharmacology. Int. J. Mol. Sci. 2016, 17, 1953. [Google Scholar] [CrossRef]
- Tyls, F.; Palenicek, T.; Horacek, J. Psilocybin—Summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Appel, J.B.; Callahan, P.M. Involvement of 5-HT receptor subtypes in the discriminative stimulus properties of mescaline. Eur. J. Pharmacol. 1989, 159, 41–46. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res. Rev. 2000, 31, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.W.; Jiang, X.L.; Winter, J.C.; Yu, A.M. Psychedelic 5-methoxy-N,N-dimethyltryptamine: Metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr. Drug Metab. 2010, 11, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Villevieille, T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef]
- Johnson, K.M.; Jones, S.M. Neuropharmacology of phencyclidine: Basic mechanisms and therapeutic potential. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 707–750. [Google Scholar] [CrossRef]
- Azmitia, E.C.; Murphy, R.B.; Whitaker-Azmitia, P.M. MDMA (ecstasy) effects on cultured serotonergic neurons: Evidence for Ca2(+)-dependent toxicity linked to release. Brain Res. 1990, 510, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lyles, J.; Cadet, J.L. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: Cellular and molecular mechanisms. Brain Res. Rev. 2003, 42, 155–168. [Google Scholar] [CrossRef]
- Preller, K.H.; Vollenweider, F.X. Phenomenology, Structure, and Dynamic of Psychedelic States. Curr. Top. Behav. Neurosci. 2018, 36, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Roseman, L.; Bolstridge, M.; Demetriou, L.; Pannekoek, J.N.; Wall, M.B.; Tanner, M.; Kaelen, M.; McGonigle, J.; Murphy, K.; et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 2017, 7, 13187. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, M.C.; Mithoefer, A.T.; Feduccia, A.A.; Jerome, L.; Wagner, M.; Wymer, J.; Holland, J.; Hamilton, S.; Yazar-Klosinski, B.; Emerson, A.; et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry 2018, 5, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Belouin, S.J.; Henningfield, J.E. Psychedelics: Where we are now, why we got here, what we must do. Neuropharmacology 2018, 142, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Inserra, A.; Campanale, A.; Cheishvili, D.; Dymov, S.; Wong, A.; Marcal, N.; Syme, R.A.; Taylor, L.; De Gregorio, D.; Kennedy, T.E.; et al. Modulation of DNA methylation and protein expression in the prefrontal cortex by repeated administration of D-lysergic acid diethylamide (LSD): Impact on neurotropic, neurotrophic, and neuroplasticity signaling. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 119, 110594. [Google Scholar] [CrossRef]
- De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; McLaughlin, R.; Maione, S.; Comai, S.; Gobbi, G. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Pharmacol. Res. 2016, 113, 81–91. [Google Scholar] [CrossRef]
- Tena, J.; Lebrilla, C.B. Glycomic profiling and the mammalian brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2022238118. [Google Scholar] [CrossRef]
- Bandtlow, C.E.; Zimmermann, D.R. Proteoglycans in the developing brain: New conceptual insights for old proteins. Physiol. Rev. 2000, 80, 1267–1290. [Google Scholar] [CrossRef]
- Marcus, J.; Dupree, J.L.; Popko, B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. J. Cell Biol. 2002, 156, 567–577. [Google Scholar] [CrossRef]
- Kleene, R.; Schachner, M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004, 5, 195–208. [Google Scholar] [CrossRef]
- Best, T.; Kemps, E.; Bryan, J. Effects of saccharides on brain function and cognitive performance. Nutr. Rev. 2005, 63, 409–418. [Google Scholar] [CrossRef]
- Ooi, C.P.; Loke, S.C.; Yassin, Z.; Hamid, T.A. Carbohydrates for improving the cognitive performance of independent-living older adults with normal cognition or mild cognitive impairment. Cochrane Database Syst. Rev. 2011, CD007220. [Google Scholar] [CrossRef]
- Murrey, H.E.; Hsieh-Wilson, L.C. The chemical neurobiology of carbohydrates. Chem. Rev. 2008, 108, 1708–1731. [Google Scholar] [CrossRef]
- Haukedal, H.; Freude, K.K. Implications of Glycosylation in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 625348. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, C.L.; Sipos, E.; Klaric, T.; Torok, B.; Bellardie, M.; Erjave, G.N.; Perkovic, M.N.; Lauc, G.; Pivac, N.; Zelena, D. Searching for glycomic biomarkers for predicting resilience and vulnerability in a rat model of posttraumatic stress disorder. Stress 2020, 23, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, S.; Tosh, N.; Urquhart, A.; Trickey, K.; Tremewan, R.; Galloway, G.; Rich, L.; Lea, R.; Malycha, P.; Mountford, C. Post-traumatic stress disorder affects fucose-alpha(1-2)-glycans in the human brain: Preliminary findings of neuro deregulation using in vivo two-dimensional neuro MR spectroscopy. Transl. Psychiatry 2019, 9, 27. [Google Scholar] [CrossRef]
- Noro, E.; Togayachi, A.; Sato, T.; Tomioka, A.; Fujita, M.; Sukegawa, M.; Suzuki, N.; Kaji, H.; Narimatsu, H. Large-Scale Identification of N-Glycan Glycoproteins Carrying Lewis x and Site-Specific N-Glycan Alterations in Fut9 Knockout Mice. J. Proteome Res. 2015, 14, 3823–3834. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Noel, M.; Lehoux, S.; Cetinbas, M.; Xavier, R.J.; Sadreyev, R.I.; Scolnick, E.M.; Smoller, J.W.; Cummings, R.D.; Mealer, R.G. Mammalian brain glycoproteins exhibit diminished glycan complexity compared to other tissues. Nat. Commun. 2022, 13, 275. [Google Scholar] [CrossRef]
- Reich, M.; Liefeld, T.; Gould, J.; Lerner, J.; Tamayo, P.; Mesirov, J.P. GenePattern 2.0. Nat. Genet. 2006, 38, 500–501. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Bult, C.J.; Blake, J.A.; Smith, C.L.; Kadin, J.A.; Richardson, J.E.; the Mouse Genome Database Group. Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 2019, 47, D801–D806. [Google Scholar] [CrossRef] [PubMed]
- Oommen, A.M.; Somaiya, N.; Vijayan, J.; Kumar, S.; Venkatachalam, S.; Joshi, L. GlycoGAIT: A web database to browse glycogenes and lectins under gastric inflammatory diseases. J. Theor. Biol. 2016, 406, 93–98. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Kamburov, A.; Pentchev, K.; Galicka, H.; Wierling, C.; Lehrach, H.; Herwig, R. ConsensusPathDB: Toward a more complete picture of cell biology. Nucleic Acids Res. 2011, 39, D712–D717. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Vo, H.T.; Laszczyk, A.M.; King, G.D. Klotho, the Key to Healthy Brain Aging? Brain Plast. 2018, 3, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Morise, J.; Takematsu, H.; Oka, S. The role of human natural killer-1 (HNK-1) carbohydrate in neuronal plasticity and disease. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 2455–2461. [Google Scholar] [CrossRef]
- Bartels, M.F.; Winterhalter, P.R.; Yu, J.; Liu, Y.; Lommel, M.; Mohrlen, F.; Hu, H.; Feizi, T.; Westerlind, U.; Ruppert, T.; et al. Protein O-Mannosylation in the Murine Brain: Occurrence of Mono-O-Mannosyl Glycans and Identification of New Substrates. PLoS ONE 2016, 11, e0166119. [Google Scholar] [CrossRef]
- Nishihara, S.; Iwasaki, H.; Nakajima, K.; Togayachi, A.; Ikehara, Y.; Kudo, T.; Kushi, Y.; Furuya, A.; Shitara, K.; Narimatsu, H. Alpha1,3-fucosyltransferase IX (Fut9) determines Lewis X expression in brain. Glycobiology 2003, 13, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Belkai, E.; Scherrmann, J.M.; Noble, F.; Marie-Claire, C. Modulation of MDMA-induced behavioral and transcriptional effects by the delta opioid antagonist naltrindole in mice. Addict. Biol. 2009, 14, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; McKinzie, A.A.; Couceyro, P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995, 15, 2471–2481. [Google Scholar] [CrossRef]
- Muller, T.E.; Fontana, B.D.; Bertoncello, K.T.; Franscescon, F.; Mezzomo, N.J.; Canzian, J.; Stefanello, F.V.; Parker, M.O.; Gerlai, R.; Rosemberg, D.B. Understanding the neurobiological effects of drug abuse: Lessons from zebrafish models. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109873. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.A.; Panksepp, J.; Bluthe, R.M.; van Honk, J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: A review of single administration studies. Front. Neuroendocrinol. 2012, 33, 17–35. [Google Scholar] [CrossRef]
- Schindler, E.A.D.; Wallace, R.M.; Sloshower, J.A.; D’Souza, D.C. Neuroendocrine Associations Underlying the Persistent Therapeutic Effects of Classic Serotonergic Psychedelics. Front. Pharmacol. 2018, 9, 177. [Google Scholar] [CrossRef]
- Zhang, Z.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Stemkowski, P.L.; Zamponi, G.W. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep. 2015, 12, 752–759. [Google Scholar] [CrossRef]
- Caputi, F.F.; Palmisano, M.; Carboni, L.; Candeletti, S.; Romualdi, P. Opioid gene expression changes and post-translational histone modifications at promoter regions in the rat nucleus accumbens after acute and repeated 3,4-methylenedioxy-methamphetamine (MDMA) exposure. Pharmacol. Res. 2016, 114, 209–218. [Google Scholar] [CrossRef]
- Scott, H.; Panin, V.M. N-glycosylation in regulation of the nervous system. Adv. Neurobiol. 2014, 9, 367–394. [Google Scholar] [CrossRef]
- Dani, N.; Broadie, K. Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev. Neurobiol. 2012, 72, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R.; Blumcke, I. Perineuronal nets--a specialized form of extracellular matrix in the adult nervous system. Brain Res. Rev. 1994, 19, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Suttkus, A.; Morawski, M.; Arendt, T. Protective Properties of Neural Extracellular Matrix. Mol. Neurobiol. 2016, 53, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef]
- De Luca, C.; Papa, M. Looking Inside the Matrix: Perineuronal Nets in Plasticity, Maladaptive Plasticity and Neurological Disorders. Neurochem. Res. 2016, 41, 1507–1515. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Domowicz, M.S. Proteoglycans in brain development and pathogenesis. FEBS Lett. 2018, 592, 3791–3805. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Glycans and glycosaminoglycans in neurobiology: Key regulators of neuronal cell function and fate. Biochem. J. 2018, 475, 2511–2545. [Google Scholar] [CrossRef]
- Schmack, K.; Bosc, M.; Ott, T.; Sturgill, J.F.; Kepecs, A. Striatal dopamine mediates hallucination-like perception in mice. Science 2021, 372, eabf4740. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatr. Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef]
- Hanson, K.; Fisher, K.; Hooper, N.M. Exploiting the neuroprotective effects of alpha-klotho to tackle ageing- and neurodegeneration-related cognitive dysfunction. Neuronal Signal. 2021, 5, NS20200101. [Google Scholar] [CrossRef]
- Lukasiewicz, K.; Baker, J.J.; Zuo, Y.; Lu, J. Serotonergic Psychedelics in Neural Plasticity. Front. Mol. Neurosci. 2021, 14, 748359. [Google Scholar] [CrossRef]
- Rudin, D.; Liechti, M.E.; Luethi, D. Molecular and clinical aspects of potential neurotoxicity induced by new psychoactive stimulants and psychedelics. Exp. Neurol. 2021, 343, 113778. [Google Scholar] [CrossRef]
- Nakki, R.; Koistinaho, J.; Sharp, F.R.; Sagar, S.M. Cerebellar toxicity of phencyclidine. J. Neurosci. 1995, 15, 2097–2108. [Google Scholar] [CrossRef]
- Choudhury, D.; Autry, A.E.; Tolias, K.F.; Krishnan, V. Ketamine: Neuroprotective or Neurotoxic? Front. Neurosci. 2021, 15, 672526. [Google Scholar] [CrossRef] [PubMed]
- Zeldich, E.; Chen, C.D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Tucker Zhou, T.B.; Harris, D.A.; Abraham, C.R. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Hunt, C.R.; Marsh, B.; Bacon, A.; Pope, R.; Vanderplank, P.; Wynick, D. Galanin acts as a neuroprotective factor to the hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 5105–5110. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Pappas, C.; Tajiri, N.; Borlongan, C.V. Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci. Rep. 2016, 6, 35659. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Klaus, J.; Young, J.; Koerner, I.; Sheldahl, L.C.; Hurn, P.D.; Martinez-Murillo, F.; Alkayed, N.J. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc. Natl. Acad. Sci. USA 2006, 103, 14489–14494. [Google Scholar] [CrossRef]
- Roth-Deri, I.; Green-Sadan, T.; Yadid, G. Beta-endorphin and drug-induced reward and reinforcement. Prog. Neurobiol. 2008, 86, 1–21. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Q.; Li, L. Isolation and characterization of glycosylated neuropeptides. Methods Enzymol. 2019, 626, 147–202. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Srivastava, D.P.; Allen, J.A.; Strachan, R.T.; Roth, B.L.; Penzes, P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc. Natl. Acad. Sci. USA 2009, 206, 19575–19580. [Google Scholar] [CrossRef] [PubMed]
- Marona-Lewicka, D.; Thisted, R.A.; Nichols, D.E. Distinct temporal phases in the behavioral pharmacology of LSD: Dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology 2005, 180, 427–435. [Google Scholar] [CrossRef]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT2A Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef]
- Muschamp, J.W.; Regina, M.J.; Hull, E.M.; Winter, J.C.; Rabin, R.A. Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004, 1023, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Inserra, A.; De Gregorio, D.; Rezai, T.; Lopez-Canul, M.G.; Comai, S.; Gobbi, G. Lysergic acid diethylamide differentially modulates the reticular thalamus, mediodorsal thalamus, and infralimbic prefrontal cortex: An in vivo electrophysiology study in male mice. J. Psychopharmacol. 2021, 35, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Razi, A.; Zeidman, P.; Stämpfli, P.; Friston, K.J.; Vollenweider, F.X. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc. Natl. Acad. Sci. USA 2019, 116, 2743–2748. [Google Scholar] [CrossRef] [PubMed]


| Psychedelic Molecules | Structure | Serotonergic Signalling | Glutamate Signalling | Dopamine Signalling | Cholinergic/Adrenergic Signalling | References |
|---|---|---|---|---|---|---|
| DMT: N,N-dimethyltryptamine | 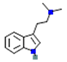 | 5-HT2A, 5-HT1A and 5-HT2c receptors (stimulatory) | ↑ postsynaptic glutamate | ↑ the release of dopamine as well as degradation | ↓ Acetylcholine in corpus striatum | [10] |
| LSD: (5R,8R)-(+)-lysergic acid-N,N-diethylamide |  | 5-HT2 (stimulatory) and 5-HT1 (inhibitory). Also binds other 5-HT1 subtypes | ↑ postsynaptic glutamate | agonist activity at dopamine D2 and D4 receptor | ↑ Acetylcholine | [11] |
| Psilocybin | 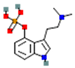 | high affinity at 5-HT2 receptors (stimulatory) | ↑ postsynaptic glutamate | ↑ dopamine levels | [12] | |
| Mescaline | 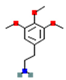 | high affinity at 5-HT2A. receptors (stimulatory) | ↑ postsynaptic glutamate | induced release of dopamine | [13,14] | |
| 5-HO-DMT: N,N-Dimethyl-5-Hydroxytryptamine |  | high affinity for the 5-HT1A receptor (stimulatory) | [15] | |||
| 5-MeO-DMT: 5-Methoxy-N,N-Dimethyltryptamine |  | 5-HT2A >5-HT2C >5-HT1A receptors (stimulatory) | ||||
| Ketamine | 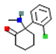 | Inhibits serotonin uptake and thereby ↑ the concentration | Antagonizes NMDA and non-NMDA receptors | Inhibits dopamine uptake and thereby increases the concentration | Inhibition of catecholamines uptake, ketamine provokes a hyperadrenergic state (release of norepinephrine, dopamine, and serotonin). | [16] |
| Phencyclidine | 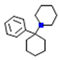 | Inhibits serotonin uptake and thereby ↑ the concentration | Antagonizes NMDA | Inhibits dopamine uptake and thereby increases the concentration | Inhibition of norepinephrine reuptake and binds to muscarinic Ach receptor | [17] |
| MDMA: 3,4-Methylenedioxymethamphetamine | 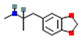 | Enhanced serotonin release followed by substantial decrease | ↑ dopamine release | ↑ norepinephrine release | [18,19] |
| Gene Symbol | GSE138802 | GSE73799 | GSE73799 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ketamine vs. Saline | Phencyclidine vs. Saline | ||||||||
| Pre-Limbic Cortex | 1 h | 2 h | 4 h | 8 h | 1 h | 2 h | 4 h | 8 h | |
| Striatum | Striatum | ||||||||
| Neuro-Regulatory Genes | |||||||||
| Kl Klotho [Cleaved into: Klotho peptide] | 0.0 | −0.5 | 1.1 | 0.0 | 0.0 | 1.2 | 0.0 | 0.8 | 0.5 |
| Cdkn1a—Cyclin-dependent kinase inhibitor 1 | 0.0 | 0.5 | 0.0 | 0.0 | −0.6 | 0.0 | 0.9 | 0.0 | −0.7 |
| Pomc—Pro-opiomelanocortin (POMC) (Corticotropin-lipotropin) | 0.0 | −0.5 | 0.0 | 0.0 | 0.7 | −0.6 | 0.0 | 0.0 | 0.6 |
| Klf4—Krueppel-like factor 4 | 0.6 | 0.0 | −0.7 | −0.4 | 0.0 | 0.5 | 0.0 | −0.5 | 0.0 |
| Ncam1—Neural cell adhesion molecule 1 | 0.0 | 0.0 | 0.0 | 0.0 | −0.9 | 0.0 | 0.9 | 0.0 | −1.1 |
| Aplp1—Amyloid beta precursor like protein 1 | 0.0 | 0.0 | 0.0 | 0.0 | −0.9 | 0.0 | 0.8 | 0.0 | −1.1 |
| Scg5—Neuroendocrine protein 7B2 (Secretogranin V) | 0.0 | 0.0 | 0.0 | 0.0 | −0.8 | 0.0 | 0.8 | 0.0 | −1.0 |
| Ptprz1—Receptor-type tyrosine-protein phosphatase zeta | 0.0 | 0.0 | 0.0 | 0.0 | −0.8 | 0.0 | 0.7 | 0.0 | −0.9 |
| Cartpt—Cocaine- and amphetamine-regulated transcript protein | 1.9 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 |
| Gal—Galanin peptides | 5.4 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 |
| Oxt—Oxytocin-neurophysin 1 | 7.7 | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 1.5 | 0.0 |
| Avp—Vasopressin-neurophysin 2-copeptin | 7.6 | 0.0 | 0.0 | 1.1 | 0.0 | 0.0 | 0.0 | 1.3 | 0.0 |
| Chrna10—Cholinergic receptor, nicotinic, alpha polypeptide 10 | 5.2 | −0.5 | −0.4 | 0.0 | 0.0 | −0.6 | 0.0 | 0.0 | 0.0 |
| Pde1b—Calcium/calmodulin-dependent 3’,5’-cyclic nucleotide phosphodiesterase 1B | 1.0 | 0.0 | 0.0 | 0.0 | −1.0 | 0.0 | 0.0 | 0.0 | −1.1 |
| Syn1—Synapsin-1 (Synapsin I) | 0.0 | 0.0 | 0.0 | 0.0 | −0.8 | 0.0 | 0.0 | 0.8 | −0.9 |
| Ppfia3—Liprin-alpha-3 | 0.0 | 0.0 | 0.0 | −0.5 | 0.0 | 0.0 | 0.0 | −0.5 | −0.7 |
| Syt6—Synaptotagmin-6 (Synaptotagmin VI) | 2.0 | 0.0 | 0.0 | −0.6 | 0.0 | −0.4 | 0.0 | −0.6 | 0.0 |
| Col18a1—Collagen alpha-1(XVIII) chain | 0.6 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 |
| B3gat1—Galactosylgalactosylxylosylprotein 3-beta-glucuronosyltransferase 1 | 0.0 | −0.4 | 0.0 | −0.5 | −0.9 | −0.4 | 0.0 | −0.4 | −1.2 |
| Fkbp5—Peptidyl-prolyl cis-trans isomerase FKBP5 | 0.0 | 0.0 | 0.0 | 0.7 | −0.5 | 0.0 | 0.0 | 0.5 | 0.0 |
| Sytl2—Synaptotagmin-like protein 2 | −0.7 | −0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 |
| Gnas—Guanine nucleotide-binding protein G(s) subunit alpha isoforms short | 0.0 | 0.0 | −0.5 | −0.5 | 0.0 | 0.0 | −0.5 | −0.7 | 0.0 |
| Sstr5—Somatostatin receptor type 5 | 0.0 | 0.0 | −0.4 | −0.5 | 0.0 | 0.0 | −0.5 | −0.7 | 0.0 |
| Glycogenes | |||||||||
| Hexb—Beta-hexosaminidase subunit beta | 0.0 | 0.0 | 0.0 | 0.0 | −0.8 | 0.0 | 0.8 | −0.8 | −0.8 |
| Kera—Keratocan (KTN) | 0.0 | 0.0 | 0.0 | −0.6 | 0.5 | 0.0 | −0.5 | −0.6 | 0.0 |
| Ogn—Mimecan (Osteoglycin) | 0.0 | 0.0 | 0.0 | 0.0 | −0.6 | 0.6 | 0.0 | −0.5 | −0.7 |
| Hs6st2—Heparan-sulfate 6-O-sulfotransferase 2 | 0.5 | 0.0 | 0.0 | 0.0 | −0.8 | 0.0 | 0.0 | −0.8 | −0.9 |
| Alg6—Dolichyl pyrophosphate Man9GlcNAc2 alpha-1,3-glucosyltransferase | 0.0 | −0.5 | 0.0 | 0.0 | −0.7 | 0.0 | 0.0 | 0.0 | −0.8 |
| Calr3—Calreticulin-3 (Calreticulin-2) | 0.0 | −0.5 | 0.0 | −0.6 | 0.3 | 0.0 | 0.0 | −0.9 | 0.4 |
| Col9a2—Collagen alpha-2(IX) chain | 0.0 | 0.0 | 0.0 | 0.0 | −0.5 | 0.0 | 0.0 | −0.5 | −0.6 |
| Mgat5—Alpha-1,6-mannosylglycoprotein 6-beta-N-acetylglucosaminyltransferase A | 0.0 | −0.6 | −0.5 | 0.0 | −0.4 | 0.0 | −0.8 | 0.4 | 0.0 |
| B3gat1—Galactosylgalactosylxylosylprotein 3-beta-glucuronosyltransferase 1 | 0.0 | −0.4 | 0.0 | −0.5 | −0.9 | −0.4 | 0.0 | −0.4 | −1.2 |
| Alg3—Dol-P-Man:Man(5)GlcNAc(2)-PP-Dol alpha-1,3-mannosyltransferase | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.5 | −0.5 |
| Glt8d2—Glycosyltransferase 8 domain-containing protein 2 | −0.9 | −0.6 | 0.0 | 0.0 | 0.0 | −0.5 | 0.0 | 0.0 | 0.0 |
| Tpst2—Protein-tyrosine sulfotransferase 2 | 0.0 | 0.0 | 0.0 | 0.0 | −0.8 | 0.0 | 0.7 | 0.0 | −0.9 |
| Dcn—Decorin (Bone proteoglycan II) | 0.0 | 0.0 | 1.0 | 0.0 | −0.6 | 0.0 | 0.0 | 0.0 | −0.6 |
| Bgn—Biglycan (Bone/cartilage proteoglycan I) | 0.8 | 0.0 | 0.0 | 0.0 | −0.7 | 0.0 | 0.0 | 0.0 | −0.7 |
| Ndst1—Bifunctional heparan sulfate N-deacetylase/N-sulfotransferase 1 | 0.0 | 0.5 | 0.0 | 0.0 | −0.6 | 0.0 | 0.0 | 0.0 | −0.8 |
| Tmtc4—Protein O-mannosyl-transferase TMTC4 | 0.0 | 0.0 | −0.7 | 0.0 | 0.0 | 0.0 | −0.9 | −0.5 | 0.0 |
| Ugt1a2—UDP-glucuronosyltransferase 1-2 | 0.0 | −0.3 | 0.0 | −0.8 | 0.0 | 0.6 | 0.0 | −0.7 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oommen, A.M.; Roberts, K.J.; Joshi, L.; Cunningham, S. Transcriptomic Analysis of Glycosylation and Neuroregulatory Pathways in Rodent Models in Response to Psychedelic Molecules. Int. J. Mol. Sci. 2023, 24, 1200. https://doi.org/10.3390/ijms24021200
Oommen AM, Roberts KJ, Joshi L, Cunningham S. Transcriptomic Analysis of Glycosylation and Neuroregulatory Pathways in Rodent Models in Response to Psychedelic Molecules. International Journal of Molecular Sciences. 2023; 24(2):1200. https://doi.org/10.3390/ijms24021200
Chicago/Turabian StyleOommen, Anup M., Katherine J. Roberts, Lokesh Joshi, and Stephen Cunningham. 2023. "Transcriptomic Analysis of Glycosylation and Neuroregulatory Pathways in Rodent Models in Response to Psychedelic Molecules" International Journal of Molecular Sciences 24, no. 2: 1200. https://doi.org/10.3390/ijms24021200
APA StyleOommen, A. M., Roberts, K. J., Joshi, L., & Cunningham, S. (2023). Transcriptomic Analysis of Glycosylation and Neuroregulatory Pathways in Rodent Models in Response to Psychedelic Molecules. International Journal of Molecular Sciences, 24(2), 1200. https://doi.org/10.3390/ijms24021200








