Abstract
In this research, we investigated the structural and biological properties of phosphate glasses (PGs) after the addition of V2O5. A xV2O5∙(100 − x)[CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 16 mol% was synthesized via a conventional melt-quenching technique. Several analysis techniques (dissolution tests, pH, SEM-EDS, FT-IR, and EPR) were used to obtain new experimental data regarding the structural behavior of the system. In vitro tests were conducted to assess the antitumor character of V2O5-doped glass (x = 16 mol%) compared to the matrix (x = 0 mol%) and control (CTRL-) using several tumoral cell lines (A375, A2780, and Caco-2). The characterization of PGs showed an overall dissolution rate of over 90% for all vitreous samples (M and V1–V7) and the high reactivity of this system. EPR revealed a well-resolved hyperfine structure (hfs) typical of vanadyl ions in a C4v symmetry. FT-IR spectra showed the presence of all structural units expected for P2O5, as well as very clear depolymerization of the vitreous network induced by V2O5. The MTT assay indicated that the viability of tumor cells treated with V7-glass extract was reduced to 50% when the highest concentration was used (10 µg/mL) compared to the matrix treatment (which showed no cytotoxic effect at any concentration). Moreover, the matrix treatment (without V2O5) provided an optimal environment for tumor cell attachment and proliferation. In conclusion, the two types of treatment investigated herein were proven to be very different from a statistical point of view (p < 0.01), and the in vitro studies clearly underline the cytotoxic potential of vanadium ions from phosphate glass (V7) as an antitumor agent.
1. Introduction
One of the most promising research directions for compounds containing vanadium is their potential anticancer activity [1]. According to the World Health Organization (WHO), in 2020, there were 19.3 million cases of cancer and almost 10.0 million deaths worldwide, which increased the burden on medical systems all over the world [2]. Moreover, cancer is a condition that can develop from almost any cell and therefore anywhere in the body, and it continues to be a leading cause of death worldwide. Cancers that most often affect women include colorectal, skin, and ovarian cancers [3].
Vitreous materials have numerous possible compositions and a wide range of applications [4,5,6]. Among these, a special class of glasses is phosphate glasses (PGs). PGs have been developed and intensively studied, mainly in the biomedical field (such as for surgical implants used in tissue engineering, wound dressings composed of bioactive glass microfibers for wound healing and the promotion of angiogenesis, bioactive glass-based composite biomaterials, drug delivery through antibacterial ions (silver and copper ions), and dental implants) [1,4,7,8,9] and in technological applications (such as IC packaging, thick-film technology, glass–metal sealing, flat-panel display sealing, as a host matrix for the vitrification of radioactive waste, and as a host material for lasers) [10,11,12,13]. The properties that make PGs promising candidates in biomedical applications are closely related to their molecular structure, which is generally described using the Qn formalism, where n represents the number of bridging oxygen atoms in each tetrahedron [12,13]. Their greatest advantage is their solubility in aqueous solutions and the possibility of being tuned over many orders of magnitude by tailoring the glass composition [14,15]. Another benefit from a biomedical standpoint is the fact that these glasses can be doped with ions routinely found inside the body. Once dissolved in a biological fluid, they can initiate a wide range of responses by releasing ions into the local environment [15].
Many PGs are materials with excellent bioresorbability [16,17] and very high biocompatibility with living tissues [11,18], offering great potential for tissue engineering applications.
The addition of transition metal oxides (TMOs) in the composition of phosphate glass typically leads to the depolymerization of the vitreous phosphate network and to the formation of new P–O–TM bonds [13]. These structural modifications are linked to the amount of doping and have a strong influence on the physical, chemical, and magnetic properties of these glasses [19,20,21,22]. Moreover, including TMOs in the P2O5 network not only improves their properties but can also have beneficial effects from a biological perspective [23].
Vanadium (V) is one of the most suitable transition metals that can be incorporated into PGs, as it is characterized by a partially filled 3d layer and can exist in at least two valence states in these glasses [24]. These valence states are influenced by synthesis conditions, the raw chemicals used, and the total amount of V2O5 in the glass [25].
In recent years, numerous studies have reported antitumoral activity for many vanadium-containing compounds [26]. Thus, vanadium compounds exert their antitumor effects through the following molecular and cellular mechanisms:
- (i)
- Fragmentation activities that directly target DNA. Studies conducted by Mohamadi et al. [27] through cyclic voltammetry, competitive fluorescence assays, and electronic absorption spectroscopy showed a groove binding of a complex containing vanadium to salmon sperm DNA, which was also accompanied by a partial insertion of the ligand between the base stacks of the DNA. Furthermore, this mononuclear diketone-based oxido vanadium (IV) complex showed significant cytotoxicity against multiple cancer cell lines (i.e., breast, liver and colon).
- (ii)
- Disruption of the redox balance due to an increase in reactive oxygen species (ROS). Reactive oxygen species are well known to promote a multitude of aspects in tumor development and progression and are detected in all cancers cells. Vanadium salts such as sodium metavanadate (NaVO3) or vanadium (IV) sulfate oxide (VOSO4) have been reported to induce a significant increase in ROS level in human lung cancer cells [28,29]. Furthermore, a polyacrylate derivative of peroxovanadate was shown to inhibit the growth of lung carcinoma cells [30]. Finally, a vanadium dioxide nanocoating (VO2-modified) quartz surface prepared by Li et al. [31] increased intracellular ROS levels in cholangiocarcinoma cells.
- (iii)
- Apoptosis or programmed cell death. Apoptosis serves the purpose of removing DNA-damaged cells that could lead to carcinogenesis. This process is disturbed in cancer cells, which have a mechanism of avoiding it. The literature reports that this mechanism was induced in an oral squamous cell carcinoma cell line [32] and in human anaplastic thyroid carcinoma cells [33] by sodium orthovanadate (Na3VO4). In gastric cancer lines, a vanadium complex used by Wang et al. [34] also induced apoptosis. Furthermore, in human breast cancer cell lines, this effect was obtained through a vanadium complex combined with the flavonoid quercetin [35].
- (iv)
- Cell cycle arrest. It is already well known that cancer cells are characterized by a dysregulation of the cell cycle, resulting in aberrant cell proliferation and their high genetic instability [36]. Sodium metavanadate was reported to cause G2/M cell cycle arrest in prostate cancer cells in a study conducted by Liu et al. [37]. A similar G2/M cell cycle arrest mechanism was described for the effect of sodium metavanadate on papillary thyroid carcinoma-derived cells [38], for an oxido vanadium complex containing a quinolinium cation in pancreatic cancer cell lines [26], and for an oxido vanadium complex with phenanthroline in hepatocellular carcinoma cell lines [39]. All these antitumoral effects are mediated by the cyclin/CDK complexes, which play a key role in checkpoints of the cell cycle.
Against this background, in the present study, we evaluated the effects of vanadium on the structural and biological properties of a xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 16 mol%. The structural properties of the glass system were examined by a variety of techniques, namely electron paramagnetic resonance (EPR), Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS). pH measurements and dissolution tests were used to evaluate both the release behavior and bioactivity of this glass system. Furthermore, in order to explore and quantify the antitumoral character of vanadium ions from the phosphate glass, we used in vitro biological tests and a tetrazolium-based MTT assay, respectively, on three different human cancer cell lines (A375, A2780, and Caco-2).
2. Results and Discussion
2.1. Structural Characterization
2.1.1. Dissolution Tests
Pure or high-phosphorus-content glasses are characterized by relatively low chemical durability compared to silicate- and borate-based glasses [40].
According to the literature, the dissolution of PGs in aqueous media involves two pivotal stages, namely hydration (softening stage) and hydrolysis (breaking stage) [41].
As a result of the hydration reaction, P–O–P bonds in the hydrated layer break, and a hydrolysis reaction takes place (second stage). This stage is a process of degradation of the glass network (from outside to inside) and is highly dependent on ambient pH [40].
In our case, the solubility of the glasses with xV2O5∙(100 − x)[CaF2∙3P2O5∙CaO], where x = 0.25, 0.5, 1, 2, 4, 8, and 16 mol%, was increased for all compositions (Figure 1). The doping (V2O5) of the structure of phosphate glasses did not affect their dissolution rate. After 14 days of incubation, the glass samples showed a mass loss of over 90% of their initial mass.
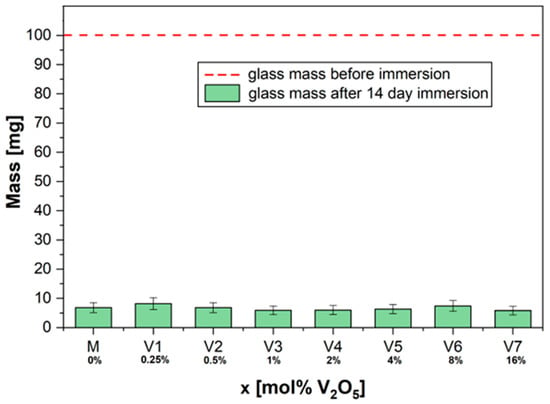
Figure 1.
The solubility of glass powders: dry mass before and after immersion for 14 days in 10 mL deionized water.
No considerable change in chemical durability was observed for the sample with the highest doping concentration (x = 16 mol%).
Bunker et al. [42] developed a general description of the dissolution reactions between PGs and water. In our case, water hydrated the phosphate anions by reacting with the metal V–O–P bonds that link neighboring P anions according to the following equation:
─P─ O− (Ca2+; Vn) O− ─ P + 2H2O → ─ P─OH HO─P─ + (Ca2+; Vn) (OH)2
| |
(F−) (F−)
n = oxidation states (2+, 3+, 4+ or 5+)
| |
(F−) (F−)
n = oxidation states (2+, 3+, 4+ or 5+)
The results presented here can be explained by the action of water molecules (H+, H3O+) that diffuse into the surface structure and break the weakest P–O–P or P–O–V chains. The size and the shape of the glass, as well as the glass surface area, play a dominant role in ion exchange; this corrosion property is determined by the ratio between the area and the volume of the glass [41]. The dissolution/corrosion behavior of glasses with the CaO-P2O5-CaF2:V2O5 depends on the network strength, which is based mainly on the glass composition.
2.1.2. pH Evolution
During 21 days of immersion, the evolution of pH values in a phosphate buffer solution (PBS) was measured at different time intervals; the results are illustrated in Figure 2. All investigated compositions caused a decrease in the pH of PBS. A significantly strong decrease was visible on the first day. Then, a slower decrease over the rest of the incubation time was noticed. According to the procedure, several measurements of pH were performed, with the most important control points being: on day 0, an initial pH value of 7.43; on day 9 pH values ranging from 7.18 to 6.10; and on day 21, pH values ranging from 6.97 to 5.94.
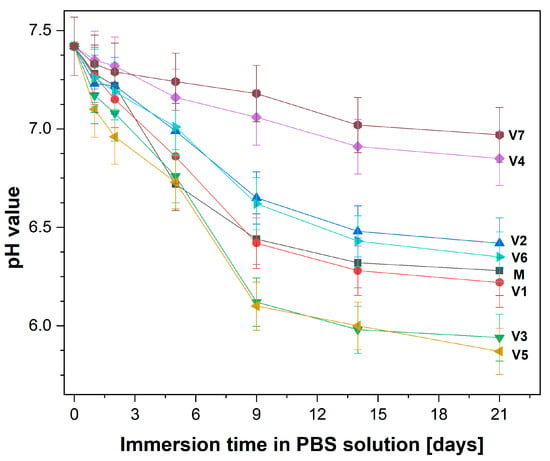
Figure 2.
pH evolution of phosphate-buffered saline (PBS) solution as a result of glass sample immersion over 21 days at 37 °C.
According to the literature, the pH values produced by the presence of PGs are a consequence of their dissolution, which generates some acidic species as a result of an ion exchange process [42,43,44].
In our research, the pH gradually decreased as immersion time increased. This might be due to the formation of phosphoric acid in relation to H2PO4− entities [44]. Therefore, phosphate ions at the glass surface are released into the PBS solution through the breakage of P−O−P/P−O−V bonds associated with the PO4 units.
H3PO4 + H2O ↔ H2PO4− + H3O+ (K1 = 7.1 × 10−3)
H2PO4− + H2O ↔ HPO42− + H3O+ (K2 = 6.3 × 10−8)
HPO42− + H2O ↔ PO43− + H3O+ (K3 = 4.4 × 10−13)
Additionally, the fluorine content can have a notable impact on the pH values due to the ion exchange process [45].
Similarly, just as Ca2+ ions on the glass surface combine with the PBS solution in exchange for H+ ions from the solution (from the dissociation of water into H+ and OH−) [46], F− ions are exchanged for OH− groups. Thus, hydroxyl is removed from the solution, and a weak acid–hydrofluoric acid is formed. If the fluoride content increases, more hydrofluoric acid is formed, and the pH increase is less noticeable [47].
2.1.3. SEM-EDS
The surface morphologies of two relevant samples (M and V7) are shown in Figure 3. SEM analyses showed that all glasses have numerous microparticles with irregular shapes and a wide distribution of sizes. No spherical particles were observed.
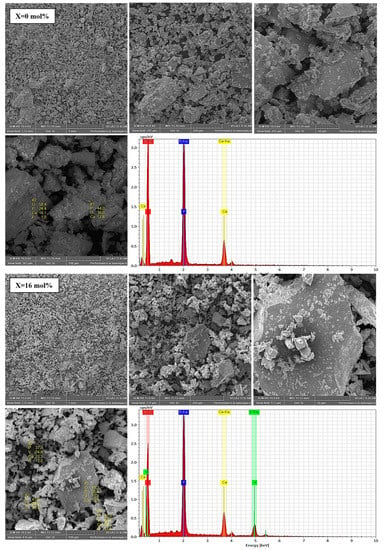
Figure 3.
SEM micrographs and EDS analysis for glass samples with x = 0 and 16 mol% V2O5 used in the dissolution experiments. Colour legend: O (red), Ca (yellow), V (green) and P (blue).
The presence of vanadium was confirmed by EDS analysis in all studied phosphate glasses, except for the host glass (matrix), which was free of doping. In addition, all constituent elements (P, O, Ca, and V) were confirmed in quantities analogous to the initial nominal composition (Table 1).

Table 1.
The chemical elements present in the surface of glass powders evaluated by EDS spectroscopy.
A decrease in both oxygen and calcium occurred in samples V1 to V7 as the doping concentration increased, whereas the amount of phosphorus remained relatively unchanged, with range of values between 28.50–33.49 wt%.
To confirm the homogeneous structure of the studied glasses, SEM characterizations combined with EDS were performed. EDS mapping analysis was performed for the entire glass system. A representative image of V6 glass is shown in Figure 4; the red, blue, yellow, and green dots correspond to O, P, Ca, and V distribution states, respectively.
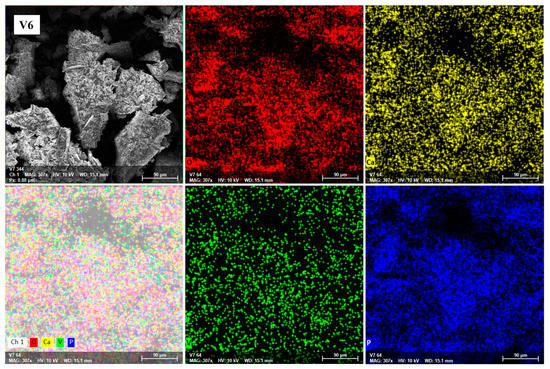
Figure 4.
EDS mapping of O (red), Ca (yellow), V (green), and P (blue) of glass powder with x = 8 mol% V2O5 before the dissolution experiments.
Our data show a good chemical homogeneity of the detected elements in all glasses (M and V1–V7). Moreover, the results of the elemental analysis are in accordance with the compositional elements used in their preparation, supporting and confirming the existence of strict stoichiometric ratios among the constituent atoms.
2.1.4. EPR
The EPR spectra of a vanadium-doped xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 16 mol% are presented in Figure 5A.
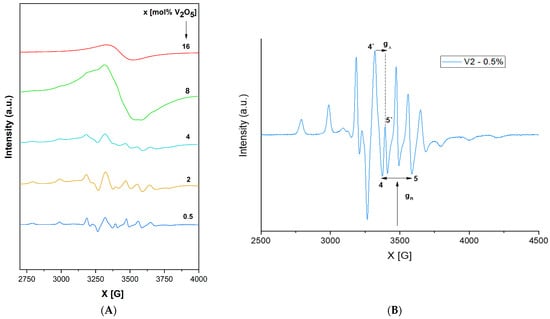
Figure 5.
(A). EPR spectra of a xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 16 mol%. (B). Individual EPR spectra of a xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system with x = 0. 5 mol%.
According to our previous research on V2O5-doped phosphate glasses [48,49,50], vanadium oxide acts both as a network modifier for low- and medium-vanadium-content glasses and a network former for higher-content glasses (due to the similarity of VO4 tetrahedral units to phosphate units).
Vanadium has five valence electrons that can be lost and in glasses or glass ceramics and can exist in different ionic forms (i.e., V3+, V4+, and V5+) [49,50,51].
For the glass system investigated in the present study, as shown in Figure 5A,B, EPR spectra show a well-resolved hfs (hyperfine structure) for 0.25 ≤ x ≤ 4 mol %, with a structure based on 16 lines (8 in the parallel and 8 in the perpendicular band) attributed to the interaction of a 3d1 spin electron with 51V nuclear spin (I = 7/2). The spectral was generated based on a spin Hamiltonian appropriate for these spectra previously reported in [52,53,54,55]. EPR Hamiltonian parameters, MO coefficients, the Fermi contact coefficient (K), and the dipolar hyperfine coupling parameter (P) were calculated using the LCAO-MO model developed by Kivelson and Lee [56] for vanadyl ions.
The EPR data obtained in the present study (Table 2A) indicate that g‖ < g⊥ < ge (ge = 2.0023) and A‖ > A⊥, revealing that V4+ ions incorporated in the phosphate network are present in the form of vanadyl ions (VO2+) [52,53,57] in a pyramidal site such as C4v symmetry (an octahedral coordination with a tetragonal compression). The prevalent axial distortion of the VO2+ octahedral oxygen complex along the V = O bond is assumed to be the cause of relatively constant values for all the studied glasses, showing only a very slight variation with the content [58]. In [53], the value of A tensor was connected with the covalence degree between the paramagnetic ion and its ligands. The smaller the A value, the higher the covalence degree. The present results indicate a consistently higher A‖ value compared with A⊥ and therefore a great ionicity of the bonds in their axial direction. This statement is also supported by the values of MO coefficients (Table 2B), which show an ionic character for in-plane σ and π bonds (β22 ~ 1) but a stronger covalency for out-of-plane vanadyl oxygen (επ2 ~ 0.6), which is also consistent with the square-pyramidal coordination of vanadium ions in these glasses. K is a dimensionless Fermi contact interaction parameter [52] representing the amount of unpaired electron density at the vanadium nucleus location. The value obtained for the present glass system (k ~ 0.7) is in agreement with the literature [52,54,57,58,59], indicating a poor contribution of the vanadium 4s orbital to the vanadyl bond. The calculated dipolar hyperfine coupling parameter value (P ~ 142 cm−1) is also in agreement with the literature [26,52,54,57,58,59].

Table 2.
(A). EPR parameter and MO coefficients for a xV2O5∙ (100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 4 mol%. (B). MO coefficients for a xV2O5∙ (100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 4 mol%.
With x < 4 mol% content of vanadium ions, the network is dominated by polymeric phosphate long-range structures with isolated paramagnetic ions in a specific position. As the content of vanadium ions increases (x ≥ 4 mol %), phosphate network depolymerization is fastened, and the isolated distribution of metallic ions is affected, allowing the appearance of dipole–dipole interactions between them. EPR data suggest the network-modifier role of V2O5 for x ≥ 4 mol %. For x > 4 mol %, the EPR spectra appear as a superposition of two signals, one with a well-resolved hfs structure typical of isolated ions and a second broad line typical of clustered ions. Figure 6 illustrates the decrease in the broad line with increasing vanadium concentration over 4 mol %, which correlates with previously reported data [54], indicating that superexchange magnetic interactions appear in V4+–O2–V4+ chains; this is consistent with the network-former role of vanadium oxide, which isolates the phosphate structures [26]. It was previously reported [48] that the presence of one fluorine ion may coordinate V4+ ion transposition with “yl” oxygen and substitute the 6th. This would lead to increased g‖ and A‖ values and a broadening of the vanadium hfs lines. This effect was not observed in the present glass system, although it contains fluorine ions.
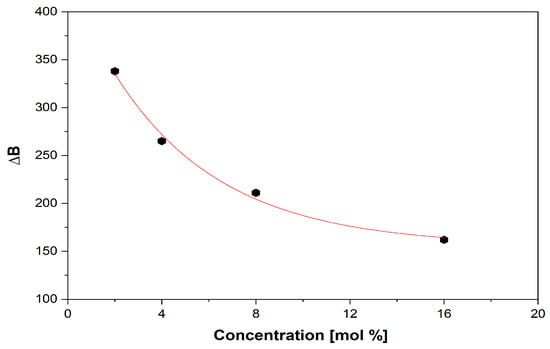
Figure 6.
Decrease in the broad line (∆B) with increasing vanadium content.
2.1.5. FT-IR
IR spectra of the xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 16 mol% in the range of 400–1400 cm−1 are presented in Figure 7. For the purposes of comparison and discussion, the relative intensities of the assigned vibrational-band IR spectra were normalized [60].
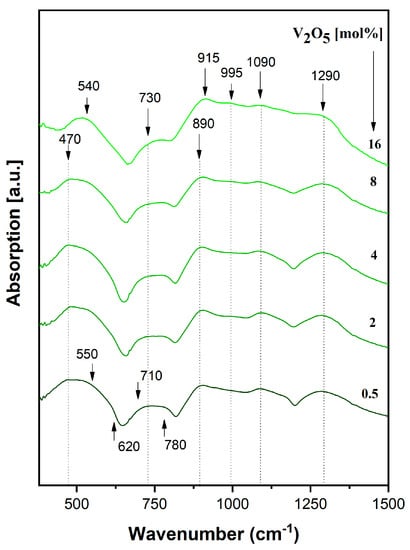
Figure 7.
IR spectra of a xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system.
Phosphate networks traditionally indicate specific bands in the 450–1300 cm−1 [26,47,49,61,62,63,64], which are clearly visible in the present glass system starting with less concentrated vanadium (x = 0.5 mol %):
- (a)
- Bands at ~ 475 cm−1, 550 cm−1, and 620 cm−1 are attributed to PO2 bending vibrations in P–O–P and O–P–O chains in PO4 groups and ring phosphate units;
- (b)
- Bands at ~ 710 cm−1 and ~780 cm−1 are attributed to P–O–P symmetrical stretching vibrations in the ring phosphate units;
- (c)
- Bands at ~ 890 cm−1 and ~ 915 cm−1 are assigned to asymmetrical stretching vibrations in the ring phosphate units;
- (d)
- Bands at ~ 995 cm−1 and ~ 1090 cm−1 are attributed to PO2 stretching vibrations due to free oxygen atoms and a solid-state effect; and
- (e)
- The band at ~1290 cm−1 belongs to the stretching vibrations of the phosphate network P = O double bond.
No significant changes were observed in the shape of IR spectra with up to 4 mol% vanadium content.
As vanadium content increases over 4 mol%, the band at 550 cm−1 increases in intensity and it is shifted to 540 cm−1 due to the superposition of phosphate bands and lattice vibrations of the V2O5 oxide network, especially at 16 mol % vanadium content. The bands at 620 and 710 cm−1 decrease in intensity until disappearance in the glasses with 16 mol %, due to the depolymerization effect of vanadium oxide. The band at 780 cm−1 is shifted towards 730 cm−1, mainly as a result of changes in the P–O–P length and a disorder effect produced by the depolymerization process induced by high vanadium content. The band at 890 cm−1 increases in intensity. Stretching vibrations in V–O bonds of VO4 tetrahedra are superpositioned over the stretching P–O–P vibration modes—an effect that is visible in the IR spectra over 4 mol %.
With a high content of vanadium introduced in the phosphate network (16 mol %), the bands at 915 cm−1, 995 cm−1, and 1090 cm−1 are better-located and shaped. The bands at 915 cm−1 and 995 cm−1 result from the superposition of P–O–P stretching vibration modes over V–O vibrations in VO2 trigonal by pyramids, and the band at 1090 cm−1 derives from the superposition of phosphate vibrations with V = O double-bond vibrations. The band at ~ 1290 cm−1 slowly shifts at 1300 cm−1 with high vanadium content and decreases in intensity due to V–O = P bonds, which weaken the double P = O bond in the process of network depolymerization [26,48,50,61,64,65,66,67].
Both EPR spectra and coefficients, as well as IR spectra, show the very clear vanadium oxide depolymerization effect.
2.2. In Vitro Biological Evaluation (Antitumoral Activity MTT Assay)
For in vitro biological evaluation, two glass samples (V7 and M) were selected from the xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system with 0 ≤ x ≤ 16 mol%. The V7 glass sample (x = 16 mol% V2O5) was chosen due to its high solubility and the increased amount of doping. The M glass sample (x = 0 mol%) was chosen as the standard. The extracts were obtained as described in Section 3.3.3 and used as two treatments (Figure 8): (i) V2O5 treatment (V7 glass extract) and (ii) matrix treatment (M glass extract).
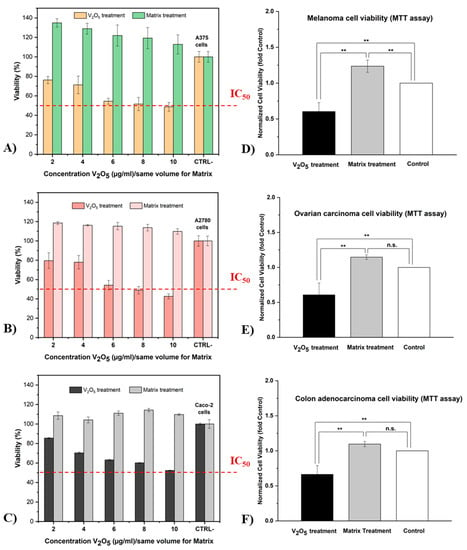
Figure 8.
Percentage of viability of three different tumor cell lines (A, A375; B, A2780; and C, Caco-2) after 24 h incubation with glass extracts V7 and M in different concentrations (n = 5; error bars represent min–max values) andsignificant differences between the two treatments and the control (D,E) and within treatments (F) (mean ± SD, ** p < 0.01, n.s. = non-significant).
As shown in Figure 8A–C, an MTT assay was applied to several different human tumor lines (A375, A2780, and Caco-2) in order to highlight the antitumor character of vanadium ions from the V7 glass sample. Furthermore, dose–response experiments were performed for a limited concentration range (2, 4, 6, 8, and 10 μg/mL) with a relatively short incubation period (24 h) for both treatments. Cell viability without treatment/extract was chosen as the control (CTRL, 100%).
The results of the MTT assay showed that the V2O5 treatment exerted a strong antiproliferative effect on all tumor cell lines. This treatment caused a slight inhibition of tumor cell growth from the first concentration of 2 μg/mL V2O5. In addition, with increasing concentration, the efficacy of V2O5 treatment became increasingly evident, reaching a rate of inhibition of over 50% at the highest concentration (10 μg/mL V2O5) for the A375 and A2780 cell lines. According to ISO 10993-5 (in vitro cytotoxicity test), cell viability is determined as a percentage of the control; if cell viability is less than 70%, the material is cytotoxic [68]. In this case, concentrations of 6, 8, and 10 μg/mL V2O5 were considered to be cytotoxic for the studied tumor lines.
All types of cancer cells exhibit an increase in metabolic activity; therefore, redox homeostasis is of crucial importance for their survival. Disruption of this homeostasis with the purpose of triggering ROS-induced apoptotic signaling can be achieved with vanadium ions due to their increased redox potential. As demonstrated by Guerrero-Palomo et al. [29], this redox process is influenced by the oxidation state of the vanadium cation presented in the coordination sphere of the complex.
Concerning the matrix treatment (without vanadium), we can say that it did not interfere with cell viability and was therefore not cytotoxic for these tumor lines. Moreover, it provided an incentive physiological environment for cell attachment and therefore helped in cell development. Compared with V2O5 treatment and the CTRL-, it produced a considerable proliferative effect on melanoma, ovarian, and colon carcinoma cells. Additionally, as the concentration (dose) increased, the viability of tumor cells was slightly affected, although still not less than 100%. This effect can be explained by the composition of the matrix extract, which contains the main elements of glass M (trace elements such as Ca, P, and F). These elements are also naturally found in the human body and are biocompatible with biological systems [69].
According to statistical analyses (see Figure 8D–F), highly significant differences in cytotoxicity were found between the two types of treatment (p < 0.01). These differences were validated for all tested tumor cell lines. Surprisingly, no statistically significant differences were found between the matrix treatment and the control (cells grown without extract) for the A2780 and Caco-2 cell lines. However, significant differences were found between the matrix treatment and the control for the A375 line.
Therefore, our results suggest that the proliferation of the three tumor lines (A375, A2780, and Caco-2) was strongly affected by the applied V2O5-based treatment in a manner dependent on the concentration of V2O5 ions. Furthermore, the matrix treatment (without V2O5) provided an optimal environment for cell attachment and proliferation.
The incorporation of vanadium ions in various types of bioactive glasses and bioceramics is attractive for various biomedical applications, including drug delivery, tissue engineering, and cancer therapy. As shown throughout our work, V2O5-containing glass exerts in vitro strong cytotoxic effects against tumor cell lines A375, A2780, and Caco-2. However, owing to the lack of coherence of the chemical structure and the mechanism of action of vanadium ions, in vivo prediction is difficult to realize because experimental (chemical and biological) conditions are always more complex and significantly different from the conditions in clinical trials.
3. Materials and Methods
3.1. Preparation of Bioactive Glasses
The raw materials used in the present investigation, CaF2, P2O5, V2O5 (Alfa Aesar, Karlsruhe, Germany), and CaCO3 (Petr Švec—PENTA, Prague, Czech Republic), were of reagent-grade purity. The glass samples comprising xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] with 0 ≤ x ≤ 16 mol% were prepared by weighting suitable proportions of each component. The powders were mixed and melted in Al2O3 crucibles at 1170 °C in an electric furnace (PLF 110/6 Chamber Furnace) for 15 min. Homogenized melts were quenched to room temperature (RT) by pouring them on a stainless-steel plate and quickly pressing it with another plate. The prepared glass samples were mechanically (crushed) and chemically (dissolved) processed according to the analysis package used.
3.2. Sample Characterization
3.2.1. Dissolution Test
We used dissolution tests in order to better understand the dynamics of doping the phosphate glasses. These tests were performed for all the powder samples under the same experimental conditions, deionized water as an immersion liquid at a temperature of 37 °C, which we chose to ensure a close resemblance to the temperature of the human body. The relative weight loss (Wloss) of each sample after immersion was expressed as a percentage and calculated according to the following equation:
where mi is the initial weight of each sample, and mf is the weight after immersion in 10 mL deionized water.
For each sample, 100 mg was immersed in 10 mL of deionized water at 37 °C with continuous orbital shaking (300 rpm) on a titer plate shaker (Heidolph™ Titramax 1000, Hamburg, Germany) for 14 days. After incubation, the resulting suspensions were decanted and vacuum-filtered using Whatman-41 filter paper. The resulting sediment was then dried at RT for 3 days and weighted using a Precise XT220A analytical balance with a self-calibration system (SCS).
3.2.2. pH Evolution
In order to assess pH changes over time, powdered glass (100 ± 0.5 mg) was immersed in 10 mL isotonic buffer (PBS, phosphate-buffered saline, pH 7.42) for up to 21 days at 37 °C. The PBS was synthesized according to AAT Bioquest, Inc. [70] (Table 3). The pH values were measured immediately after immersion for different incubation periods (1, 2, 5, 9, 14, and 21 days) using a digital pH meter (Titroline easy, Schott, Mainz, Germany).

Table 3.
The reagents used in the preparation of the 1 L buffer solution (PBS, pH 7.4).
The electrode was calibrated using standard pH values of 4.01, 7.00, and 10.01 before taking the pH measurements. Values were obtained with an estimated error of ± 0.02.
3.2.3. SEM-EDS
To evaluate their composition and morphology, the glasses were analyzed using an SEM VEGA3 SBU-EasyProbe scanning electron microscope (Tescan, Bron, Czech Republic) with an energy-dispersive X-ray spectroscopy Quantax 200 EDS detector (Bruker, Berlin, Germany). The glass samples (powdered) were mounted on an aluminum stud using a double-sided adhesive carbon tape and measured in duplicate at RT. All quantitative EDS elementary data were calculated assuming the stoichiometry of the oxides and normalization to 100%.
3.2.4. EPR
EPR measurements of powder samples were carried out at RT with a Bruker Biospin EMX spectrometer operating in the X band (9–10 GHz). The EPR parameters were set at 100 KHz modulation frequency, microwave power of 10 mW, modulation amplitude of 3 G, time constant of 2.56 ms, scan time of 61 s, and receiver gain of 103. To avoid the alteration of the glass structure due to ambient conditions, especially humidity, samples were powdered and enclosed in tubular holders of the same caliber. Equal quantities of samples were studied.
3.2.5. FT-IR
Fourier transform infrared (FT-IR) absorption spectra were recorded using a JASCO 4100 spectrometer with a spectral range of 350 cm−1 to 4000 cm−1. Detection was carried out with a DLATGS detector with a KBr window at RT. The samples were prepared using the KBr pellet technique and measured at a resolution of 4 cm−1 with 256 scans/sample.
3.3. In Vitro Cytotoxicity Tests
3.3.1. Cell Culture
Three cancer cell lines were studied: the A-375 (CRL-1619™) human melanoma cell line, the Caco-2 (HTB-37™) human colon adenocarcinoma cell line from the ATCC (American Type Culture Collection, Rockville, MD, USA), and the A2780 human ovarian carcinoma cell line from the ECACC (European Collection of Authenticated Cell Cultures, Salisbury, UK).
All cell lines were initially grown and then cultured using fresh medium supplemented as recommended by the suppliers; the A375 cell line was maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5 g/L glucose 2 mM L-glutamine supplemented with 10% fetal bovine serum (FBS) and without antibiotics; the Caco-2 cell line was cultivated in Minimum Essential Medium (MEM), which contained 2 mM L-glutamine and % FBS without antibiotics; and the A2780 cell line was maintained in Roswell Park Memorial Institute (RPMI)–1640 medium supplemented with 2 mM L-glutamine, 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin as antibiotics. Cells were plated in 25 cm2 tissue culture flasks and grown under standard conditions: 37 °C, 95% humidity, and 5% CO2 in air.
3.3.2. Cell Subculture
To subculture the cells, they were divided, and the culture medium was replaced with fresh medium every 2–3 days as follows. First, the old medium was removed, and the cells were rinsed briefly with phosphate-buffered saline (PBS) at least 2 times. Five hundred milliliters of trypsin was then added, and the flask was incubated at 37 °C under 5% CO2 for 5–10 min. After the cells had detached from the lower part of the flask, 2 mL of fresh medium was added for trypsin inactivation. The slurry was then collected in a 15 mL tube and centrifuged at 800 g at RT for 10 min. After centrifugation, the supernatant was removed, and the pellet was mixed with 1 mL of fresh medium. The new culture was divided into two parts and transferred to two new flasks with 10 mL of fresh medium.
3.3.3. Preparation of Extracts
Based on structural analyses and dissolution tests, two glass samples were chosen for in vitro bioactivity assessment (V7 and M). Each sample was extracted in deionized water (Milli-Q, Millipore GmbH, Schwalbach, Germany) with a concentration of 10 mg powder sample per 1 mL deionized water and incubated for 10 days at 37 °C with discontinuous stirring. After incubation, the formed colloidal solutions were centrifuged at 9000 rpm at RT for 10 min and filtered using a Whatman paper filter. Moreover, the obtained extracts were refiltered through a 0.22 μm Millex-GP syringe filter unit (Millipore, catalog number SLGP05010) and diluted with culture medium to obtain five serial concentrations of extract (2, 4, 6, 8, and 10 V2O5 μg/mL). For the matrix sample (without doping), we used the same volumetric quantities as those for extract V7.
3.3.4. MTT Cell Proliferation Assay
The cells were grown until they were confluent. Then, they were trypsinized, and two hundred microliters of suspension containing 5 × 104 cells was seeded in each well of a 96-well microtiter plate (Eppendorf, Germany) and incubated overnight at 37 °C with 5% CO2. On the following day, the medium was replaced, and the cells were treated with the two glass extracts (M and V7) in various concentrations (2, 4, 6, 8, and 10 µg/mL) and maintained at 37 °C with 5% CO2 for 24 h. After the incubation period, the old medium was removed from the wells and rinsed with 150 µL PBS (Ca- and Mg-enriched). Then, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) was added, resulting in a final volume of 150 µL per well. The microtiter plate was then incubated again for 2 h at 37 °C with 5% CO2. After incubation, the MTT was removed, 150 µL of dimethyl sulfoxide (DMSO) was added over each well, and the cell viability was measured using a microplate reader (Synergy HT Multi-Mode BioTek, USA) at 550 and 630 nm (for background). The data are presented as the percentage of viable cells calculated according to Equation (2).
where ODsis the optical density (in units) for the sample, ODb is the optical density for the blank wells, and ODc is the optical density for the control wells. The results are expressed as percent survival relative to an untreated control (CTRL-), and the experiment was performed with five repetitions for each treatment concentration to ensure a better and more accurate prediction.
% Cell Viability = [(ODs − ODb)/(ODc − ODb)] × 100
3.3.5. Statistical Analysis
All biological experiments were performed at least twice. Data are expressed as survival rate versus untreated control (CTRL-) for the cell viability of each treatment and mean ± standard deviation (SD) for each treatment type. For cell culture experiments, one replication represents the average of five wells containing glass-extract-treated or -untreated cells. In order to determine significant differences between types of treatments, a one-way analysis of variance (ANOVA) was performed, followed by Tukey’s HSD multiple range test. The significance of differences was defined at the 1% level (p < 0.01).
4. Conclusions
V2O5-doped phosphate glasses were successfully prepared by the conventional melt-quenching method with a chemical composition of xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] (where x = 0, 0.5, 1, 2, 4, 8, and 16 mol%) by varying the vanadium pentoxide content.
Dissolution tests demonstrates that the addition of V2O5 does not affect the dissolution rate of the prepared materials.
All glasses caused a decrease in the pH value of the PBS solution, proving the reactivity of the system. This phenomenon was caused by a rapid ion exchange between network-modifier ions (here, F− and VnO2n+1) attached to non-bridging oxygen ions (NBOs) and HO from the solution.
SEM analysis illustrated that the structure and the homogeneity of vanadium-containing phosphate glasses do not change with doping concentration. EDS analysis confirmed the presence of all elements (P, O, Ca, and V) according to the nominal composition.
EPR revealed a well-resolved hyperfine structure (hfs) typical of vanadyl ions in a C4v symmetry for glasses with 0.25 ≤ x ≤ 4 mol % V2O5. Additionally, with x > 4 mol% V2O5, the spectra showed the superposition of two EPR signals, one due to an hfs structure and the other consisting of a broad line typical of clustered ions.
FT-IR spectra of the xV2O5∙(100 − x) [CaF2∙3P2O5∙CaO] glass system showed the presence of all structural units characteristic of P2O5 in the glass structure, as well as a very clear depolymerization effect due to vanadium oxide (V2O5).
MTT assay showed that V2O5-based treatment has a strong antitumoral effect on cell growth, whereas matrix treatment does not interfere with cell viability and is therefore not cytotoxic for tested cell lines. In addition, the V2O5-based treatment has a visible inhibitory effect on tumor cells (from the lowest concentration used, i.e., 2 μg/mL V2O5) that increases directly proportionally to V2O5 concentration. Statistical analyses showed significant differences in cytotoxicity between the two types of treatment (p < 0.01).
In conclusion, the use of vanadium-containing bioglass particles should be encouraged for the development of new biomaterials and devices with biomedical applications, including innovative drug delivery methods and effective new cancer therapy options.
Author Contributions
Conceptualization, R.Ș. and C.L.; Methodology, R.Ș., M.Z., D.S. and C.L.; Simulation work, D.S., C.L., M.Z., G.D. and R.Ș.; Validation, C.L., D.S., G.D., M.Z. and R.Ș.; Formal Analysis, N.S.V., C.L., G.D., M.Z. and R.Ș.; Resources, R.Ș. and D.S.; Writing—original draft preparation, C.L., N.S.V. and R.Ș.; Writing—review and editing, N.S.V., C.L. and R.Ș.; Supervision, R.Ș. and D.S.D.; Funding acquisition, D.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rehder, D. The Potentiality of Vanadium in Medicinal Applications. Inorg. Chim. Acta 2020, 504, 119445. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 March 2022).
- Cancer Facts for Women|Most Common Cancers in Women. Available online: https://www.cancer.org/healthy/cancer-facts/cancer-facts-for-women.html (accessed on 2 March 2022).
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M. Bio-Ceramics with Clinical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 978-1-118-40672-4. [Google Scholar]
- Scholze, H. Glass: Nature, Structure, and Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4613-9069-5. [Google Scholar]
- Lepry, W.C.; Nazhat, S.N. A Review of Phosphate and Borate Sol–Gel Glasses for Biomedical Applications. Adv. NanoBiomed. Res. 2021, 1, 2000055. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Patel, U.; Kennedy, A.R.; Macri-Pellizzeri, L.; Sottile, V.; Grant, D.M.; Scammell, B.E.; Ahmed, I. Porous Calcium Phosphate Glass Microspheres for Orthobiologic Applications. Acta Biomater. 2018, 72, 396–406. [Google Scholar] [CrossRef]
- Szewczyk, A.; Skwira, A.; Konopacka, A.; Sądej, R.; Prokopowicz, M. Mesoporous Silica-Bioglass Composite Pellets as Bone Drug Delivery System with Mineralization Potential. Int. J. Mol. Sci. 2021, 22, 4708. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Migneco, C.; Fiume, E.; Miola, M.; Ferraris, S.; Spriano, S.; Ferraris, M.; Verné, E. Glasses and Glass–Ceramics for Biomedical Applications. In Ceramics, Glass and Glass-Ceramics: From Early Manufacturing Steps Towards Modern Frontiers; Baino, F., Tomalino, M., Tulyaganov, D., Eds.; PoliTO Springer Series; Springer International Publishing: Cham, Switzerland, 2021; pp. 153–201. ISBN 978-3-030-85776-9. [Google Scholar]
- Ibrahim, A.; Sadeq, M.S. Influence of Cobalt Oxide on the Structure, Optical Transitions and Ligand Field Parameters of Lithium Phosphate Glasses. Ceram. Int. 2021, 47, 28536–28542. [Google Scholar] [CrossRef]
- Alhasni, B. Insight into the Structure of Magnesium and Sodium Mixed Phosphate Glasses: A Molecular Dynamics Study. J. Non-Cryst. Solids 2022, 578, 121338. [Google Scholar] [CrossRef]
- Stefan, R.; Simedru, D.; Popa, A.; Ardelean, I. Structural Investigations of V2O5-P2O5-CaO Glass System by FT-IR and EPR Spectroscopies. J. Mater. Sci. 2012, 47, 3746–3751. [Google Scholar] [CrossRef]
- Edathazhe, A.B.; Shashikala, H.D. Dissolution Studies of Na2O-BaO-CaO-P2O5 Glasses in Deionized Water under Semi-Dynamic Conditions for Bioactive Applications. Mater. Today: Proc. 2018, 5, 21241–21247. [Google Scholar] [CrossRef]
- Lee, S.; Nagata, F.; Kato, K.; Nakano, T.; Kasuga, T. Structures and Dissolution Behaviors of Quaternary CaO-SrO-P2O5-TiO2 Glasses. Materials 2021, 14, 1736. [Google Scholar] [CrossRef] [PubMed]
- Lapa, A.; Cresswell, M.; Jackson, P.; Boccaccini, A.R. Phosphate Glass Fibres with Therapeutic Ions Release Capability—A Review. Adv. Appl. Ceram. 2020, 119, 1–14. [Google Scholar] [CrossRef]
- Hyunh, N.B.; Palma, C.S.D.; Rahikainen, R.; Mishra, A.; Azizi, L.; Verne, E.; Ferraris, S.; Hytönen, V.P.; Sanches Ribeiro, A.; Massera, J. Surface Modification of Bioresorbable Phosphate Glasses for Controlled Protein Adsorption. ACS Biomater. Sci. Eng. 2021, 7, 4483–4493. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive Calcium Phosphate–Based Glasses and Ceramics and Their Biomedical Applications: A Review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- Ningthemcha, R.K.N.; Mondal, R.; Das, A.S.; Debnath, S.; Kabi, S.; Singh, L.S.; Biswas, D. The Effect of Transition Metal and Heavy Metal Incorporation on the Structural, Optical and Electrical Properties of Zinc-Phosphate Ternary Glassy System: A Comparative Study. Mater. Chem. Phys. 2022, 278, 125672. [Google Scholar] [CrossRef]
- Karimi, M.; Mehdizadeh, A.; Hekmatshoar, M.H.; Vafaei, S. A New Method for Controlling Structural, Electrical and Optical Properties of Phosphate Glasses, Containing Transition Metal Ions (TMIs). J. Non-Cryst. Solids 2019, 525, 119693. [Google Scholar] [CrossRef]
- Vedeanu, N.S.; Magdas, D.A. The Influence of Some Transition Metal Ions in Lead- and Calcium-Phosphate Glasses. J. Alloys Compd. 2012, 534, 93–96. [Google Scholar] [CrossRef]
- Arya, S.K.; Danewalia, S.S.; Arora, M.; Singh, K. Effect of Variable Oxidation States of Vanadium on the Structural, Optical, and Dielectric Properties of B 2 O 3 –Li 2 O–ZnO–V 2 O 5 Glasses. J. Phys. Chem. B 2016, 120, 12168–12176. [Google Scholar] [CrossRef] [PubMed]
- Lusvardi, G.; Sgarbi Stabellini, F.; Salvatori, R. P2O5-Free Cerium Containing Glasses: Bioactivity and Cytocompatibility Evaluation. Materials 2019, 12, 3267. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Li, J.; Pu, X.; Wang, J.; Huang, Z.; Yin, G. Effects of Incorporated Vanadium and Its Chemical States on Morphology and Mesostructure of Mesoporous Bioactive Glass Particles. Microporous Mesoporous Mater. 2021, 319, 111061. [Google Scholar] [CrossRef]
- Cicconi, M.R.; Lu, Z.; Uesbeck, T.; van Wüllen, L.; Brauer, D.S.; de Ligny, D. Influence of Vanadium on Optical and Mechanical Properties of Aluminosilicate Glasses. Front. Mater. 2020, 7, 161. [Google Scholar] [CrossRef]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Mohamadi, M.; Ebrahimipour, S.Y.; Torkzadeh-Mahani, M.; Foro, S.; Akbari, A. A Mononuclear Diketone-Based Oxido-Vanadium(IV) Complex: Structure, DNA and BSA Binding, Molecular Docking and Anticancer Activities against MCF-7, HPG-2, and HT-29 Cell Lines. RSC Adv. 2015, 5, 101063–101075. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Palomo, G.; Rendón-Huerta, E.P.; Montaño, L.F.; Fortoul, T.I. Vanadium Compounds and Cellular Death Mechanisms in the A549 Cell Line: The Relevance of the Compound Valence. J. Appl. Toxicol. 2019, 39, 540–552. [Google Scholar] [CrossRef]
- Chatterjee, N.; Anwar, T.; Islam, N.S.; Ramasarma, T.; Ramakrishna, G. Growth Arrest of Lung Carcinoma Cells (A549) by Polyacrylate-Anchored Peroxovanadate by Activating Rac1-NADPH Oxidase Signalling Axis. Mol. Cell Biochem. 2016, 420, 9–20. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.; Zhou, H.; Jin, P.; Cheung, K.M.C.; Chu, P.K.; Yeung, K.W.K. Vanadium Dioxide Nanocoating Induces Tumor Cell Death through Mitochondrial Electron Transport Chain Interruption. Glob. Chall. 2019, 3, 1800058. [Google Scholar] [CrossRef]
- Khalil, A.A.; Jameson, M.J. Sodium Orthovanadate Inhibits Proliferation and Triggers Apoptosis in Oral Squamous Cell Carcinoma in Vitro. Biochem. Mosc. 2017, 82, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jiang, W.; Li, D.; Gu, M.; Liu, K.; Dong, L.; Wang, C.; Jiang, H.; Dai, W. Sodium Orthovanadate inhibits growth and triggers apoptosis of human anaplastic thyroid carcinoma cells in vitro and in vivo. Oncol. Lett. 2019, 17, 4255–4262. [Google Scholar] [CrossRef]
- Lu, L.-P.; Suo, F.-Z.; Feng, Y.-L.; Song, L.-L.; Li, Y.; Li, Y.-J.; Wang, K.-T. Synthesis and Biological Evaluation of Vanadium Complexes as Novel Anti-Tumor Agents. Eur. J. Med. Chem. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, S.; Chakraborty, T. Vanadium Quercetin Complex Attenuates Mammary Cancer by Regulating the P53, Akt/MTOR Pathway and Downregulates Cellular Proliferation Correlated with Increased Apoptotic Events. Biometals 2018, 31, 647–671. [Google Scholar] [CrossRef]
- Williams, G.H.; Stoeber, K. The Cell Cycle and Cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Liu, Y.-J.; Wang, Q.; Yang, X.-G.; Wang, K. Reactive-Oxygen-Species-Mediated Cdc25C Degradation Results in Differential Antiproliferative Activities of Vanadate, Tungstate, and Molybdate in the PC-3 Human Prostate Cancer Cell Line. J. Biol. Inorg. Chem. 2012, 17, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.P.; Videira, A.; Soares, P.; Máximo, V. Orthovanadate-Induced Cell Death in RET/PTC1-Harboring Cancer Cells Involves the Activation of Caspases and Altered Signaling through PI3K/Akt/MTOR. Life Sci. 2011, 89, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhao, H.; Tao, L.; Li, X.; Zhou, Z.; Sun, Y.; Chen, C.; Wei, D.; Liu, Y.; Diao, G. Synthesis, in Vitro Cytotoxicity, and Structure–Activity Relationships (SAR) of Multidentate Oxidovanadium(IV) Complexes as Anticancer Agents. Dalton Trans. 2018, 47, 10035–10045. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, H.; Yano, T.; Myochin, M.; Matsuyama, K.; Okita, T.; Miyamoto, S. Chemical Durability of Iron-Phosphate Glass as the High Level Waste from Pyrochemical Reprocessing. Procedia Chem. 2012, 7, 764–771. [Google Scholar] [CrossRef]
- Çelikbilek Ersundu, M.; Kuzu, B.; Ersundu, A.E. Structural Properties and Dissolution Behavior of New Generation Controlled Release Phosphate Glass Fertilizers. J. Non-Cryst. Solids 2022, 576, 121239. [Google Scholar] [CrossRef]
- Bunker, B.C.; Arnold, G.W.; Wilder, J.A. Phosphate Glass Dissolution in Aqueous Solutions. J. Non Cryst. Solids 1984, 64, 291–316. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Farag, M.M.; Knowles, J.C. Dissolution and Drug Release Profiles of Phosphate Glasses Doped with High Valency Oxides. J. Mater. Sci. Mater. Med. 2016, 27, 108. [Google Scholar] [CrossRef]
- Es-Soufi, H.; Bih, L.; Benzineb, M. Study of Tungsten Phosphate Glasses Containing Fe2O3. New J. Glass Ceram. 2019, 9, 33–49. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; O’Donnell, M.D.; Law, R.V.; Hill, R.G. Fluoride-Containing Bioactive Glasses: Effect of Glass Design and Structure on Degradation, PH and Apatite Formation in Simulated Body Fluid. Acta Biomater. 2010, 6, 3275–3282. [Google Scholar] [CrossRef]
- Kiran, P.; Ramakrishna, V.; Trebbin, M.; Udayashankar, N.K.; Shashikala, H.D. Effective Role of CaO/P2O5 Ratio on SiO2-CaO-P2O5 Glass System. J. Adv. Res. 2017, 8, 279–288. [Google Scholar] [CrossRef]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High Phosphate Content Significantly Increases Apatite Formation of Fluoride-Containing Bioactive Glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Vedeanu, N.; Stanescu, R.; Filip, S.; Ardelean, I.; Cozar, O. IR and ESR Investigations on V2O5–P2O5–BaO Glass System with Opto-Electronic Potential. J. Non-Cryst. Solids 2012, 358, 1881–1885. [Google Scholar] [CrossRef]
- Ardelean, I.; Cozar, O.; Vedeanu, N.; Rusu, D.; Andronache, C. EPR Study of V2O5–P2O5–Li2O Glass System. J. Mater. Sci. Mater. Electron. 2007, 18, 963–966. [Google Scholar] [CrossRef]
- Vedeanu, N.; Cozar, O.; Ardelean, I. IR and EPR Investigations of V2O5-P 2O5-CaF2 Glass System. J. Optoelectron. Adv. Mater. 2007, 9, 698–701. [Google Scholar]
- Saddeek, Y.B.; Shaaban, E.R.; Aly, K.A.; Sayed, I.M. Characterization of Some Lead Vanadate Glasses. J. Alloys Compd. 2009, 478, 447–452. [Google Scholar] [CrossRef]
- Rameshkumar, V.; Chakradhar, R.; Murali, A.; Gopal, N.; Lakshmanarao, J. A Study of Electron Paramagnetic Resonance and Optical Absorption Spectra of VO2+ Ions in Alkali Barium Phosphate Glasses. Int. J. Mod. Phys. B 2012, 17, 3033–3047. [Google Scholar] [CrossRef]
- Hejda, P.; Holubova, J.; Černošek, Z.; Cernoskova, E. The Structure and Properties of Vanadium Zinc Phosphate Glasses. J. Non-Cryst. Solids 2017, 462, 65–71. [Google Scholar] [CrossRef]
- Vedeanu, N.S.; Cozar, I.B.; Stanescu, R.; Stefan, R.; Vodnar, D.; Cozar, O. Structural Investigation of V2O5–P2O5–K2O Glass System with Antibacterial Potential. Bull. Mater. Sci. 2016, 39, 697–702. [Google Scholar] [CrossRef]
- Seth, V.P.; Gupta, S.; Jindal, A.; Gupta, S.K. ESR of Vanadyl Ions in Li2O · BaO · B2O3 Glasses. J. Non-Cryst. Solids 1993, 162, 263–267. [Google Scholar] [CrossRef]
- Kivelson, D.; Lee, S. ESR Studies and the Electronic Structure of Vanadyl Ion Complexes. J. Chem. Phys. 1964, 41, 1896–1903. [Google Scholar] [CrossRef]
- Vedeanu, N.; Cozar, O.; Stanescu, R.; Cozar, I.B.; Ardelean, I. Structural Investigation of New Vanadium–Bismuth–Phosphate Glasses by IR and ESR Spectroscopy. J. Mol. Struct. 2013, 1044, 323–327. [Google Scholar] [CrossRef]
- Ardelean, I.; Cozar, O.; Ilonca, G.; Simon, V.; Mih, V.; Craciun, C.; Simon, S. EPR and Magnetic Susceptibility Studies on V2 O5-P2O5-PbO Glasses. J. Mater. Sci. Mater. Electron. 2000, 11, 401–404. [Google Scholar] [CrossRef]
- Kumar, R.V.S.S.S.N.; Bungala, C.J.; Chandrasekhar, A.; Reddy, B.; Reddy, Y.; Rao, P. Optical Absorption and EPR Spectral Studies on Vanadyl Doped Zinc Phosphate Glass. J. Alloys Compd. 1999, 287, 84–86. [Google Scholar] [CrossRef]
- Sekiya, T.; Mochida, N.; Soejima, A. Raman Spectra of Binary Tellurite Glasses Containing Tri- or Tetra-Valent Cations. J. Non-Cryst. Solids 1995, 191, 115–123. [Google Scholar] [CrossRef]
- Rada, S.; Culea, M.; Rada, M.; Culea, E. Effect of the Introduction of Vanadium Pentaoxide in Phospho-Tellurite Glasses Containing Gadolinium Ions. J. Mater. Sci. 2008, 43, 6122–6125. [Google Scholar] [CrossRef]
- Doweidar, H.; Moustafa, Y.M.; El-Egili, K.; Abbas, I. Infrared Spectra of Fe2O3–PbO–P2O5 Glasses. Vib. Spectrosc. 2005, 37, 91–96. [Google Scholar] [CrossRef]
- Bergo, P.; Reis, S.T.; Pontuschka, W.M.; Prison, J.M.; Motta, C.C. Dielectric Properties and Structural Features of Barium-Iron Phosphate Glasses. J. Non-Cryst. Solids 2004, 336, 159–164. [Google Scholar] [CrossRef]
- Lucacel Ciceo, R.; Todea, M.; Dudric, R.; Buhai, A.; Simon, V. Structural Effect of Cobalt Ions Added to a Borophosphate-Based Glass System. J. Non Cryst. Solids 2018, 481, 562–567. [Google Scholar] [CrossRef]
- Magdas, D.A.; Vedeanu, N.S.; Toloman, D. Study on the Effect of Vanadium Oxide in Calcium Phosphate Glasses by Raman, IR and UV–Vis Spectroscopy. J. Non-Cryst. Solids 2015, 428, 151–155. [Google Scholar] [CrossRef]
- Broglia, G.; Mugoni, C.; Du, J.; Siligardi, C.; Montorsi, M. Lithium Vanado-Phosphate Glasses: Structure and Dynamics Properties Studied by Molecular Dynamics Simulations. J. Non-Cryst. Solids 2014, 403, 53–61. [Google Scholar] [CrossRef]
- Ferraa, S.; Barebita, H.; Moutataouia, M.; Nimour, A.; Elbadaoui, A.; Baach, B.; Guedira, T. Effect of Barium Oxide on Structural Features and Thermal Properties of Vanadium Phosphate Glasses. Chem. Phys. Lett. 2021, 765, 138304. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(En), Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 2 January 2023).
- Kermani, F.; Mollazadeh Beidokhti, S.; Baino, F.; Gholamzadeh-Virany, Z.; Mozafari, M.; Kargozar, S. Strontium- and Cobalt-Doped Multicomponent Mesoporous Bioactive Glasses (MBGs) for Potential Use in Bone Tissue Engineering Applications. Materials 2020, 13, 1348. [Google Scholar] [CrossRef] [PubMed]
- PBS (Phosphate Buffered Saline) (1X, pH 7.4) Preparation and Recipe|AAT Bioquest. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/pbs-phosphate-buffered-saline (accessed on 30 August 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).