The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review
Abstract
1. Introduction
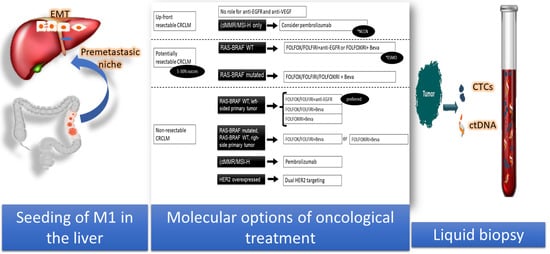
2. Metastatic Niche
3. Impact of the Metastasis Molecular Pattern in the Choice of Chemotherapy
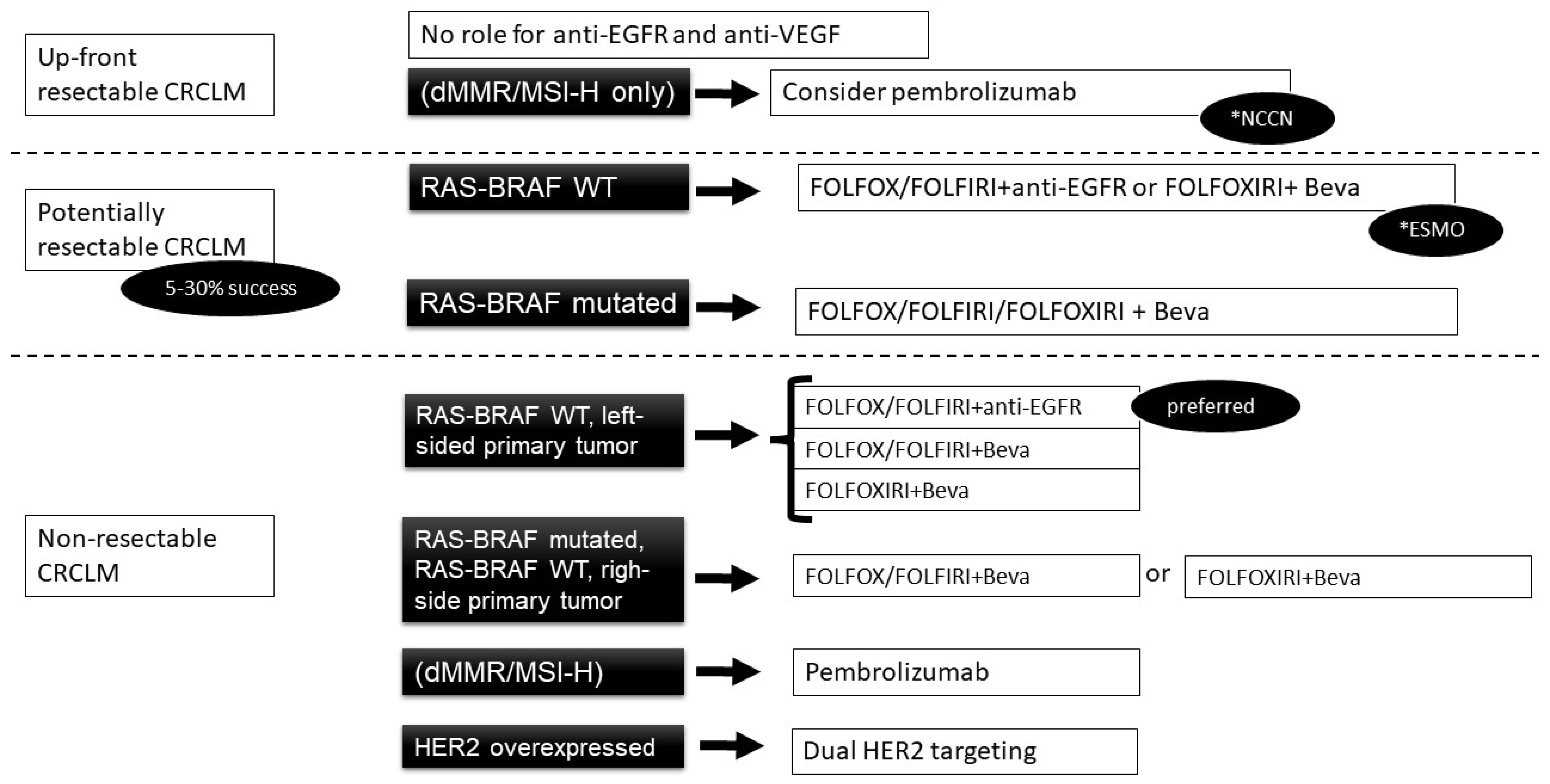
3.1. Current Indications for MCA
3.1.1. RAS Wild-Type Cancer
3.1.2. Anti-VEGF Agents
3.1.3. BRAF V600 Mutation
3.1.4. Different BRAF Mutations
3.1.5. Mismatch Repair
3.1.6. HER-2 Amplification
3.2. Initially Unresectable Metastatic Disease
3.3. Resectable Metastases
3.4. Unresectable Metastases
4. The Impact of the Molecular Profile on Follow-Up
4.1. Surveillance for Metastatic Liver Disease
4.2. Circulating Biomarkers
- (a)
- Proteins
- (b)
- Nucleic Acids
- (c)
- Exosomes and microvesicles
- (d)
- Circulating cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEA | carcinoembryonic antigen |
| cfDNA | circulating free DNA |
| CRC | colorectal cancer |
| CRCLM | colorectal cancer liver metastasis |
| CTCs | circulating tumour cells |
| ctDNA | circulating tumour DNA |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| EV | extracellular vesicles |
| MCA | monoclonal antibodies |
| miRNA | micro RNA |
| OS | overall survival |
| PFS | progression free survival |
| TEP | tumour-educated platelet |
| VEGF | vascular endothelial growth factor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Adam, R.; Köhne, C.H.; Pozzo, C.; Poston, G.; Ychou, M.; Rougier, P. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur. J. Cancer 2006, 42, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Borner, M.M. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer—Too good to be true? Editorial. Ann. Oncol. 1999, 10, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Recently Updated NCCN Clinical Practice Guidelines in OncologyTM. Available online: https://www.nccn.org/professionals/physician_gls/recently_updated.aspx (accessed on 19 March 2020).
- Nordlinger, B.; Guiget, M.; Vaillant, J.C.; Balladur, P.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef]
- Iwatsuki, S.; Dvorchik, I.; Madariaga, J.R.; Wallis Marsh, J.; Dodson, F.; Bonham, A.C.; Geller, D.A.; Gayowski, T.J.; Fung, J.J.; Starzl, T.E. Hepatic resection for metastatic colorectal adenocarcinoma: A proposal of a prognostic scoring system. J. Am. Coll. Surg. 1999, 189, 291–299. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Vauthey, J.N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A.; Dale, P.S.; Howard, R.J.; et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef]
- Fernandez, F.G.; Drebin, J.A.; Linehan, D.C.; Dehdashti, F.; Siegel, B.A.; Strasberg, S.M.; Fong, Y.; Wanebo, H.J.; Henderson, J.M.; Pinson, C.W. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann. Surg. 2004, 240, 438–450. [Google Scholar] [CrossRef]
- Muratore, A.; Zorzi, D.; Bouzari, H.; Amisano, M.; Massucco, P.; Sperti, E.; Capussotti, L. Asymptomatic colorectal cancer with un-resectable liver metastases: Immediate colorectal resection or up-front systemic chemotherapy? Ann. Surg. Oncol. 2007, 14, 766–770. [Google Scholar] [CrossRef]
- Alberts, S.R.; Horvath, W.L.; Sternfeld, W.C.; Goldberg, R.M.; Mahoney, M.R.; Dakhil, S.R.; Levitt, R.; Rowland, K.; Nair, S.; Sargent, D.J.; et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J. Clin. Oncol. 2005, 23, 9243–9249. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Weeks, J.C.; Catalano, P.J.; Cronin, A.; Finkelman, M.D.; Mack, J.W.; Keating, N.L.; Schrag, D. Patients’ expectations about effects of chemotherapy for advanced cancer. N. Engl. J. Med. 2012, 367, 1616–1625. [Google Scholar] [CrossRef]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; Garcia Alfonso, P.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab Plus mFOLFOX-6 or FOLFOXIRI in Patients with Initially Unresectable Liver Metastases From Colorectal Cancer: The OLIVIA Multinational Randomised Phase II Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef]
- Ye, L.C.; Liu, T.S.; Ren, L.; Wei, Y.; Zhu, D.X.; Zai, S.Y.; Ye, Q.H.; Yu, Y.; Xu, B.; Qin, X.Y.; et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013, 31, 1931–1938. [Google Scholar] [CrossRef]
- Drew, J.; Machesky, L.M. The liver metastatic niche: Modelling the extracellular matrix in metastasis. Dis. Model. Mech. 2021, 14, dmm048801. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Xiang, D.M.; Sun, W.; Ning, B.F.; Zhou, T.F.; Li, X.F.; Zhong, W.; Cheng, Z.; Xia, M.Y.; Wang, X.; Deng, X.; et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut 2018, 67, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Celià-Terrassa, T.; Kang, Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016, 30, 892–908. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef]
- Ali, S.R.; Jordan, M.; Nagarajan, P.; Amit, M. Nerve Density and Neuronal Biomarkers in Cancer. Cancers 2022, 14, 4817. [Google Scholar] [CrossRef]
- Naba, A.; Pearce, O.M.T.; Del Rosario, A.; Ma, D.; Ding, H.; Rajeeve, V.; Cutillas, P.R.; Balkwill, F.R.; Hynes, R.O. Characterization of the Extracellular Matrix of Normal and Diseased Tissues Using Proteomics. J. Proteome Res. 2017, 16, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, P.B.; Colpaert, C.; Salgado, R.; Royers, R.; Hellemans, H.; Van Den Heuvel, E.; Goovaerts, G.; Dirix, L.Y.; Van Marck, E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 2001, 195, 336–342. [Google Scholar] [CrossRef]
- Feng, W.; Huang, W.; Chen, J.; Qiao, C.; Liu, D.; Ji, X.; Xie, M.; Zhang, T.; Wang, Y.; Sun, M.; et al. CXCL12-mediated HOXB5 overexpression facilitates Colorectal Cancer metastasis through transactivating CXCR4 and ITGB3. Theranostics 2021, 11, 2612–2633. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Kafatos, G.; Taylor, A.; Gastanaga, V.M.; Oliner, K.S.; Hechmati, G.; Terwey, J.H.; Van Krieken, J.H. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: A pooled analysis of randomised controlled trials. Eur. J. Cancer 2015, 51, 1704–1713. [Google Scholar] [CrossRef]
- Baselga, J. The EGFR as a target for anticancer therapy—Focus on cetuximab. Eur. J. Cancer 2001, 37 (Suppl. S4), 16–22. [Google Scholar] [CrossRef] [PubMed]
- Han, C.B.; Li, F.; Ma, J.T.; Zou, H.W. Concordant KRAS mutations in primary and metastatic colorectal cancer tissue specimens: A meta-analysis and systematic review. Cancer Investig. 2012, 30, 741–747. [Google Scholar] [CrossRef]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.I.; Tebbutt, N.C.; Kabbinavar, F.; Giantonio, B.J.; Guan, Z.-Z.; Mitchell, L.; Waterkamp, D.; Tabernero, J. Efficacy and safety of bevacizumab in metastatic colorectal cancer: Pooled analysis from seven randomized controlled trials. Oncologist 2013, 18, 1004–1012. [Google Scholar] [CrossRef]
- Clark, J.; Sanoff, H. Systemic Chemotherapy for Metastatic Colorectal Cancer: General Principles—UpToDate. 2022. Available online: https://www.uptodate.com/contents/systemic-chemotherapy-for-metastatic-colorectal-cancer-general-principles?search=GeneralPrincipleslivermetastasis&source=search_result&selectedTitle=5~150&usage_type=default&display_rank=5 (accessed on 25 September 2022).
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef]
- Cohen, R.; Liu, H.; Fiskum, J.; Adams, R.; Chibaudel, B.; Maughan, T.S.; Van Cutsem, E.; Venook, A.; Douillard, J.Y.; Heinemann, V.; et al. BRAF V600E Mutation in First-Line Metastatic Colorectal Cancer: An Analysis of Individual Patient Data From the ARCAD Database. J. Natl. Cancer Inst. 2021, 113, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. Non-V600 BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. 2017, 35, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, E.; Yoshino, T.; Yamazaki, K.; Muro, K.; Yamaguchi, K.; Nishina, T.; Yuki, S.; Shitara, K.; Bando, H.; Mimaki, S.; et al. Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: The Biomarker Research for anti-EGFR monoclonal Antibodies by Comprehensive Cancer genomics (BREAC) study. Br. J. Cancer 2017, 117, 1450–1458. [Google Scholar] [CrossRef]
- Johnson, B.; Loree, J.M.; Jacome, A.A.; Mendis, S.; Syed, M.; Morris II, V.K.; Parseghian, C.M.; Dasari, A.; Pant, S.; Raymond, V.M.; et al. Atypical, Non-V600 BRAF Mutations as a Potential Mechanism of Resistance to EGFR Inhibition in Metastatic Colorectal Cancer. JCO Precis. Oncol. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.Y.; Thant, C.N.; De Stanchina, E.; et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 7089–7097. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet. Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Karan, C.; Tan, E.; Sarfraz, H.; Knepper, T.C.; Walko, C.M.; Felder, S.; Kim, R.; Sahin, I.H. Human Epidermal Growth Factor Receptor 2-Targeting Approaches for Colorectal Cancer: Clinical Implications of Novel Treatments and Future Therapeutic Avenues. JCO Oncol. Pract. 2022, 18, 545–554. [Google Scholar] [CrossRef]
- Park, D.I.; Kang, M.S.; Oh, S.J.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Han, W.K.; Kim, H.; et al. HER-2/neu overexpression is an independent prognostic factor in colorectal cancer. Int. J. Colorectal Dis. 2007, 22, 491–497. [Google Scholar] [CrossRef]
- Petrelli, F.; Barni, S. Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: A meta-analysis. Int. J. Colorectal Dis. 2012, 27, 997–1004. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1535–1546. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Maughan, T.S.; Adams, R.A.; Smith, C.G.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Idziaszczyk, S.; Harris, R.; Fisher, D.; Kenny, S.L.; et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011, 377, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Tang, W.; Ren, L.; Liu, T.; Ye, Q.; Wei, Y.; He, G.; Lin, Q.; Wang, X.; Wang, M.; Liang, F.; et al. Bevacizumab Plus mFOLFOX6 Versus mFOLFOX6 Alone as First-Line Treatment for RAS Mutant Unresectable Colorectal Liver-Limited Metastases: The BECOME Randomized Controlled Trial. J. Clin. Oncol. 2020, 38, 3175–3184. [Google Scholar] [CrossRef]
- Modest, D.P.; Martens, U.M.; Riera-Knorrenschild, J.; Greeve, J.; Florschütz, A.; Wessendorf, S.; Ettrich, T.; Kanzler, S.; Nörenberg, D.; Ricke, J.; et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: The randomized, open-label, phase II Volfi study (AIO KRK0109). J. Clin. Oncol. 2019, 37, 3401–3411. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; Griffiths, G.O.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet. Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef]
- Clark, J.; Sanoff, H. Systemic Therapy for Nonoperable Metastatic Colorectal Cancer: Selecting the Initial Therapeutic Approache. Available online: https://www.uptodate.com/contents/systemic-therapy-for-nonoperable-metastatic-colorectal-cancer-selecting-the-initial-therapeutic-approach?search=unresectablelivermetastasiscolorectalinitial&source=search_result&selectedTitle=6~150&usage_type=default& (accessed on 25 September 2022).
- Hochster, H.S.; Hart, L.L.; Ramanathan, R.K.; Childs, B.H.; Hainsworth, J.D.; Cohn, A.L.; Wong, L.; Fehrenbacher, L.; Abubakr, Y.; Saif, M.W.; et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J. Clin. Oncol. 2008, 26, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Batziou, C.; Trafalis, D.; Koutantos, J.; Batzios, S.; Stathopoulos, J.; Legakis, J.; Armakolas, A. Treatment of colorectal cancer with and without bevacizumab: A phase III study. Oncology 2010, 78, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Passardi, A.; Nanni, O.; Tassinari, D.; Turci, D.; Cavanna, L.; Fontana, A.; Ruscelli, S.; Mucciarini, C.; Lorusso, V.; Ragazzini, A.; et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: Final results for first-line treatment from the ITACa randomized clinical trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Köhne, C.H.; Hofheinz, R.; Mineur, L.; Letocha, H.; Greil, R.; Thaler, J.; Fernebro, E.; Gamelin, E.; DeCosta, L.; Karthaus, M. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 65–72. [Google Scholar] [CrossRef]
- Berlin, J.; Posey, J.; Tchekmedyian, S.; Hu, E.; Chan, D.; Malik, I.; Yang, L.; Amado, R.G.; Randolph Hecht, J. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin. Colorectal Cancer 2007, 6, 427–432. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.Z.; Wu, Q.; et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E.; et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Colorectal Cancer. NICE guideline NG151. 2020. Available online: https://www.nice.org.uk/guidance/ng151 (accessed on 25 September 2022).
- Lee, J.H.; Lee, S.-W. The Roles of Carcinoembryonic Antigen in Liver Metastasis and Therapeutic Approaches. Gastroenterol. Res. Pract. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, L.; Song, J.; Wang, G.; Li, P.; Li, W.; Luo, P.; Sun, X.; Wu, J.; Liu, Y.; et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer 2021. [Google Scholar] [CrossRef]
- Pericleous, S.; Bhogal, R.H.; Mavroeidis, V.K. The Role of Circulating Biomarkers in the Early Detection of Recurrent Colorectal Cancer Following Resection of Liver Metastases. Front. Biosci. 2022, 27, 189. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Hong, S.; Weng, J. Synergistic diagnostic value of circulating tumor cells and tumor markers CEA/CA19-9 in colorectal cancer. Scand. J. Gastroenterol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Skovlund, E.; Sorbye, H.; Bolstad, N.; Johannes Nustad, K.; Glimelius, B.; Pfeiffer, P.; Kure, E.H.; Johansen, J.S.; Magne Tveit, K.; et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: A BRAF-mutant subset with high CA 19-9 level and poor outcome. Br. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Kumar, U.; Kumari, R. Clinical Significance and Role of TK1, CEA, CA 19-9 and CA 72-4 levels in Diagnosis of Colorectal Cancers. Asian Pac. J. Cancer Prev. 2020, 21, 3133–3136. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Ratajczak, K.; Jakiela, S. Toward early cancer detection: Focus on biosensing systems and biosensors for an anti-apoptotic protein survivin and survivin mRNA. Biosens. Bioelectron. 2019, 137, 58–71. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Hairpin-Hairpin Molecular Beacon Interactions for Detection of Survivin mRNA in Malignant SW480 Cells. ACS Appl. Mater. Interfaces 2018, 10, 17028–17039. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Optical Biosensing System for the Detection of Survivin mRNA in Colorectal Cancer Cells Using a Graphene Oxide Carrier-Bound Oligonucleotide Molecular Beacon. Nanomater 2018, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Nakamura, Y.; Taniguchi, H.; Odegaard, J.I.; Nomura, S.; Kojima, M.; Sugimoto, M.; Konishi, M.; Gotohda, N.; Takahashi, S.; et al. Impact of Preoperative Circulating Tumor DNA Status on Survival Outcomes After Hepatectomy for Resectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2021, 28, 4744–4755. [Google Scholar] [CrossRef]
- Bolhuis, K.; van ’t Erve, I.; Mijnals, C.; Delis-Van Diemen, P.M.; Huiskens, J.; Komurcu, A.; Lopez-Yurda, M.; van den Broek, D.; Swijnenburg, R.J.; Meijer, G.A.; et al. Postoperative circulating tumour DNA is associated with pathologic response and recurrence-free survival after resection of colorectal cancer liver metastases. EBioMedicine 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.I.; Wang, Y.; Cohen, J.I.; Li, L.I.; Hong, W.I.; Christie, M.; Li Wong, H.I.; Kosmider, S.I.; Wong, R.I.; Thomson, B.I.; et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: A prospective cohort study. PLoS Med. 2021, 18, e1003620. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Han, L.; Shi, W.J.; Xie, Y.B.; Zhang, Z.G. Diagnostic value of four serum exosome microRNAs panel for the detection of colorectal cancer. World J. Gastrointest. Oncol. 2021, 13, 970–979. [Google Scholar] [CrossRef]
- Hu, H.Y.; Yu, C.H.; Zhang, H.H.; Zhang, S.Z.; Yu, W.Y.; Yang, Y.; Chen, Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int. J. Biol. Macromol. 2019, 132, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Han, B.; Gao, S.; Wang, X.; Wang, Z.; Wang, F.; Zhang, J.; Xu, D.; Sun, B. Exosome-encapsulated microRNAs as circulating biomarkers for colorectal cancer. Oncotarget 2017, 8, 60149–60158. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Gu, R.H.; Yan, B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J. Cell. Biochem. 2018, 120, 1457–1463. [Google Scholar] [CrossRef]
- Plantureux, L.; Crescence, L.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Effects of platelets on cancer progression. Thromb. Res. 2018, 164 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.E.; Zurakowski, D.; Italiano, J.E.; Michel, L.V.; Connors, S.; Oenick, M.; D’Amato, R.J.; Klement, G.L.; Folkman, J. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 2012, 15, 265–273. [Google Scholar] [CrossRef]
- Qian, W.; Ge, X.X.; Wu, J.; Gong, F.R.; Wu, M.Y.; Xu, M.D.; Lian, L.; Wang, W.J.; Li, W.; Tao, M. Prognostic evaluation of resectable colorectal cancer using platelet-associated indicators. Oncol. Lett. 2019, 18, 571–580. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Q.; Li, D.Z.; Zhou, X.; Yu, D.S.; Zhong, J. TIMP1 mRNA in tumor-educated platelets is diagnostic biomarker for colorectal cancer. Aging 2019, 11, 8998–9012. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Rahbari, N.N.; Büchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Yu, H.; Ma, L.; Zhu, Y.; Li, W.; Ding, L.; Gao, H. Significant diagnostic value of circulating tumour cells in colorectal cancer. Oncol. Lett. 2020, 20, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lalmahomed, Z.S.; Mostert, B.; Onstenk, W.; Kraan, J.; Ayez, N.; Gratama, J.W.; Grünhagen, D.; Verhoef, C.; Sleijfer, S. Prognostic value of circulating tumour cells for early recurrence after resection of colorectal liver metastases. Br. J. Cancer 2015, 112, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.A.; McNamara, K.; Al-Sukhni, E.; Diskin, J.; Chan, D.; Ash, C.; Lowes, L.E.; Allan, A.L.; Zogopoulos, G.; Moulton, C.A.; et al. Central, But Not Peripheral, Circulating Tumor Cells are Prognostic in Patients Undergoing Resection of Colorectal Cancer Liver Metastases. Ann. Surg. Oncol. 2016, 23, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Liu, L.R.; Yang, X.Y.; Liu, F.; Zhang, Z.G. Serum CA19-9 as a marker of circulating tumor cells in first reflux blood of colorectal cancer patients. Oncotarget 2017, 8, 67918–67932. [Google Scholar] [CrossRef] [PubMed]
| With Cetuximab | ||||||||||||
| Name | DOI | Treatment | KRAS WT LLD patients | Follow-up | PFS | p | OS | p | Resection R0 | p | Treat response | p |
| OPUS 2011 | 10.1093/annonc/mdq632 | FOLFOX-Cetuxi vs. FOLFOX | 48(25/23) | HR 0.64 | 0.39 | HR 0.93 | 0.85 | 16% vs. 4.3% (RR 3.68) | 0.23 | RR 1.94 | 0.02 | |
| CRYSTAL 2011, Van Cutsem | 10.1200/JCO.2010.33.5091 | FOLFIRI-Cetuxi vs. FOLFIRI | 140 (68/72) | 29.7 Mo | HR 0.56 | 0.04 | HR 0.85 | 0.43 | 13.2% vs. 5.5% (RR 2.38) | 0.13 | RR 1.59 | 0.003 |
| MRC Coin 2011 | 10.1016/S0140-6736(11)60613-2 | CAPOX or FOLFOX-Cetuxi vs. CAPOX or FOLFOX | 178 (87/91) | 21 Mo | HR 0.68 | 0.03 | NR | NR | 15% vs. 13% (RR 1.13) | 0.74 | NR | NR |
| Le-Chi Ye 2013 | 10.1200/JCO.2012.44.8308 | FOLFIRI or FOLFOX- Cetuxi vs. FOLFIRI of FOLFOX | 70/68 | 25 Mo | HR 0.60 | 0.004 | HR 0.54 | 0.013 | 25.7% vs. 7.4% (OR 4.37) | 0.004 | 57.1% vs. 29.4% | 0.001 |
| With Panitumumab | ||||||||||||
| Name | DOI | Treatment | KRAS WT LLD patients | Follow-up | PFS | p | OS | p | Resection R0 | p | Treat response | p |
| Douillard 2010 | 10.1200/JCO.2009.27.4860 | FOLFOX-Pani vs. FOLFOX | 116 (60/56) | 13.2 Mo | HR 0.82 | 0.43 | HR 0.93 | 0.81 | 27.8% vs. 17.5% (RR 1.59) | 0.19 | NR | NR |
| With Bevacizumab | ||||||||||||
| Name | DOI | Treatment | KRAS mutated LLD patients | Follow-up | PFS | p | OS | p | Resection R0 | p | Treat response | p |
| BECOME | 10.1200/JCO.20.174 | FOLFOX+Beva vs. FOLFOX | 241 (121/120) | 37 Mo | HR 0.49 | 0.001 | HR 0.71 | 0.31 | 22.3% vs. 5.8% | 0.01 | 54.5% vs. 36.7% | 0.001 |
| Conversion Chemotherapy without Limited Liver Disease | ||||||||||||
| Name | DOI | Treatment | KRAS WT patients | Follow-up | PFS | p | OS | p | Resection | p | Treat response | p |
| VOLFI | 10.1200/JCO.1901340 | FOLFOXIRI-Pani vs. FOLFOXIRI | 96 (63/33) | 44.2 Mo vs. 63.3 Mo | HR 1.07 | 0.76 | HR 0.67 | 0.12 | 33% vs. 12.1% (OR 3.63) | 0.02 | OR 4.469 | 0.004 |
| Salz 2008 | 10.1200/JCO.2007.14.9930 | CAPOX or FOLOFOX-Beva vs. CAPOX or FOLFOX | 700 vs. 701 (no KRAS specified) | 15.6 Mo | HR 0.83 | 0.0023 | HR 0.89 | 0.769 | 8.4% vs. 6.1% | NR | OR 0.9 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavel, M.-C.; Ramirez-Maldonado, E.; Pueyo-Périz, E.; Memba, R.; Merino, S.; Geoghegan, J.; Jorba, R. The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1127. https://doi.org/10.3390/ijms24021127
Pavel M-C, Ramirez-Maldonado E, Pueyo-Périz E, Memba R, Merino S, Geoghegan J, Jorba R. The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review. International Journal of Molecular Sciences. 2023; 24(2):1127. https://doi.org/10.3390/ijms24021127
Chicago/Turabian StylePavel, Mihai-Calin, Elena Ramirez-Maldonado, Eva Pueyo-Périz, Robert Memba, Sandra Merino, Justin Geoghegan, and Rosa Jorba. 2023. "The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review" International Journal of Molecular Sciences 24, no. 2: 1127. https://doi.org/10.3390/ijms24021127
APA StylePavel, M.-C., Ramirez-Maldonado, E., Pueyo-Périz, E., Memba, R., Merino, S., Geoghegan, J., & Jorba, R. (2023). The Impact of Molecular Biology in the Seeding, Treatment Choices and Follow-Up of Colorectal Cancer Liver Metastases—A Narrative Review. International Journal of Molecular Sciences, 24(2), 1127. https://doi.org/10.3390/ijms24021127