Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts
Abstract
1. Introduction
2. Anatomical Imaging Techniques
2.1. Confocal Laser Scanning Microscopy (CLSM)
2.1.1. Reflectance Confocal Microscopy (RCM)
2.1.2. Fluorescence Confocal Microscopy (FCM)
2.2. Multiphoton Microscopy (MPM)
2.3. Optical Coherence Tomography (OCT)
2.4. High-Frequency Ultrasound (HFUS)
2.5. Terahertz Pulsed Imaging (TPI)
2.6. Magnetic Resonance Imaging (MRI)
2.7. Near-Infrared (NIR) Bioimaging
3. Molecular Imaging Techniques
3.1. Single Photon Emission Computed Tomography (SPECT)
3.2. Positron Emission Tomography (PET)
3.3. Biological Targets for Cutaneous Melanoma Molecular Imaging
3.3.1. Imaging the Immune Environment
Indoleamine 2,3-Dioxygenase (IDO)
PD-1/PD-L1
3.3.2. Imaging Integrins
3.3.3. The Non-Invasive Visualization of Fibroblast Activation Protein (FAP)
3.3.4. Imaging the Melanocortin-1 Receptor (MC1R)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, H.; White, A.C.; Borowsky, A.D. New insights into the functions of Cox-2 in skin and esophageal malignancies. Exp. Mol. Med. 2020, 52, 538–547. [Google Scholar] [CrossRef]
- Kim, Y.; He, Y.-Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Dobre, E.-G.; Constantin, C.; Neagu, M. Skin Cancer Research Goes Digital: Looking for Biomarkers within the Droplets. J. Pers. Med. 2022, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Schiferle, E.B.; Cheon, S.Y.; Ham, S.; Son, H.G.; Messerschmidt, J.L.; Lawrence, D.P.; Cohen, J.V.; Flaherty, K.T.; Moon, J.J.; Lian, C.G.; et al. Rejection of benign melanocytic nevi by nevus-resident CD4(+) T cells. Sci. Adv. 2021, 7, eabg4498. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, N.; Simonacci, F.; Greco, M.P.; Grignaffini, E.; Raposio, E. Single center evidence for the treatment of basal cell carcinoma of the head and neck. Acta Biomed. 2019, 90, 77–82. [Google Scholar] [CrossRef]
- Kavasi, R.M.; Neagu, M.; Constantin, C.; Munteanu, A.; Surcel, M.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Matrix Effectors in the Pathogenesis of Keratinocyte-Derived Carcinomas. Front. Med. 2022, 9, 1258. [Google Scholar] [CrossRef]
- Fontanillas, P.; Alipanahi, B.; Furlotte, N.A.; Johnson, M.; Wilson, C.H.; Agee, M.; Bell, R.K.; Bryc, K.; Elson, S.L.; Hinds, D.A.; et al. Disease risk scores for skin cancers. Nat. Commun. 2021, 12, 160. [Google Scholar] [CrossRef]
- Skin Cancer as a Major Public Health Problem—The Surgeon General’s Call to Action to Prevent Skin Cancer—NCBI Bookshelf, US Department of Health and Human Services ed.; Office of the Surgeon General (US): Washington, DC, USA, 2014.
- Ekwueme, D.U.; Guy, G.P.J.; Li, C.; Rim, S.H.; Parelkar, P.; Chen, S.C. The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity-United States, 2000 to 2006. J. Am. Acad. Dermatol. 2011, 65, S133–S143. [Google Scholar] [CrossRef]
- Yilmaz, A.; Gencoglan, G.; Varol, R.; Demircali, A.A.; Keshavarz, M.; Uvet, H. MobileSkin: Classification of Skin Lesion Images Acquired Using Mobile Phone-Attached Hand-Held Dermoscopes. J. Clin. Med. 2022, 11, 5102. [Google Scholar] [CrossRef]
- Elmore, J.G.; Barnhill, R.L.; Elder, D.E.; Longton, G.M.; Pepe, M.S.; Reisch, L.M.; Carney, P.A.; Titus, L.J.; Nelson, H.D.; Onega, T.; et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: Observer accuracy and reproducibility study. BMJ 2017, 357, j2813. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Garfinkel, J.; Zhang, X.; Wu, D.; Zhang, Y.; de Haan, K.; Wang, H.; Liu, T.; Bai, B.; Rivenson, Y.; et al. Biopsy-free in vivo virtual histology of skin using deep learning. Light Sci. Appl. 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.; Shoukry, M.; Ashary, M.A.; Kasbi, A.A.; Baksh, M.; Gabriel, E. Advanced Tumor Imaging Approaches in Human Tumors. Cancers 2022, 14, 1549. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Cho, J.Y.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Won, C.H. Emerging Minimally Invasive Technologies for the Detection of Skin Cancer. J. Pers. Med. 2021, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Sun, J.; Cai, W. Anatomical and molecular imaging of skin cancer. Clin. Cosmet. Investig. Dermatol. 2008, 1, 1–17. [Google Scholar] [CrossRef]
- Wong, A.N.M.; McArthur, G.A.; Hofman, M.S.; Hicks, R.J. The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 67–77. [Google Scholar] [CrossRef]
- Decristoforo, C.; Faintuch-Linkowski, B.; Rey, A.; von Guggenberg, E.; Rupprich, M.; Hernandez-Gonzales, I.; Rodrigo, T.; Haubner, R. [99mTc]HYNIC-RGD for imaging integrin alphavbeta3 expression. Nucl. Med. Biol. 2006, 33, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.K.; Huang, X.; Moberg, V.E.; Barkey, N.M.; Sondak, V.K.; Tian, H.; Morse, D.L.; Vagner, J. Synthesis and characterization of a melanoma-targeted fluorescence imaging probe by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. Bioconjug. Chem. 2012, 23, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, L.; de Jong, D.; Dercle, L.; Hosten, B.; Braumuller, B.; Das, J.P.; Deng, A.; Moya-Plana, A.; A’Keen, C.; Yeh, R.; et al. Translating Molecules into Imaging-The Development of New PET Tracers for Patients with Melanoma. Diagnostics 2022, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.L.; Pandharipande, P.V.; Lee, J.M.; Lehman, C.D.; Lee, C.I. Imaging-Based Screening: Understanding the Controversies. Am. J. Roentgenol. 2014, 203, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.-Y.; Törnberg, S.; Elfström, K.M.; Liu, X.; Nyström, L.; Jonsson, H. Overdiagnosis in the population-based organized breast cancer screening program estimated by a non-homogeneous multi-state model: A cohort study using individual data with long-term follow-up. Breast Cancer Res. 2018, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.J.; Mainprize, J.G. Overdetection of Breast Cancer. Curr. Oncol. 2022, 29, 311. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, A.A.G.; Dieng, M.; Khanna, N.; Lord, S.J.; Dalton, J.; Menzies, A.M.; Turner, R.M.; Allen, J.; Saw, R.P.M.; Nieweg, O.E.; et al. False-Positive Results and Incidental Findings with Annual CT or PET/CT Surveillance in Asymptomatic Patients with Resected Stage III Melanoma. Ann. Surg. Oncol. 2019, 26, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dickinson, J.A.; Thériault, G.; Grad, R.; Groulx, S.; Wilson, B.J.; Szafran, O.; Bell, N.R. Overdiagnosis: Causes and consequences in primary health care. Can. Fam. Physician 2018, 64, 654–659. [Google Scholar] [PubMed]
- Levine, A.; Markowitz, O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018, 4, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- De Barcaui, E.O.; Carvalho, A.C.P.; Lopes, F.P.P.L.; Piñeiro-Maceira, J.; Barcaui, C.B. High frequency ultrasound with color Doppler in dermatology. An. Bras. Dermatol. 2016, 91, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lindley-Hatcher, H.; Chen, X.; Pickwell-MacPherson, E. THz Sensing of Human Skin: A Review of Skin Modeling Approaches. Sensors 2021, 21, 3624. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Yang, H.-J.; Choi, M.; Son, J.-H. Effective demethylation of melanoma cells using terahertz radiation. Biomed. Opt. Express 2019, 10, 4931–4941. [Google Scholar] [CrossRef]
- Zimny, A.; Zińska, L.; Bladowska, J.; Neska-Matuszewska, M.; Sąsiadek, M. Intracranial lesions with high signal intensity on T1-weighted MR images—Review of pathologies. Polish J. Radiol. 2013, 78, 36–46. [Google Scholar] [CrossRef]
- Diaconeasa, A.; Boda, D.; Neagu, M.; Constantin, C.; Căruntu, C.; Vlădău, L.; Guţu, D. The role of confocal microscopy in the dermato-oncology practice. J. Med. Life 2011, 4, 63–74. [Google Scholar] [PubMed]
- Ilie, M.A.; Caruntu, C.; Lupu, M.; Lixandru, D.; Tampa, M.; Georgescu, S.-R.; Bastian, A.; Constantin, C.; Neagu, M.; Zurac, S.A.; et al. Current and future applications of confocal laser scanning microscopy imaging in skin oncology. Oncol. Lett. 2019, 17, 4102–4111. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance Confocal Microscopy for in vivo Skin Imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.; Star, P.; Guitera, P. Advances in the use of reflectance confocal microscopy in melanoma. Melanoma Manag. 2018, 5, MMT04. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Caruntu, A.; Scheau, C.; Nicolae, I.; Matei, A.; Caruntu, C.; et al. Current Perspectives on the Role of Matrix Metalloproteinases in the Pathogenesis of Basal Cell Carcinoma. Biomolecules 2021, 11, 903. [Google Scholar] [CrossRef]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Caruntu, C.; Ghita, M.A.; Voiculescu, V.; Voiculescu, S.; Rosca, A.E.; Caruntu, A.; Moraru, L.; Popa, I.M.; Calenic, B.; et al. Gene Expression and Proteome Analysis as Sources of Biomarkers in Basal Cell Carcinoma. Dis. Markers 2016, 2016, 9831237. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Caruntu, A.; Caruntu, C. A Systematic Review and Meta-Analysis of the Accuracy of in vivo Reflectance Confocal Microscopy for the Diagnosis of Primary Basal Cell Carcinoma. J. Clin. Med. 2019, 8, 1462. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Caruntu, C.; Popa, M.I.; Voiculescu, V.M.; Zurac, S.; Boda, D. Vascular patterns in basal cell carcinoma: Dermoscopic, confocal and histopathological perspectives. Oncol. Lett. 2019, 17, 4112–4125. [Google Scholar] [CrossRef] [PubMed]
- Kadouch, D.J.; Schram, M.E.; Leeflang, M.M.; Limpens, J.; Spuls, P.I.; de Rie, M.A. In vivo confocal microscopy of basal cell carcinoma: A systematic review of diagnostic accuracy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Voiculescu, V.M.; Caruntu, A.; Tebeica, T.; Caruntu, C. Preoperative Evaluation through Dermoscopy and Reflectance Confocal Microscopy of the Lateral Excision Margins for Primary Basal Cell Carcinoma. Diagnostics 2021, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Casari, A.; Pepe, P.; Moscarella, E.; Zalaudek, I.; Argenziano, G.; Pellacani, G. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology 2012, 225, 264–270. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Liopyris, K.; Aleissa, S.; Rajadhyaksha, M.; Cohen, G.; Marghoob, A.A.; Rossi, A.M.; Barker, C.A. In vivo imaging characterization of basal cell carcinoma and cutaneous response to high-dose ionizing radiation therapy: A prospective study of reflectance confocal microscopy, dermoscopy, and ultrasonography. J. Am. Acad. Dermatol. 2021, 84, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Sierra, H.; Yélamos, O.; Cordova, M.; Chen, C.-S.J.; Rajadhyaksha, M. Reflectance confocal microscopy-guided laser ablation of basal cell carcinomas: Initial clinical experience. J. Biomed. Opt. 2017, 22, 085005. [Google Scholar] [CrossRef]
- Ahlgrimm-Siess, V.; Horn, M.; Koller, S.; Ludwig, R.; Gerger, A.; Hofmann-Wellenhof, R. Monitoring efficacy of cryotherapy for superficial basal cell carcinomas with in vivo reflectance confocal microscopy: A preliminary study. J. Dermatol. Sci. 2009, 53, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Fabbrocini, G.; Costa, C.; Scalvenzi, M. Reflectance Confocal Microscopy Identification of Subclinical Basal Cell Carcinoma after Vismodegib Treatment: Report of a Case. Dermatol. Ther. 2021, 11, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Lallas, A.; Kyrgidis, A.; Rabinovitz, H.; Moscarella, E.; Ciardo, S.; Zalaudek, I.; Oliviero, M.; Losi, A.; Gonzalez, S.; et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J. Am. Acad. Dermatol. 2014, 71, 716–724.e1. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Boda, D.; Caruntu, C.; Zurac, S.; Giurcaneanu, C. A Retrospective Study of the Diagnostic Accuracy of in vivo Reflectance Confocal Microscopy for Basal Cell Carcinoma Diagnosis and Subtyping. J. Clin. Med. 2019, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Woliner-van der Weg, W.; Peppelman, M.; Elshot, Y.S.; Visch, M.B.; Crijns, M.B.; Alkemade, H.A.C.; Bronkhorst, E.M.; Adang, E.; Amir, A.; Gerritsen, M.J.P.; et al. Biopsy outperforms reflectance confocal microscopy in diagnosing and subtyping basal cell carcinoma: Results and experiences from a randomized controlled multicentre trial. Br. J. Dermatol. 2021, 184, 663–671. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Shin, S.; Jung, S.-H.; Park, Y.M.; Park, G.S.; Lee, S.H.; Chung, Y.-J. Genomic Progression of Precancerous Actinic Keratosis to Squamous Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 528–538.e8. [Google Scholar] [CrossRef] [PubMed]
- Moscarella, E.; Rabinovitz, H.; Zalaudek, I.; Piana, S.; Stanganelli, I.; Oliviero, M.C.; Lallas, A.; Ardigo, M.; Cota, C.; Catricalà, C.; et al. Dermoscopy and reflectance confocal microscopy of pigmented actinic keratoses: A morphological study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Curiel-Lewandrowski, C.; Myrdal, C.N.; Saboda, K.; Hu, C.; Arzberger, E.; Pellacani, G.; Legat, F.J.; Ulrich, M.; Hochfellner, P.; Oliviero, M.C.; et al. In vivo Reflectance Confocal Microscopy as a Response Monitoring Tool for Actinic Keratoses Undergoing Cryotherapy and Photodynamic Therapy. Cancers 2021, 13, 5488. [Google Scholar] [CrossRef] [PubMed]
- Peppelman, M.; Nguyen, K.P.; Hoogedoorn, L.; van Erp, P.E.J.; Gerritsen, M.-J.P. Reflectance confocal microscopy: Non-invasive distinction between actinic keratosis and squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Caruntu, A.; Boda, D.; Caruntu, C. In vivo Reflectance Confocal Microscopy-Diagnostic Criteria for Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. J. Clin. Med. 2020, 9, 1987. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.S.; Rocha, L.; Bezerra, A.P.C.; Nico, M.M.S.; Lourenço, S.V. Reflectance confocal microscopy (RCM)-based criteria for progression of lower-lip squamous cell carcinoma: A prospective study. Oral. Oncol. 2022, 125, 105674. [Google Scholar] [CrossRef]
- Nguyen, K.P.; Peppelman, M.; Hoogedoorn, L.; Van Erp, P.E.J.; Gerritsen, M.-J.P. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: A systematic review. Eur. J. Dermatol. 2016, 26, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Branzan, A.L.; Landthaler, M.; Szeimies, R.-M. In vivo confocal scanning laser microscopy in dermatology. Lasers Med. Sci. 2007, 22, 73–82. [Google Scholar] [CrossRef]
- Chung, V.Q.; Dwyer, P.J.; Nehal, K.S.; Rajadhyaksha, M.; Menaker, G.M.; Charles, C.; Jiang, S.B. Use of ex vivo confocal scanning laser microscopy during Mohs surgery for nonmelanoma skin cancers. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2004, 30, 1470–1478. [Google Scholar] [CrossRef]
- Stevenson, A.D.; Mickan, S.; Mallett, S.; Ayya, M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol. Pract. Concept. 2013, 3, 19–27. [Google Scholar] [CrossRef]
- Pezzini, C.; Kaleci, S.; Chester, J.; Farnetani, F.; Longo, C.; Pellacani, G. Reflectance confocal microscopy diagnostic accuracy for malignant melanoma in different clinical settings: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2268–2279. [Google Scholar] [CrossRef]
- Pellacani, G.; Pepe, P.; Casari, A.; Longo, C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: A longitudinal prospective study. Br. J. Dermatol. 2014, 171, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Stanganelli, I.; Longo, C.; Mazzoni, L.; Magi, S.; Medri, M.; Lanzanova, G.; Farnetani, F.; Pellacani, G. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br. J. Dermatol. 2015, 172, 365–371. [Google Scholar] [CrossRef]
- Durkin, J.R.; Tchanque-Fossuo, C.N.; Rose, A.N.; Elwood, H.R.; Stepenaskie, S.; Barbosa, N.S. Surgical Margin Mapping of Melanoma in situ Using in vivo Reflectance Confocal Microscopy Mosaics. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2021, 47, 605–608. [Google Scholar] [CrossRef]
- Ribero, S.; Marra, E.; Tomasini, C.F.; Fierro, M.T.; Bombonato, C.; Longo, C. Confocal microscopy and dermoscopy for the monitoring of BRAF inhibitor therapy of melanoma skin metastases. Br. J. Dermatol. 2017, 176, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Hibler, B.P.; Yélamos, O.; Cordova, M.; Sierra, H.; Rajadhyaksha, M.; Nehal, K.S.; Rossi, A.M. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis 2017, 99, 346–352. [Google Scholar] [PubMed]
- Cinotti, E.; Labeille, B.; Debarbieux, S.; Carrera, C.; Lacarrubba, F.; Witkowski, A.M.; Moscarella, E.; Arzberger, E.; Kittler, H.; Bahadoran, P.; et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.; Quadri, M.; Farnetani, F.; Ciardo, S.; Palazzo, E.; Lotti, R.; Cesinaro, A.M.; Fabbiani, L.; Vaschieri, C.; Puviani, M.; et al. In vivo Melanoma Cell Morphology Reflects Molecular Signature and Tumor Aggressiveness. J. Investig. Dermatol. 2022, 142, 2205–2216.e6. [Google Scholar] [CrossRef] [PubMed]
- Giambrone, D.; Alamgir, M.; Masud, A.; Bronsnick, T.; Rao, B. The diagnostic accuracy of in vivo confocal microscopy in clinical practice. J. Am. Acad. Dermatol. 2015, 73, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Kose, K.; Gou, M.; Yélamos, O.; Cordova, M.; Rossi, A.M.; Nehal, K.S.; Flores, E.S.; Camps, O.; Dy, J.G.; Brooks, D.H.; et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: An approach to address challenges in imaging living tissue and extend field of view. Sci. Rep. 2017, 7, 10759. [Google Scholar] [CrossRef] [PubMed]
- Malciu, A.M.; Lupu, M.; Voiculescu, V.M. Artificial Intelligence-Based Approaches to Reflectance Confocal Microscopy Image Analysis in Dermatology. J. Clin. Med. 2022, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Arginelli, F.; Farnetani, F.; Ciardo, S.; Bertoni, L.; Manfredini, M.; Zerbinati, N.; Longo, C.; Pellacani, G. Clinical Applications of in vivo and ex vivo Confocal Microscopy. Appl. Sci. 2021, 11, 1979. [Google Scholar] [CrossRef]
- Rey-Barroso, L.; Peña-Gutiérrez, S.; Yáñez, C.; Burgos-Fernández, F.J.; Vilaseca, M.; Royo, S. Optical Technologies for the Improvement of Skin Cancer Diagnosis: A Review. Sensors 2021, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Ragazzi, M.; Rajadhyaksha, M.; Nehal, K.; Bennassar, A.; Pellacani, G.; Malvehy Guilera, J. In vivo and ex vivo Confocal Microscopy for Dermatologic and Mohs Surgeons. Dermatol. Clin. 2016, 34, 497–504. [Google Scholar] [CrossRef]
- Sahu, A.; Cordero, J.; Wu, X.; Kossatz, S.; Harris, U.; Franca, P.D.D.; Kurtansky, N.R.; Everett, N.; Dusza, S.; Monnier, J.; et al. Combined PARP1-Targeted Nuclear Contrast and Reflectance Contrast Enhance Confocal Microscopic Detection of Basal Cell Carcinoma. J. Nucl. Med. 2022, 63, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Gareau, D.S.; Karen, J.K.; Dusza, S.W.; Tudisco, M.; Nehal, K.S.; Rajadhyaksha, M. Sensitivity and specificity for detecting basal cell carcinomas in Mohs excisions with confocal fluorescence mosaicing microscopy. J. Biomed. Opt. 2009, 14, 34012. [Google Scholar] [CrossRef]
- Ruini, C.; Vladimirova, G.; Kendziora, B.; Salzer, S.; Ergun, E.; Sattler, E.; French, L.E.; Hartmann, D. Ex-vivo fluorescence confocal microscopy with digital staining for characterizing basal cell carcinoma on frozen sections: A comparison with histology. J. Biophotonics 2021, 14, e202100094. [Google Scholar] [CrossRef]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B.; Ten Hagen, T.L.M. An adapted dorsal skinfold model used for 4D intravital followed by whole-mount imaging to reveal endothelial cell-pericyte association. Sci. Rep. 2021, 11, 20389. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Ten Hagen, T.L.M. Intravital Microscopy of Tumor-associated Vasculature Using Advanced Dorsal Skinfold Window Chambers on Transgenic Fluorescent Mice. J. Vis. Exp. 2018, 131, e55115. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kang, C.; Fang, F. Biometric Measurement of Anterior Segment: A Review. Sensors 2020, 20, 4285. [Google Scholar] [CrossRef]
- Themstrup, L.; Jemec, G.B.E. Chapter 6—Optical Coherence Tomography for Skin Cancer and Actinic Keratosis; Hamblin, M.R., Avci, P., Gupta, G.K.B.T.-I.D., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 59–67. ISBN 978-0-12-802838-4. [Google Scholar]
- Alawi, S.A.; Kuck, M.; Wahrlich, C.; Batz, S.; McKenzie, G.; Fluhr, J.W.; Lademann, J.; Ulrich, M. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—A practical approach. Exp. Dermatol. 2013, 22, 547–551. [Google Scholar] [CrossRef]
- Ulrich, M.; von Braunmuehl, T.; Kurzen, H.; Dirschka, T.; Kellner, C.; Sattler, E.; Berking, C.; Welzel, J.; Reinhold, U. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: An observational study. Br. J. Dermatol. 2015, 173, 428–435. [Google Scholar] [CrossRef]
- Rao, D.S.S.; Jensen, M.; Grüner-Nielsen, L.; Olsen, J.T.; Heiduschka, P.; Kemper, B.; Schnekenburger, J.; Glud, M.; Mogensen, M.; Israelsen, N.M.; et al. Shot-noise limited, supercontinuum-based optical coherence tomography. Light Sci. Appl. 2021, 10, 133. [Google Scholar] [CrossRef]
- Ferrante di Ruffano, L.; Dinnes, J.; Deeks, J.J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Takwoingi, Y.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 12, CD013189. [Google Scholar] [CrossRef]
- Welzel, J.; Schuh, S.; De Carvalho, N.; Themstrup, L.; Ulrich, M.; Jemec, G.B.E.; Holmes, J.; Pellacani, G. Dynamic optical coherence tomography shows characteristic alterations of blood vessels in malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1087–1093. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chen, Z. Advances in Doppler optical coherence tomography and angiography. Transl. Biophotonics 2019, 1, e201900005. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography-Practical applications in dermatology and comparison with established imaging methods. Ski. Res. Technol. Off. J. Int. Soc. Bioeng. Ski. Int. Soc. Digit. Imaging Ski. Int. Soc. Ski. Imaging 2021, 27, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Ruini, C.; Schuh, S.; Gust, C.; Kendziora, B.; Frommherz, L.; French, L.E.; Hartmann, D.; Welzel, J.; Sattler, E. Line-field optical coherence tomography: In vivo diagnosis of basal cell carcinoma subtypes compared with histopathology. Clin. Exp. Dermatol. 2021, 46, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Kaestle, R.; Sattler, E.C.; Welzel, J. Optical coherence tomography of actinic keratoses and basal cell carcinomas—Differentiation by quantification of signal intensity and layer thickness. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography May Enhance Monitoring of Superficial Basal Cell Carcinoma Treated with Imiquimod 5% Cream: A Pilot Study. Cancers 2021, 13, 4913. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, O.; Schwartz, M.; Feldman, E.; Bienenfeld, A.; Bieber, A.K.; Ellis, J.; Alapati, U.; Lebwohl, M.; Siegel, D.M. Evaluation of Optical Coherence Tomography as a Means of Identifying Earlier Stage Basal Cell Carcinomas while Reducing the Use of Diagnostic Biopsy. J. Clin. Aesthet. Dermatol. 2015, 8, 14–20. [Google Scholar]
- Sahu, A.; Yélamos, O.; Iftimia, N.; Cordova, M.; Alessi-Fox, C.; Gill, M.; Maguluri, G.; Dusza, S.W.; Navarrete-Dechent, C.; González, S.; et al. Evaluation of a Combined Reflectance Confocal Microscopy-Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018, 154, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.A.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; Elagin, V.V.; Kuznetsov, S.S.; Gelikonov, G.V.; Ksenofontov, S.Y.; Zagaynova, E.V.; Feldchtein, F.I.; et al. Accurate early prediction of tumour response to PDT using optical coherence angiography. Sci. Rep. 2019, 9, 6492. [Google Scholar] [CrossRef]
- Markowitz, O.; Schwartz, M. The Use of Noninvasive Optical Coherence Tomography to Monitor the Treatment Progress of Vismodegib and Imiquimod 5% Cream in a Transplant Patient with Advanced Basal Cell Carcinoma of the Nose. J. Clin. Aesthet. Dermatol. 2016, 9, 37–41. [Google Scholar]
- Schuh, S.; Holmes, J.; Ulrich, M.; Themstrup, L.; Jemec, G.B.E.; De Carvalho, N.; Pellacani, G.; Welzel, J. Imaging Blood Vessel Morphology in Skin: Dynamic Optical Coherence Tomography as a Novel Potential Diagnostic Tool in Dermatology. Dermatol. Ther. 2017, 7, 187–202. [Google Scholar] [CrossRef]
- Gust, C.; Schuh, S.; Welzel, J.; Daxenberger, F.; Hartmann, D.; French, L.E.; Ruini, C.; Sattler, E.C. Line-Field Confocal Optical Coherence Tomography Increases the Diagnostic Accuracy and Confidence for Basal Cell Carcinoma in Equivocal Lesions: A Prospective Study. Cancers 2022, 14, 1082. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Mo, Y.; Wen, Y.-Q.; Cheng, M.-J.; Huo, S.-T.; Chen, X.-J.; Chen, Q. Optical coherence tomography for the diagnosis of malignant skin tumors: A meta-analysis. J. Biomed. Opt. 2018, 23, 020902. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.L.M.; Suppa, M.; Marneffe, A.; Miyamoto, M.; Jemec, G.B.E.; Del Marmol, V. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Themstrup, L.; Pellacani, G.; Welzel, J.; Holmes, J.; Jemec, G.B.E.; Ulrich, M. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, O.; Wang, K.; Levine, A.; Schwartz, M.; Minhas, S.; Feldman, E.; Siegel, D.M. Noninvasive Long-term Monitoring of Actinic Keratosis and Field Cancerization Following Treatment with Ingenol Mebutate Gel 0.015. J. Clin. Aesthet. Dermatol. 2017, 10, 28–33. [Google Scholar]
- Themstrup, L.; Banzhaf, C.; Mogensen, M.; Jemec, G.B.E. Cryosurgery Treatment of Actinic Keratoses Monitored by Optical Coherence Tomography: A Pilot Study. Dermatology 2012, 225, 242–247. [Google Scholar] [CrossRef]
- Gambichler, T.; Schmid-Wendtner, M.H.; Plura, I.; Kampilafkos, P.; Stücker, M.; Berking, C.; Maier, T. A multicentre pilot study investigating high-definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 537–541. [Google Scholar] [CrossRef]
- Lai, P.-Y.; Shih, T.-Y.; Chang, C.-H.; Kuo, W.-C. Applications of Multi-Contrast Optical Coherence Tomography in Assessment of Dysplastic Nevi to Malignant Melanoma. Front. Phys. 2022, 10, 845958. [Google Scholar] [CrossRef]
- Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-Field Confocal Optical Coherence Tomography: A New Tool for the Differentiation between Nevi and Melanomas? Cancers 2022, 14, 1140. [Google Scholar] [CrossRef]
- Sahu, A.; Oh, Y.; Peterson, G.; Cordova, M.; Navarrete-Dechent, C.; Gill, M.; Alessi-Fox, C.; Gonzalez, S.; Phillips, W.; Wilson, S.; et al. In vivo optical imaging-guided targeted sampling for precise diagnosis and molecular pathology. Sci. Rep. 2021, 11, 23124. [Google Scholar] [CrossRef]
- Levy, J.; Barrett, D.L.; Harris, N.; Jeong, J.J.; Yang, X.; Chen, S.C. High-frequency ultrasound in clinical dermatology: A review. Ultrasound J. 2021, 13, 24. [Google Scholar] [CrossRef]
- Meikle, B.; Kimble, R.M.; Tyack, Z. Ultrasound measurements of pathological and physiological skin thickness: A scoping review protocol. BMJ Open 2022, 12, e056720. [Google Scholar] [CrossRef] [PubMed]
- Ihnatsenka, B.; Boezaart, A.P. Ultrasound: Basic understanding and learning the language. Int. J. Shoulder Surg. 2010, 4, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Halip, I.-A.; Vâţă, D.; Statescu, L.; Salahoru, P.; Patraşcu, A.I.; Temelie Olinici, D.; Tarcau, B.; Popescu, I.-A.; Mocanu, M.; Constantin, A.-M.; et al. Assessment of Basal Cell Carcinoma Using Dermoscopy and High Frequency Ultrasound Examination. Diagnostics 2022, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Grajdeanu, I.-A.; Vata, D.; Statescu, L.; Adriana Popescu, I.; Porumb-Andrese, E.; Ionela Patrascu, A.; Stincanu, A.; Taranu, T.; Crisan, M.; Gheuca Solovastru, L. Use of imaging techniques for melanocytic naevi and basal cell carcinoma in integrative analysis (Review). Exp. Ther. Med. 2020, 20, 78–86. [Google Scholar] [CrossRef] [PubMed]
- De Barcaui, E.O.; Carvalho, A.C.P.; Valiante, P.M.; Piñeiro-Maceira, J.; Barcaui, C.B. High-frequency (22-MHz) ultrasound for assessing the depth of basal cell carcinoma invasion. Ski. Res. Technol. 2021, 27, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Khlebnikova, A.; Molochkov, V.; Selezneva, E.; Belova, L.; Bezugly, A.; Sedova, T.; Molochkov, A. Basal cell carcinoma invasion depth determined with 30 and 75 MHz high-frequency ultrasound and histopathology—A comparative study. Med. Ultrason. 2020, 22, 31–36. [Google Scholar] [CrossRef]
- Wang, L.F.; Zhu, A.Q.; Wang, Q.; Li, X.L.; Yan, J.N.; Li, M.X.; Jin, F.S.; Chen, S.T.; Guo, L.H.; Xu, H.X. Value of High-Frequency Ultrasound for Differentiating Invasive Basal Cell Carcinoma from Non-invasive Types. Ultrasound Med. Biol. 2021, 47, 2910–2920. [Google Scholar] [CrossRef]
- Medhat Kabsha, A.; Eguiluz-Meléndez, A.; Gushken, F.; Rosa, G.; Elazaly, H.F.; de Oliveira, L.; Zanon Zotin, M.; Vásquez, M.; RG Ferreira, L.; Tarantino, A. Efficacy of posaconazole for treatment of basal cell carcinoma. Princ. Pract. Clin. Res. J. 2019, 5, 67–73. [Google Scholar] [CrossRef]
- Chen, Z.-T.; Yan, J.-N.; Zhu, A.-Q.; Wang, L.-F.; Wang, Q.; Li, L.; Guo, L.-H.; Li, X.-L.; Xu, H.-X. High-frequency ultrasound for differentiation between high-risk basal cell carcinoma and cutaneous squamous cell carcinoma. Ski. Res. Technol. Off. J. Int. Soc. Bioeng. Ski. Int. Soc. Digit. Imaging Ski. Int. Soc. Ski. Imaging 2022, 28, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Combalia, A.; Carrera, C. Squamous Cell Carcinoma: An Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020066. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Wortsman, J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J. Am. Acad. Dermatol. 2010, 62, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, X.; Wang, P.; Guo, L.; Li, C.; Xu, M.; Zhang, G.; Wang, X. Dermoscopy and ultrosound monitoring actinic keratosis with cutaneous squamous cell carcinoma: A case report and literature review. Photodiagnosis Photodyn. Ther. 2022, 37, 102709. [Google Scholar] [CrossRef] [PubMed]
- Marmur, E.S.; Berkowitz, E.Z.; Fuchs, B.S.; Singer, G.K.; Yoo, J.Y. Use of high-frequency, high-resolution ultrasound before Mohs surgery. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2010, 36, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, I.; Trovato, P.; Granata, V.; Picone, C.; Fusco, R.; Setola, S.V.; Mattace Raso, M.; Caracò, C.; Ascierto, P.A.; Sandomenico, F.; et al. Imaging Assessment of Interval Metastasis from Melanoma. J. Pers. Med. 2022, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Bessoud, B.; Lassau, N.; Koscielny, S.; Longvert, C.; Avril, M.-F.; Duvillard, P.; Rouffiac, V.; Leclère, J.; Roche, A. High-frequency sonography and color Doppler in the management of pigmented skin lesions. Ultrasound Med. Biol. 2003, 29, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Lauwers-Cances, V.; Lourari, S.; Laurent, J.; Konstantinou, M.-P.; Lagarde, J.-M.; Krief, B.; Batatia, H.; Lamant, L.; Paul, C. High-frequency ultrasonography but not 930-nm optical coherence tomography reliably evaluates melanoma thickness in vivo: A prospective validation study. Br. J. Dermatol. 2014, 171, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, A.; Chung, D.; London, K.; Uren, R.; Thompson, J.; Nieweg, O. Ultrasound Examination of the Lymphatic Drainage Area and Regional Lymph Nodes in Melanoma Patients with In-Transit Metastases. Ann. Surg. Oncol. 2021, 28, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Sanki, A.; Uren, R.F.; Moncrieff, M.; Tran, K.L.; Scolyer, R.A.; Lin, H.-Y.; Thompson, J.F. Targeted high-resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5614–5619. [Google Scholar] [CrossRef]
- Solivetti, F.M.; Di Luca Sidozzi, A.; Pirozzi, G.; Coscarella, G.; Brigida, R.; Eibenshutz, L. Sonographic evaluation of clinically occult in-transit and satellite metastases from cutaneous malignant melanoma. Radiol. Med. 2006, 111, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Nikitkina, A.I.; Bikmulina, P.Y.; Gafarova, E.R.; Kosheleva, N.V.; Efremov, Y.M.; Bezrukov, E.A.; Butnaru, D.V.; Dolganova, I.N.; Chernomyrdin, N.V.; Cherkasova, O.P.; et al. Terahertz radiation and the skin: A review. J. Biomed. Opt. 2021, 26, 043005. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Le, W. Advances of terahertz technology in neuroscience: Current status and a future perspective. iScience 2021, 24, 103548. [Google Scholar] [CrossRef]
- D’Arco, A.; Di Fabrizio, M.; Dolci, V.; Petrarca, M.; Lupi, S. THz Pulsed Imaging in Biomedical Applications. Condens. Matter 2020, 5, 25. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical Applications of Terahertz Spectroscopy and Imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Zaytsev, K.I.; Dolganova, I.N.; Chernomyrdin, N.V.; Katyba, G.M.; Gavdush, A.A.; Cherkasova, O.P.; Komandin, G.A.; Shchedrina, M.A.; Khodan, A.N.; Ponomarev, D.S.; et al. The progress and perspectives of terahertz technology for diagnosis of neoplasms: A review. J. Opt. 2019, 22, 013001. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.J.; Son, J.H. Toward Clinical Cancer Imaging Using Terahertz Spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–9. [Google Scholar] [CrossRef]
- Yu, C.; Fan, S.; Sun, Y.; Pickwell-Macpherson, E. The potential of terahertz imaging for cancer diagnosis: A review of investigations to date. Quant. Imaging Med. Surg. 2012, 2, 33–45. [Google Scholar] [CrossRef]
- Woodward, R.M.; Cole, B.E.; Wallace, V.P.; Pye, R.J.; Arnone, D.D.; Linfield, E.H.; Pepper, M. Terahertz pulse imaging in reflection geometry of human skin cancer and skin tissue. Phys. Med. Biol. 2002, 47, 3853–3863. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.P.; Fitzgerald, A.J.; Shankar, S.; Flanagan, N.; Pye, R.; Cluff, J.; Arnone, D.D. Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo. Br. J. Dermatol. 2004, 151, 424–432. [Google Scholar] [CrossRef]
- Ramos-Soto, D.I.; Singh, A.K.; Saucedo-Casas, E.; Castro-Camus, E.; Alfaro-Gomez, M. Visualization of moisturizer effects in stratum corneum in vitro using THz spectroscopic imaging. Appl. Opt. 2019, 58, 6581–6585. [Google Scholar] [CrossRef]
- Rahman, A. Terahertz Reconstructive Imaging: A novel technique to differentiate healthy and diseased human skin. Br. J. Cancer Res. 2019, 2, 228–232. [Google Scholar] [CrossRef]
- Zaytsev, K.I.; Chernomyrdin, N.V.; Kudrin, K.G.; Gavdush, A.A.; Nosov, P.A.; Yurchenko, S.O.; Reshetov, I.V. In vivo terahertz pulsed spectroscopy of dysplastic and non-dysplastic skin nevi. J. Phys. Conf. Ser. 2016, 735, 12076. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Sun, P. Edge detection on terahertz pulse imaging of dehydrated cutaneous malignant melanoma embedded in paraffin. Front. Optoelectron. 2019, 12, 317–323. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.; Lee, S.-H.; Kim, Y.A.; Son, J.-H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103. [Google Scholar] [CrossRef]
- Cheon, H.; Paik, J.H.; Choi, M.; Yang, H.-J.; Son, J.-H. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci. Rep. 2019, 9, 6413. [Google Scholar] [CrossRef] [PubMed]
- Dobre, E.-G.; Constantin, C.; Costache, M.; Neagu, M. Interrogating Epigenome toward Personalized Approach in Cutaneous Melanoma. J. Pers. Med. 2021, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Taday, P.F.; Pepper, M.; Arnone, D.D. Selected Applications of Terahertz Pulses in Medicine and Industry. Appl. Sci. 2022, 12, 6169. [Google Scholar] [CrossRef]
- Son, J.-H.; Oh, S.J.; Cheon, H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019, 125, 190901. [Google Scholar] [CrossRef]
- Bittoun, J.; Saint-Jalmes, H.; Querleux, B.G.; Darrasse, L.; Jolivet, O.; Idy-Peretti, I.; Wartski, M.; Richard, S.B.; Leveque, J.L. In vivo high-resolution MR imaging of the skin in a whole-body system at 1.5 T. Radiology 1990, 176, 457–460. [Google Scholar] [CrossRef]
- Richard, S.; Querleux, B.; Bittoun, J.; Idy-Peretti, I.; Jolivet, O.; Cermakova, E.; Lévêque, J.L. In vivo proton relaxation times analysis of the skin layers by magnetic resonance imaging. J. Investig. Dermatol. 1991, 97, 120–125. [Google Scholar] [CrossRef]
- Richard, S.; Querleux, B.; Bittoun, J.; Jolivet, O.; Idy-Peretti, I.; de Lacharriere, O.; Leveque, J.L. Characterization of the skin in vivo by high resolution magnetic resonance imaging: Water behavior and age-related effects. J. Investig. Dermatol. 1993, 100, 705–709. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kato, H.; Noda, Y.; Kobayashi, K.; Miyazaki, T.; Hyodo, F.; Matsuo, M. Imaging findings of malignant skin tumors: Radiological–pathological correlation. Insights Imaging 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Cavallaro, M.; Vinci, S.L.; Mormina, E.; Blandino, A.; Marino, M.A.; Granata, F.; Tessitore, A.; Galletta, K.; D’Angelo, T.; et al. Magnetism of materials: Theory and practice in magnetic resonance imaging. Insights Imaging 2021, 12, 179. [Google Scholar] [CrossRef]
- De Kerviler, E.; Cuenod, C.A.; Clément, O.; Halimi, P.; Frija, G.; Frija, J. What is bright on T1 MRI scans? J. Radiol. 1998, 79, 117–126. [Google Scholar]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Hara, A.; Suzui, N.; Miyazaki, T.; Matsuyama, K.; Seishima, M.; Matsuo, M. Magnetic Resonance Imaging Findings Differentiating Cutaneous Basal Cell Carcinoma from Squamous Cell Carcinoma in the Head and Neck Region. Korean J. Radiol. 2020, 21, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Uduma, F.U.; Titalom, K. Intra-orbital malignant melanoma: Role of MR imaging (a case report and literature review). Glob. J. Health Sci. 2011, 4, 253–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, S.E.; Beer, K.T.; Banic, A.; Steinbach, L.S.; Martin, M.; Friedrich, E.E.; Stauffer, E.; Vock, P.; Greiner, R.H. MRI of merkel cell carcinoma: Histologic correlation and review of the literature. AJR Am. J. Roentgenol. 2005, 185, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, V.; Jafarieh, S.; Rembielak, A. The role of imaging in head and neck cancer: An overview of different imaging modalities in primary diagnosis and staging of the disease. J. Contemp. Brachytherapy 2020, 12, 512–518. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Basal Cell Skin Cancer, Version 2.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf (accessed on 24 September 2022).
- Fisher, K.; Jude, S.; Gastman, B.; Grekin, R.C.; Helen Diller Family, U.; Grossman, K.; Ho, A.L.; Sloan Kettering Cancer Center Karl Lewis, M.D.; Lydiatt, D.D.; Buffett Cancer Center Jane Messina, P.; et al. NCCN Clinical Practice Guidelines in Oncology: Squamous Cell Skin Cancer, Version 2.2018. 2018. Available online: https://oncolife.com.ua/doc/nccn/Squamous_Cell_Skin_Cancer.pdf (accessed on 24 September 2022).
- Summers, P.; Saia, G.; Colombo, A.; Pricolo, P.; Zugni, F.; Alessi, S.; Marvaso, G.; Jereczek-Fossa, B.A.; Bellomi, M.; Petralia, G. Whole-body magnetic resonance imaging: Technique, guidelines and key applications. Ecancermedicalscience 2021, 15, 1164. [Google Scholar] [CrossRef]
- Kechidi, R.; Aubry, S. Vivo Magnetic Resonance Imaging of the Skin BT—Agache’s Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants; Humbert, P., Fanian, F., Maibach, H.I., Agache, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 477–486. ISBN 978-3-319-32383-1. [Google Scholar]
- Kim, J.; Kim, J.Y.; Chun, K.A.; Jee, W.-H.; Sung, M.-S. MR imaging manifestations of skin tumors. Eur. Radiol. 2008, 18, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.-H.; Chou, A.S.-B.; Hsu, Y.-H.; Lee, S.-K.; Lee, C.-C.; Yen, P.-S.; Ling, C.-M.; Lee, W.-H.; Lin, C.-C.; Chang, P.-Y. Marjolin’s ulcer: MR appearance. AJR Am. J. Roentgenol. 2006, 186, 819–820. [Google Scholar] [CrossRef]
- Chen, L.; Zhai, L.; Al-Kzayer, L.F.Y.; Sarsam, S.N.; Liu, T.; Alzakar, R.H.; Nakazawa, Y. Neurocutaneous Melanosis in Association with Large Congenital Melanocytic Nevi in Children: A Report of 2 Cases with Clinical, Radiological, and Pathogenetic Evaluation. Front. Neurol. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Goulart, C.R.; Mattei, T.A.; Ramina, R. Cerebral melanoma metastases: A critical review on diagnostic methods and therapeutic options. ISRN Surg. 2011, 2011, 276908. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Hara, A.; Suzui, N.; Miyazaki, T.; Matsuyama, K.; Seishima, M.; Matsuo, M. MR imaging findings for differentiating cutaneous malignant melanoma from squamous cell carcinoma. Eur. J. Radiol. 2020, 132, 109212. [Google Scholar] [CrossRef] [PubMed]
- Eggen, A.C.; Wind, T.T.; Bosma, I.; Kramer, M.C.A.; van Laar, P.J.; van der Weide, H.L.; Hospers, G.A.P.; Jalving, M. Value of screening and follow-up brain MRI scans in patients with metastatic melanoma. Cancer Med. 2021, 10, 8395–8404. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.R.S.; Chiang, V.L.; Tran, T.; Yu, J.B.; Weiss, S.A.; Goldberg, S.B.; Aboian, M.S.; Kluger, H.M.; Mahajan, A. Incidence and characteristics of metastatic intracranial lesions in stage III and IV melanoma: A single institute retrospective analysis. J. Neurooncol. 2021, 154, 197–203. [Google Scholar] [CrossRef]
- Lester, S.C.; Taksler, G.B.; Kuremsky, J.G.; Lucas, J.T.J.; Ayala-Peacock, D.N.; Randolph, D.M., 2nd; Bourland, J.D.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Clinical and economic outcomes of patients with brain metastases based on symptoms: An argument for routine brain screening of those treated with upfront radiosurgery. Cancer 2014, 120, 433–441. [Google Scholar] [CrossRef]
- Wong, A.; Gunraj, H.; Sivan, V.; Haider, M.A. Synthetic correlated diffusion imaging hyperintensity delineates clinically significant prostate cancer. Sci. Rep. 2022, 12, 3376. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, L.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; McLean, M.A.; Priest, A.N.; Gill, A.B.; Scott, F.; Patterson, I.; Carmo, B.; Riemer, F.; Kaggie, J.D.; Frary, A.; et al. Multiparametric MRI of early tumor response to immune checkpoint blockade in metastatic melanoma. J. Immunother. Cancer 2021, 9, e003125. [Google Scholar] [CrossRef]
- Umemura, Y.; Wang, D.; Peck, K.K.; Flynn, J.; Zhang, Z.; Fatovic, R.; Anderson, E.S.; Beal, K.; Shoushtari, A.N.; Kaley, T.; et al. DCE-MRI perfusion predicts pseudoprogression in metastatic melanoma treated with immunotherapy. J. Neurooncol. 2020, 146, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, M.; van Dijke, C.F.; Wielopolski, P.A.; ten Hagen, T.L.M.; Veenland, J.F.; Preda, A.; Loeve, A.J.; Eggermont, A.M.M.; Krestin, G.P. MR angiography of tumor-related vasculature: From the clinic to the micro-environment. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2005, 25 (Suppl. 1), S85–S97, discussion S97–S98. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Le, N.N.; Onishi, N.; Newitt, D.C.; Wilmes, L.J.; Gibbs, J.E.; Carmona-Bozo, J.; Liang, J.; Partridge, S.C.; Price, E.R.; et al. Diffusion-Weighted MRI for Predicting Pathologic Complete Response in Neoadjuvant Immunotherapy. Cancers 2022, 14, 4436. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; De Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Wang, C.C.; Liu, H.L.; Fu, S.Y.; Yu, C.F.; Chang, C.; Chiang, C.S.; Hong, J.H. Decline of Tumor Vascular Function as Assessed by Dynamic Contrast-Enhanced Magnetic Resonance Imaging Is Associated with Poor Responses to Radiation Therapy and Chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Mu, X.; Zhang, X.-D.; Ming, D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconjug. Chem. 2020, 31, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Zhang, Y.; Jia, C.; Ji, M. Development and application of several fluorescent probes in near infrared region. Dye. Pigment. 2021, 190, 109284. [Google Scholar] [CrossRef]
- Deng, G.; Li, S.; Sun, Z.; Li, W.; Zhou, L.; Zhang, J.; Gong, P.; Cai, L. Near-infrared fluorescence imaging in the largely unexplored window of 900–1000 nm. Theranostics 2018, 8, 4116–4128. [Google Scholar] [CrossRef] [PubMed]
- Borlan, R.; Focsan, M.; Maniu, D.; Astilean, S. Interventional NIR Fluorescence Imaging of Cancer: Review on Next Generation of Dye-Loaded Protein-Based Nanoparticles for Real-Time Feedback During Cancer Surgery. Int. J. Nanomed. 2021, 16, 2147–2171. [Google Scholar] [CrossRef] [PubMed]
- Van der Vorst, J.R.; Schaafsma, B.E.; Verbeek, F.P.R.; Swijnenburg, R.J.; Hutteman, M.; Liefers, G.J.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Dose optimization for near-infrared fluorescence sentinel lymph node mapping in patients with melanoma. Br. J. Dermatol. 2013, 168, 93–98. [Google Scholar] [CrossRef]
- Ji, Y.; Jones, C.; Baek, Y.; Park, G.K.; Kashiwagi, S.; Choi, H.S. Near-infrared fluorescence imaging in immunotherapy. Adv. Drug Deliv. Rev. 2020, 167, 121–134. [Google Scholar] [CrossRef]
- Sterkenburg, A.J.; Hooghiemstra, W.T.R.; Schmidt, I.; Ntziachristos, V.; Nagengast, W.B.; Gorpas, D. Standardization and implementation of fluorescence molecular endoscopy in the clinic. J. Biomed. Opt. 2022, 27, 074704. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Stambuk, H.E.; Madajewski, B.; Montero, P.H.; Matsuura, D.; Busam, K.J.; Ma, K.; Turker, M.Z.; Sequeira, S.; Gonen, M.; et al. Use of Ultrasmall Core-Shell Fluorescent Silica Nanoparticles for Image-Guided Sentinel Lymph Node Biopsy in Head and Neck Melanoma: A Nonrandomized Clinical Trial. JAMA Netw. Open 2021, 4, e211936. [Google Scholar] [CrossRef]
- Dengel, L.T.; More, M.J.; Judy, P.G.; Petroni, G.R.; Smolkin, M.E.; Rehm, P.K.; Majewski, S.; Williams, M.B.; Slingluff, C.L.J. Intraoperative imaging guidance for sentinel node biopsy in melanoma using a mobile gamma camera. Ann. Surg. 2011, 253, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Montorfano, L.; Bordes, S.J.; Forleiter, C.; Newman, M.I. Near-Infrared Fluorescence Imaging for Sentinel Lymph Node Identification in Melanoma Surgery. Cureus 2021, 13, e14550. [Google Scholar] [CrossRef] [PubMed]
- Burnier, P.; Niddam, J.; Bosc, R.; Hersant, B.; Meningaud, J.-P. Indocyanine green applications in plastic surgery: A review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Calmeiro, J.; Carrascal, M.; Gomes, C.; Falcão, A.; Cruz, M.T.; Neves, B.M. Highlighting the Role of DC-NK Cell Interplay in Immunobiology and Immunotherapy; Chapoval, S.P., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3; ISBN 978-1-78984-417-7. [Google Scholar]
- Russell, B.L.; Sooklal, S.A.; Malindisa, S.T.; Daka, L.J.; Ntwasa, M. The Tumor Microenvironment Factors That Promote Resistance to Immune Checkpoint Blockade Therapy. Front. Oncol. 2021, 11, 641428. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kang, M.-W.; Kashiwagi, S.; Choi, H.S. NIR fluorescence imaging and treatment for cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004936. [Google Scholar] [CrossRef] [PubMed]
- Kleinovink, E.W. The Use of Light in Cancer Immunotherapy. Available online: https://scholarlypublications.universiteitleiden.nl/access/item%3A2951594/view (accessed on 17 October 2022).
- Harmsen, S.; Medine, E.I.; Moroz, M.; Nurili, F.; Lobo, J.; Dong, Y.; Turkekul, M.; Pillarsetty, N.V.K.; Ting, R.; Ponomarev, V.; et al. A dual-modal PET/near infrared fluorescent nanotag for long-term immune cell tracking. Biomaterials 2021, 269, 120630. [Google Scholar] [CrossRef]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Lyu, Y.; Huang, J.; Pu, K. Near-Infrared Fluorescent Macromolecular Reporters for Real-Time Imaging and Urinalysis of Cancer Immunotherapy. J. Am. Chem. Soc. 2020, 142, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Sharma, A.; Chang, M.J.; Lee, J.; Son, S.; Sessler, J.L.; Kang, C.; Kim, J.S. Fluorogenic reaction-based prodrug conjugates as targeted cancer theranostics. Chem. Soc. Rev. 2018, 47, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Li, J.; Zhao, X.; Pu, K.; Zhang, R. Semiconducting Polymer Nanoreporters for Near-Infrared Chemiluminescence Imaging of Immunoactivation. Adv. Mater. 2020, 32, 1906314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Ma, H.; Wang, H.; Zhang, R.; Zhang, X.-D. Activatable NIR-II organic fluorescent probes for bioimaging. Theranostics 2022, 12, 3345–3371. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Corrie, P.G.; Gallagher, F.A. MRI techniques for immunotherapy monitoring. J. Immunother. Cancer 2022, 10, e004708. [Google Scholar] [CrossRef] [PubMed]
- Imafuku, K.; Hata, H.; Kitamura, S.; Yanagi, T.; Shimizu, H. Ultrasonographic findings can identify “pseudoprogression” under nivolumab therapy. Br. J. Dermatol. 2017, 177, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Wahl, G.; Poier, N.; Graf, S.; Kiesl, D.; Lamprecht, B.; Gabriel, M. Impact of PET/CT for Assessing Response to Immunotherapy-A Clinical Perspective. J. Clin. Med. 2020, 9, 3483. [Google Scholar] [CrossRef] [PubMed]
- Basler, L.; Gabryś, H.S.; Hogan, S.A.; Pavic, M.; Bogowicz, M.; Vuong, D.; Tanadini-Lang, S.; Förster, R.; Kudura, K.; Huellner, M.W.; et al. Radiomics, Tumor Volume, and Blood Biomarkers for Early Prediction of Pseudoprogression in Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4414–4425. [Google Scholar] [CrossRef]
- Filimon, A.; Preda, I.A.; Boloca, A.F.; Negroiu, G. Interleukin-8 in Melanoma Pathogenesis, Prognosis and Therapy-An Integrated View into Other Neoplasms and Chemokine Networks. Cells 2021, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Pomper, M.G. Molecular imaging in oncology: Current impact and future directions. CA. Cancer J. Clin. 2022, 72, 333–352. [Google Scholar] [CrossRef]
- Noltes, M.E.; van Dam, G.M.; Nagengast, W.B.; van der Zaag, P.J.; Slart, R.H.J.A.; Szymanski, W.; Kruijff, S.; Dierckx, R.A.J.O. Let’s embrace optical imaging: A growing branch on the clinical molecular imaging tree. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4120–4128. [Google Scholar] [CrossRef]
- Yan, Y.-Q.; Wang, H.; Zhao, Y. Radiolabeled peptide probe for tumor imaging. Chin. Chem. Lett. 2022, 33, 3361–3370. [Google Scholar] [CrossRef]
- Lau, J.; Rousseau, E.; Kwon, D.; Lin, K.-S.; Bénard, F.; Chen, X. Insight into the Development of PET Radiopharmaceuticals for Oncology. Cancers 2020, 12, 1312. [Google Scholar] [CrossRef]
- De Silva, R.A.; Kumar, D.; Lisok, A.; Chatterjee, S.; Wharram, B.; Venkateswara Rao, K.; Mease, R.; Dannals, R.F.; Pomper, M.G.; Nimmagadda, S. Peptide-Based 68Ga-PET Radiotracer for Imaging PD-L1 Expression in Cancer. Mol. Pharm. 2018, 15, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Xenaki, K.T.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 01287. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Li, K.; Zhang, T. FcγR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy. Front. Immunol. 2019, 10, 00292. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M.; AlJammaz, I. Development of the Tumor-Specific Antigen-Derived Synthetic Peptides as Potential Candidates for Targeting Breast and Other Possible Human Carcinomas. Molecules 2019, 24, 3142. [Google Scholar] [CrossRef] [PubMed]
- Polycarpou, I.; Soultanidis, G.; Tsoumpas, C. Synergistic motion compensation strategies for positron emission tomography when acquired simultaneously with magnetic resonance imaging. Philos. Trans. R. Soc. 2021, 379, 20200207. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.F. The origins of SPECT and SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kraft, O.; Havel, M. Detection of Sentinel Lymph Nodes in Gynecologic Tumours by Planar Scintigraphy and SPECT/CT. Mol. Imaging Radionucl. Ther. 2012, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Herrmann, K.; Rekowski, J.; Jansen, P.; Schadendorf, D.; Stang, A.; Klode, J. Sentinel lymph node excision with or without preoperative hybrid single-photon emission computed tomography/computed tomography (SPECT/CT) in melanoma: Study protocol for a multicentric randomized controlled trial. Trials 2019, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Perissinotti, A.; Rietbergen, D.D.; Vidal-Sicart, S.; Riera, A.A.; Olmos, R.A.V. Melanoma & nuclear medicine: New insights & advances. Melanoma Manag. 2018, 5, MMT06. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, F.; Avallone, G.; Castellengo, F.; Merli, M.; Caliendo, V.; Senetta, R.; Lesca, A.; Deandreis, D.; Fierro, M.T.; Quaglino, P.; et al. Non-Sentinel Lymph Node Detection during Sentinel Lymph Node Biopsy in Not-Complete-Lymph-Node-Dissection Era: A New Technique for Better Staging and Treating Melanoma Patients. J. Clin. Med. 2021, 10, 4319. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, I.M.C.; Valdés Olmos, R.A.; Kroon, B.B.R.; Wouters, M.W.J.M.; van den Brekel, M.W.M.; Vogel, W.V.; Hoefnagel, C.A.; Nieweg, O.E. The Yield of SPECT/CT for Anatomical Lymphatic Mapping in Patients with Melanoma. Ann. Surg. Oncol. 2009, 16, 1537–1542. [Google Scholar] [CrossRef]
- Jimenez-Heffernan, A.; Ellmann, A.; Sado, H.; Huić, D.; Bal, C.; Parameswaran, R.; Giammarile, F.; Pruzzo, R.; Kostadinova, I.; Vorster, M.; et al. Results of a Prospective Multicenter International Atomic Energy Agency Sentinel Node Trial on the Value of SPECT/CT over Planar Imaging in Various Malignancies. J. Nucl. Med. 2015, 56, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Mucientes Rasilla, J.; Cardona Arboniés, J.; Delgado Bolton, R.; Izarduy Pereyra, L.; Salazar Andía, G.; Prieto Soriano, A.; Anula Fernández, R.; Mayol Martínez, J.; Lapeña Gutiérrez, L.; González Maté, A.; et al. SPECT-CT in sentinel node detection in patients with melanoma. Rev. Esp. Med. Nucl. 2009, 28, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Müller, M.; Geisel, M.H.; Leyh, J.; Pöppel, T.; Schadendorf, D.; Klode, J. Cost-effectiveness of preoperative SPECT/CT combined with lymphoscintigraphy vs. lymphoscintigraphy for sentinel lymph node excision in patients with cutaneous malignant melanoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1723–1731. [Google Scholar] [CrossRef]
- Moncrieff, M.; Pywell, S.; Snelling, A.; Gray, M.; Newman, D.; Beadsmoore, C.; Heaton, M.; Pawaroo, D. ASO Author Reflections: Effectiveness of SPECT/CT Imaging for Sentinel Node Biopsy Staging of Primary Cutaneous Melanoma and Patient Outcomes. Ann. Surg. Oncol. 2022, 29, 776–777. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Technetium-99m Radiopharmaceuticals: Manufacture of Kits; Technical Reports Series; International Atomic Energy Agency: Vienna, Austria, 2008; ISBN 978-92-0-100408-6. [Google Scholar]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Tomao, F.; Zullo, A.; De Marinis, F.; Pasciuti, G.; Rossi, L.; Zoratto, F.; Tomao, S. Positron Emission Tomography (PET) radiotracers in oncology—Utility of 18F-Fluoro-deoxy-glucose (FDG)-PET in the management of patients with non-small-cell lung cancer (NSCLC). J. Exp. Clin. Cancer Res. 2008, 27, 52. [Google Scholar] [CrossRef]
- Cochran, B.J.; Ryder, W.J.; Parmar, A.; Klaeser, K.; Reilhac, A.; Angelis, G.I.; Meikle, S.R.; Barter, P.J.; Rye, K.-A. Determining Glucose Metabolism Kinetics Using 18F-FDG Micro-PET/CT. J. Vis. Exp. 2017, 123, e55184. [Google Scholar] [CrossRef]
- Drake, L.R.; Hillmer, A.T.; Cai, Z. Approaches to PET Imaging of Glioblastoma. Molecules 2020, 25, 568. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.S.; Campbell, D.O.; Radu, C.G.; Czernin, J. Positron emission tomography reporter genes and reporter probes: Gene and cell therapy applications. Theranostics 2012, 2, 374–391. [Google Scholar] [CrossRef]
- Farwell, M.D.; Pryma, D.A.; Mankoff, D.A. PET/CT imaging in cancer: Current applications and future directions. Cancer 2014, 120, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Bianconi, F.; Schillaci, O.; Spanu, A.; Palumbo, B. The Role and Potential of 18F-FDG PET/CT in Malignant Melanoma: Prognostication, Monitoring Response to Targeted and Immunotherapy, and Radiomics. Diagnostics 2022, 12, 929. [Google Scholar] [CrossRef]
- Kaniyala Melanthota, S.; Kistenev, Y.V.; Borisova, E.; Ivanov, D.; Zakharova, O.; Boyko, A.; Vrazhnov, D.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Types of spectroscopy and microscopy techniques for cancer diagnosis: A review. Lasers Med. Sci. 2022, 37, 3067–3084. [Google Scholar] [CrossRef] [PubMed]
- Yew, E.; Rowlands, C.; So, P.T.C. Application of Multiphoton Microscopy in Dermatological Studies: A Mini-Review. J. Innov. Opt. Health Sci. 2014, 7, 1330010. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, M.J.; Hristu, R.; Dumitru, A.; Floroiu, I.; Costache, M.; Stanciu, S.G. Multiphoton microscopy of the dermoepidermal junction and automated identification of dysplastic tissues with deep learning. Biomed. Opt. Express 2020, 11, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Iftimia, N.; Yélamos, O.; Chen, C.-S.J.; Maguluri, G.; Cordova, M.A.; Sahu, A.; Park, J.; Fox, W.; Alessi-Fox, C.; Rajadhyaksha, M. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J. Biomed. Opt. 2017, 22, 76006. [Google Scholar] [CrossRef]
- Pinto, M.S.; Paolella, R.; Billiet, T.; Van Dyck, P.; Guns, P.-J.; Jeurissen, B.; Ribbens, A.; den Dekker, A.J.; Sijbers, J. Harmonization of Brain Diffusion MRI: Concepts and Methods. Front. Neurosci. 2020, 14, 00396. [Google Scholar] [CrossRef]
- Mamaikin, M.; Li, Y.-L.; Ridente, E.; Chen, W.T.; Park, J.-S.; Zhu, A.Y.; Capasso, F.; Weidman, M.; Schultze, M.; Krausz, F.; et al. Electric-field-resolved near-infrared microscopy. Optica 2022, 9, 616–622. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, T.; Zhu, L.; Li, Y.; Chen, L.; Qian, J. Self-confocal NIR-II fluorescence microscopy for in vivo imaging. bioRxiv 2021. [Google Scholar] [CrossRef]
- Khalil, M.M.; Tremoleda, J.L.; Bayomy, T.B.; Gsell, W. Molecular SPECT Imaging: An Overview. Int. J. Mol. Imaging 2011, 2011, 796025. [Google Scholar] [CrossRef] [PubMed]
- Momodu, J.I.; Vangu, M.D.T. F-18 Fluoro-2-Deoxyglucose Positron Emission Tomography (PET)/Computed Tomography (CT) Imaging in Melanoma: Normal Variants, Pitfalls, and Artifacts. Front. Nucl. Med. 2022, 2, 835404. [Google Scholar] [CrossRef]
- Delbeke, D.; Coleman, R.E.; Guiberteau, M.J.; Brown, M.L.; Royal, H.D.; Siegel, B.A.; Townsend, D.W.; Berland, L.L.; Parker, J.A.; Hubner, K.; et al. Procedure Guideline for Tumor Imaging with 18F-FDG PET/CT 1.0. J. Nucl. Med. 2006, 47, 885–895. [Google Scholar] [PubMed]
- O’Neill, H.; Malik, V.; Johnston, C.; Reynolds, J.V.; O’Sullivan, J. Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals 2019, 12, 16. [Google Scholar] [CrossRef]
- Burt, B.M.; Humm, J.L.; Kooby, D.A.; Squire, O.D.; Mastorides, S.; Larson, S.M.; Fong, Y. Using positron emission tomography with [18F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia 2001, 3, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Bronstein, Y.; Ross, M.I.; Askew, R.L.; Lee, J.E.; Gershenwald, J.E.; Royal, R.; Cormier, J.N. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: A meta-analysis. J. Natl. Cancer Inst. 2011, 103, 129–142. [Google Scholar] [CrossRef]
- Gellén, E.; Sántha, O.; Janka, E.; Juhász, I.; Péter, Z.; Erdei, I.; Lukács, R.; Fedinecz, N.; Galuska, L.; Remenyik, É.; et al. Diagnostic accuracy of 18F-FDG-PET/CT in early and late stages of high-risk cutaneous malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Aukema, T.S.; Valdés Olmos, R.A.; Wouters, M.W.J.M.; Klop, W.M.C.; Kroon, B.B.R.; Vogel, W.V.; Nieweg, O.E. Utility of Preoperative 18F-FDG PET/CT and Brain MRI in Melanoma Patients with Palpable Lymph Node Metastases. Ann. Surg. Oncol. 2010, 17, 2773–2778. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis—Report of the RANO/PET group. Neuro. Oncol. 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Harry, W.; Schroeder, L.T.H., III. Molecular Imaging of Brain Metastases with PET. In Metastasis; Exon Publications: Brisbane, Australia, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Crippa, F.; Leutner, M.; Belli, F.; Gallino, F.; Greco, M.; Pilotti, S.; Cascinelli, N.; Bombardieri, E. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. J. Nucl. Med. 2000, 41, 1491–1494. [Google Scholar]
- Schröer-Günther, M.A.; Wolff, R.F.; Westwood, M.E.; Scheibler, F.J.; Schürmann, C.; Baumert, B.G.; Sauerland, S.; Kleijnen, J. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: A systematic review. Syst. Rev. 2012, 1, 62. [Google Scholar] [CrossRef] [PubMed]
- Hlongwa, K.N.; Mokoala, K.M.G.; Matsena-Zingoni, Z.; Vorster, M.; Sathekge, M.M. The Use of 18F-FDG PET/CT Metabolic Parameters in Predicting Overall Survival in Patients Undergoing Restaging for Malignant Melanoma. Diagnostics 2022, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Kang, S.M.; Jeong, S.Y.; Lee, S.-W.; Lee, S.-J.; Lee, J.; Ahn, B.-C. Prognostic Value of Volumetric Parameters Measured by Pretreatment 18F FDG PET/CT in Patients with Cutaneous Malignant Melanoma. Clin. Nucl. Med. 2016, 41, e266–e273. [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; Gatidis, S.; Sekler, J.; Dittmann, H.; Pfannenberg, C.; la Fougère, C.; Nikolaou, K.; Forschner, A. Clinical and prognostic value of tumor volumetric parameters in melanoma patients undergoing 18F-FDG-PET/CT: A comparison with serologic markers of tumor burden and inflammation. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2020, 20, 44. [Google Scholar] [CrossRef]
- Schweighofer-Zwink, G.; Manafi-Farid, R.; Kölblinger, P.; Hehenwarter, L.; Harsini, S.; Pirich, C.; Beheshti, M. Prognostic value of 2-[18F]FDG PET-CT in metastatic melanoma patients receiving immunotherapy. Eur. J. Radiol. 2022, 146, 110107. [Google Scholar] [CrossRef] [PubMed]
- Küstner, T.; Vogel, J.; Hepp, T.; Forschner, A.; Pfannenberg, C.; Schmidt, H.; Schwenzer, N.F.; Nikolaou, K.; la Fougère, C.; Seith, F. Development of a Hybrid-Imaging-Based Prognostic Index for Metastasized-Melanoma Patients in Whole-Body 18F-FDG PET/CT and PET/MRI Data. Diagnostics 2022, 12, 2102. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Schöder, H.; Teng, R.; Humm, J.L.; Ni, A.; Wolchok, J.D.; Weber, W.A. Prognostic value of baseline metabolic tumor volume measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 930–939. [Google Scholar] [CrossRef]
- Galldiks, N.; Abdulla, D.S.Y.; Scheffler, M.; Wolpert, F.; Werner, J.-M.; Hüllner, M.; Stoffels, G.; Schweinsberg, V.; Schlaak, M.; Kreuzberg, N.; et al. Treatment Monitoring of Immunotherapy and Targeted Therapy Using 18F-FET PET in Patients with Melanoma and Lung Cancer Brain Metastases: Initial Experiences. J. Nucl. Med. 2021, 62, 464–470. [Google Scholar] [CrossRef]
- Manitz, J.; D’Angelo, S.P.; Apolo, A.B.; Eggleton, S.P.; Bajars, M.; Bohnsack, O.; Gulley, J.L. Comparison of tumor assessments using RECIST 1.1 and irRECIST, and association with overall survival. J. Immunother. Cancer 2022, 10, e003302. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Rossi, S.; Toschi, L.; Lopci, E. Comparison of Metabolic and Morphological Response Criteria for Early Prediction of Response and Survival in NSCLC Patients Treated With Anti-PD-1/PD-L1. Front. Oncol. 2020, 10, 01090. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Delyon, J.; Martineau, A.; Blanc, E.; Allayous, C.; Da Meda, L.; Merlet, P.; Lebbé, C.; Baroudjian, B.; Vercellino, L. 18FDG PET Assessment of Therapeutic Response in Patients with Advanced or Metastatic Melanoma Treated with First-Line Immune Checkpoint Inhibitors. Cancers 2022, 14, 3190. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Tan, Z.; Cheng, Y.; Tang, Y.; Guo, B.; Gong, J.; Ling, X.; Wang, L.; Xu, H. A method for evaluation of patient-specific lean body mass from limited-coverage CT images and its application in PERCIST: Comparison with predictive equation. EJNMMI Phys. 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Costelloe, C.M.; Chuang, H.H.; Madewell, J.E.; Ueno, N.T. Cancer Response Criteria and Bone Metastases: RECIST 1.1, MDA and PERCIST. J. Cancer 2010, 1, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Prosch, H.; Herold, C.J.; Weber, M.; Karanikas, G. Assessment of pulmonary melanoma metastases with 18F-FDG PET/CT: Which PET-negative patients require additional tests for definitive staging? Eur. Radiol. 2012, 22, 2451–2457. [Google Scholar] [CrossRef]
- Aide, N.; Iravani, A.; Prigent, K.; Kottler, D.; Alipour, R.; Hicks, R.J. PET/CT variants and pitfalls in malignant melanoma. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2022, 22, 3. [Google Scholar] [CrossRef]
- Leung, D.; Bonacorsi, S.; Smith, R.A.; Weber, W.; Hayes, W. Molecular Imaging and the PD-L1 Pathway: From Bench to Clinic. Front. Oncol. 2021, 11, 698425. [Google Scholar] [CrossRef] [PubMed]
- Wierstra, P.; Sandker, G.; Aarntzen, E.; Gotthardt, M.; Adema, G.; Bussink, J.; Raavé, R.; Heskamp, S. Tracers for non-invasive radionuclide imaging of immune checkpoint expression in cancer. EJNMMI Radiopharm. Chem. 2019, 4, 29. [Google Scholar] [CrossRef]
- Erol Fenercioğlu, Ö.; Beyhan, E.; Ergül, N.; Arslan, E.; Çermik, T.F. 18F-FDG PET/CT and 68Ga-FAPI-4 PET/CT Findings of Bilateral Knee Osteoarthritis in a Patient With Uveal Malignant Melanoma. Clin. Nucl. Med. 2022, 47, e144–e146. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Greguric, I.; Roselt, P.; Neels, O.C.; Aide, N.; Taylor, S.R.; Katsifis, A.; Dorow, D.S.; Hicks, R.J. High-contrast PET of melanoma using 18F-MEL050, a selective probe for melanin with predominantly renal clearance. J. Nucl. Med. 2010, 51, 441–447. [Google Scholar] [CrossRef]
- Miao, Y.; Quinn, T.P. Advances in Receptor-Targeted Radiolabeled Peptides for Melanoma Imaging and Therapy. J. Nucl. Med. 2021, 62, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef] [PubMed]
- Hegi-Johnson, F.; Rudd, S.; Hicks, R.J.; De Ruysscher, D.; Trapani, J.A.; John, T.; Donnelly, P.; Blyth, B.; Hanna, G.; Everitt, S.; et al. Imaging immunity in patients with cancer using positron emission tomography. NPJ Precis. Oncol. 2022, 6, 24. [Google Scholar] [CrossRef]
- Lauwerys, L.; Smits, E.; Van den Wyngaert, T.; Elvas, F. Radionuclide Imaging of Cytotoxic Immune Cell Responses to Anti-Cancer Immunotherapy. Biomedicines 2022, 10, 1074. [Google Scholar] [CrossRef]
- Stevens, M.Y.; Cropper, H.C.; Lucot, K.L.; Chaney, A.M.; Lechtenberg, K.J.; Jackson, I.M.; Buckwalter, M.S.; James, M.L. Development of a CD19 PET tracer for detecting B cells in a mouse model of multiple sclerosis. J. Neuroinflammation 2020, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Feltes, P.K.; Luft, C.; Nazario, L.R.; Jeckel, C.M.M.; Antunes, I.F.; Elsinga, P.H.; de Vries, E.F.J. Potential PET tracers for imaging of tumor-associated macrophages. EJNMMI Radiopharm. Chem. 2022, 7, 11. [Google Scholar] [CrossRef]
- Rashidian, M.; LaFleur, M.W.; Verschoor, V.L.; Dongre, A.; Zhang, Y.; Nguyen, T.H.; Kolifrath, S.; Aref, A.R.; Lau, C.J.; Paweletz, C.P.; et al. Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. Proc. Natl. Acad. Sci. USA 2019, 116, 16971–16980. [Google Scholar] [CrossRef]
- Krekorian, M.; Fruhwirth, G.O.; Srinivas, M.; Figdor, C.G.; Heskamp, S.; Witney, T.H.; Aarntzen, E.H.J.G. Imaging of T-cells and their responses during anti-cancer immunotherapy. Theranostics 2019, 9, 7924–7947. [Google Scholar] [CrossRef]
- Xin, Y.; Cai, H. Improved Radiosynthesis and Biological Evaluations of L- and D-1-[18F]Fluoroethyl-Tryptophan for PET Imaging of IDO-Mediated Kynurenine Pathway of Tryptophan Metabolism. Mol. Imaging Biol. 2017, 19, 589–598. [Google Scholar] [CrossRef]
- Komiya, T.; Huang, C.H. Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front. Oncol. 2018, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Study of IDO Inhibitor in Combination with Gemcitabine and Nab-Paclitaxel in Patients with Metastatic Pancreatic Cancer—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02077881 (accessed on 22 October 2022).
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Juhász, C.; Chugani, D.C.; Muzik, O.; Wu, D.; Sloan, A.E.; Barger, G.; Watson, C.; Shah, A.K.; Sood, S.; Ergun, E.L.; et al. In vivo Uptake and Metabolism of α-[11C]Methyl-l-Tryptophan in Human Brain Tumors. J. Cereb. Blood Flow Metab. 2005, 26, 345–357. [Google Scholar] [CrossRef]
- Michelhaugh, S.K.; Muzik, O.; Guastella, A.R.; Klinger, N.V.; Polin, L.A.; Cai, H.; Xin, Y.; Mangner, T.J.; Zhang, S.; Juhász, C.; et al. Assessment of Tryptophan Uptake and Kinetics Using 11-(2-18F-Fluoroethyl)-l-Tryptophan and α-11C-Methyl-l-Tryptophan PET imaging in mice implanted with patient-derived brain tumor xenografts. J. Nucl. Med. 2017, 58, 208–213. [Google Scholar] [CrossRef]
- Lukas, R.V.; Juhász, C.; Wainwright, D.A.; James, C.D.; Kennedy, E.; Stupp, R.; Lesniak, M.S. Imaging tryptophan uptake with positron emission tomography in glioblastoma patients treated with indoximod. J. Neurooncol. 2019, 141, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Pembrolizumab TX-Naive Distant Mets Melanoma and Use of (C11-AMT) PET at Baseline as Imaging Biomarker—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03089606 (accessed on 22 October 2022).
- Oldan, J.D.; Ollila, D.W.; Giglio, B.C.; Smith, E.; Bouchard, D.M.; Ivanovic, M.; Lee, Y.Z.; Collichio, F.A.; Meyers, M.O.; Wallack, D.E.; et al. High intratumoral tryptophan metabolism is a poor predictor of response to pembrolizumab (pembro) in metastatic melanoma (MM): Results from a prospective trial using baseline C11-labeled alpha-methyl tryptophan (C11-AMT) PET imaging for response predictio. J. Clin. Oncol. 2020, 38, 3556. [Google Scholar] [CrossRef]
- Krutzek, F.; Kopka, K.; Stadlbauer, S. Development of Radiotracers for Imaging of the PD-1/PD-L1 Axis. Pharmaceuticals 2022, 15, 747. [Google Scholar] [CrossRef]
- MacManus, M.; Rudd, S.; Roselt, P.; Wichmann, C.; Callahan, J.; John, T.; Scott, A.; Donnelly, P.; Hanna, G.; Hegi-Johnson, F. P1.10-02 ImmunoPET: A Phase 0/1 Study Characterising PD-L1 with 89Zr-Durvalumab (MEDI4736) PET/CT in Stage III NSCLC Patients Receiving Chemoradiation. J. Thorac. Oncol. 2022, 17, S107–S108. [Google Scholar] [CrossRef]
- [68Ga]Ga-NOTA-SNA002 (PD-L1 PET Tracer) for PET/CT in Patients With Solid Tumors—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05490264 (accessed on 22 October 2022).
- Verhoeff, S.R.; van de Donk, P.P.; Aarntzen, E.H.J.G.; Oosting, S.F.; Brouwers, A.H.; Miedema, I.H.C.; Voortman, J.; Menke-van der Houven van Oordt, W.C.; Boellaard, R.; Vriens, D.; et al. (89)Zr-DFO-Durvalumab PET/CT Before Durvalumab Treatment in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Nucl. Med. 2022, 63, 1523–1530. [Google Scholar] [CrossRef]
- Tabei, T.; Nakaigawa, N.; Kaneta, T.; Ikeda, I.; Kondo, K.; Makiyama, K.; Hasumi, H.; Hayashi, N.; Kawahara, T.; Izumi, K.; et al. Early assessment with 18F-2-fluoro-2-deoxyglucose positron emission tomography/computed tomography to predict short-term outcome in clear cell renal carcinoma treated with nivolumab. BMC Cancer 2019, 19, 298. [Google Scholar] [CrossRef]
- Chen, A.; Mokrane, F.-Z.; Schwartz, L.H.; Morschhauser, F.; Stamatoullas, A.; Schiano de Colella, J.-M.; Vercellino, L.; Casasnovas, O.; Chauchet, A.; Delmer, A.; et al. Early 18F-FDG PET/CT Response Predicts Survival in Relapsed or Refractory Hodgkin Lymphoma Treated with Nivolumab. J. Nucl. Med. 2020, 61, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lesniak, W.G.; Gabrielson, M.; Lisok, A.; Wharram, B.; Sysa-Shah, P.; Azad, B.B.; Pomper, M.G.; Nimmagadda, S. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget 2016, 7, 10215–10227. [Google Scholar] [CrossRef] [PubMed]
- Nienhuis, P.H.; Antunes, I.F.; Glaudemans, A.W.J.M.; Jalving, M.; Leung, D.; Noordzij, W.; Slart, R.H.J.A.; de Vries, E.F.J.; Hospers, G.A.P. 18F-BMS986192 PET Imaging of PD-L1 in Metastatic Melanoma Patients with Brain Metastases Treated with Immune Checkpoint Inhibitors: A Pilot Study. J. Nucl. Med. 2022, 63, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Hettich, M.; Braun, F.; Bartholomä, M.D.; Schirmbeck, R.; Niedermann, G. High-Resolution PET Imaging with Therapeutic Antibody-based PD-1/PD-L1 Checkpoint Tracers. Theranostics 2016, 6, 1629–1640. [Google Scholar] [CrossRef]
- Natarajan, A.; Mayer, A.T.; Xu, L.; Reeves, R.E.; Gano, J.; Gambhir, S.S. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjug. Chem. 2015, 26, 2062–2069. [Google Scholar] [CrossRef]
- Huck, B.R.; Kötzner, L.; Urbahns, K. Small Molecules Drive Big Improvements in Immuno-Oncology Therapies. Angew. Chem. Int. Ed. Engl. 2018, 57, 4412–4428. [Google Scholar] [CrossRef] [PubMed]
- Uptake and Biodistribution of 89Zirconium-Labeled Ipilimumab in Ipilimumab Treated Patients with Metastatic Melanoma—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03313323 (accessed on 22 October 2022).
- PET Imaging of LAG-3 Expression—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05346276 (accessed on 22 October 2022).
- 89Zr-DFO-REGN3767 in PET Scans in People with Diffuse Large B Cell Lymphoma (DLBCL)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04566978 (accessed on 22 October 2022).
- LAG3 PET Imaging in Advanced Solid Tumors—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04706715 (accessed on 22 October 2022).
- Kang, C.Y.; Duarte, S.E.; Kim, H.S.; Kim, E.; Park, J.; Lee, A.D.; Kim, Y.; Kim, L.; Cho, S.; Oh, Y.; et al. Artificial Intelligence-based Radiomics in the Era of Immuno-oncology. Oncologist 2022, 27, e471–e483. [Google Scholar] [CrossRef] [PubMed]
- Haubner, R.; Maschauer, S.; Prante, O. PET radiopharmaceuticals for imaging integrin expression: Tracers in clinical studies and recent developments. Biomed. Res. Int. 2014, 2014, 871609. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef]
- Beer, A.J.; Kessler, H.; Wester, H.-J.; Schwaiger, M. PET Imaging of Integrin αvβ3 Expression. Theranostics 2011, 1, 48–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.; Wang, F.; Liu, S. Radiolabeled cyclic RGD peptides as radiotracers for tumor imaging. Biophys. Rep. 2016, 2, 1–20. [Google Scholar] [CrossRef]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar] [PubMed]
- Decristoforo, C.; Hernandez Gonzalez, I.; Carlsen, J.; Rupprich, M.; Huisman, M.; Virgolini, I.; Wester, H.-J.; Haubner, R. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of alphavbeta3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1507–1515. [Google Scholar] [CrossRef]
- Jeong, J.M.; Hong, M.K.; Chang, Y.S.; Lee, Y.-S.; Kim, Y.J.; Cheon, G.J.; Lee, D.S.; Chung, J.-K.; Lee, M.C. Preparation of a promising angiogenesis PET imaging agent: 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclononane-1,4,7-triacetic acid and feasibility studies in mice. J. Nucl. Med. 2008, 49, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Knetsch, P.A.; Petrik, M.; Griessinger, C.M.; Rangger, C.; Fani, M.; Kesenheimer, C.; von Guggenberg, E.; Pichler, B.J.; Virgolini, I.; Decristoforo, C.; et al. [68Ga]NODAGA-RGD for imaging αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1303–1312. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Wester, H.-J. Be spoilt for choice with radiolabelled RGD peptides: Preclinical evaluation of 68Ga-TRAP(RGD)₃. Nucl. Med. Biol. 2013, 40, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Simeček, J.; Notni, J.; Kapp, T.G.; Kessler, H.; Wester, H.-J. Benefits of NOPO as chelator in gallium-68 peptides, exemplified by preclinical characterization of 68Ga-NOPO-c(RGDfK). Mol. Pharm. 2014, 11, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- McBride, W.J.; Sharkey, R.M.; Goldenberg, D.M. Radiofluorination using aluminum-fluoride (Al18F). EJNMMI Res. 2013, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Guo, N.; Pan, D.; Yu, C.; Weng, Y.; Luo, S.; Ding, H.; Xu, Y.; Wang, L.; Lang, L.; et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J. Nucl. Med. 2013, 54, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.J.; Grosu, A.-L.; Carlsen, J.; Kolk, A.; Sarbia, M.; Stangier, I.; Watzlowik, P.; Wester, H.-J.; Haubner, R.; Schwaiger, M. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 6610–6616. [Google Scholar] [CrossRef] [PubMed]
- Durante, S.; Dunet, V.; Gorostidi, F.; Mitsakis, P.; Schaefer, N.; Delage, J.; Prior, J.O. Head and neck tumors angiogenesis imaging with 68Ga-NODAGA-RGD in comparison to 18F-FDG PET/CT: A pilot study. EJNMMI Res. 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- 68Ga-NOTA-BBN-RGD PET/CT in Prostate Cancer Patients—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02747290 (accessed on 23 October 2022).
- Jiang, Y.; Liu, Q.; Wang, G.; Sui, H.; Wang, R.; Wang, J.; Zhu, Z. A prospective head-to-head comparison of 68Ga-NOTA-3P-TATE-RGD and 68Ga-DOTATATE in patients with gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4218–4227. [Google Scholar] [CrossRef] [PubMed]
- Study of the Angiogenesis by PET/CT in Patients With Lymphoma—Full Text View—ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02490891 (accessed on 23 October 2022).
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Ou, L.; Yang, X.; Liu, H.; Gong, W.; Zhang, C. Increased 68Ga-FAPI Activity in Malignant Melanoma of the Nasal Cavity. Clin. Nucl. Med. 2022, 47, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- The Role of 68Ga-FAPI-04 PET/CT as a Problem Solving Imaging Modality in Various Malignancies—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04441606 (accessed on 23 October 2022).
- 68Ga-FAPI PET/CT in Patients with Various Types of Cancer—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04499365 (accessed on 23 October 2022).
- Chen, Y.; Zheng, S.; Zhang, J.; Yao, S.; Miao, W. 68Ga-DOTA-FAPI-04 PET/CT imaging in radioiodine-refractory differentiated thyroid cancer (RR-DTC) patients. Ann. Nucl. Med. 2022, 36, 610–622. [Google Scholar] [CrossRef]
- Exploring the Application Value of PET Molecular Imaging Targeting FAP in Oral Squamous Cell Carcinoma—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05030597 (accessed on 23 October 2022).
- 68Ga-FAP-CHX PET/CT: Dosimetry and Preliminary Clinical Translational Studies—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05506566?term=Imaging+Targeting+FAP&cond=cancer&draw=2&rank=2 (accessed on 23 October 2022).
- 68Ga-FAPI-RGD PET/CT for Dual Integrin αvβ3 and FAP-Targeted Imaging in Patients with Various Types of Cancer and Compared with 18F-FDG—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05543317?term=Imaging+Targeting+FAP&cond=cancer&draw=2&rank=3 (accessed on 23 October 2022).
- Xu, Y.; Guan, X.; Zhou, R.; Gong, R. Melanocortin 5 receptor signaling pathway in health and disease. Cell Mol. Life Sci. 2020, 77, 3831–3840. [Google Scholar] [CrossRef] [PubMed]
- Tafreshi, N.K.; Silva, A.; Estrella, V.C.; McCardle, T.W.; Chen, T.; Jeune-Smith, Y.; Lloyd, M.C.; Enkemann, S.A.; Smalley, K.S.M.; Sondak, V.K.; et al. In vivo and in silico pharmacokinetics and biodistribution of a melanocortin receptor 1 targeted agent in preclinical models of melanoma. Mol. Pharm. 2013, 10, 3175–3185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hossain, M.R.; Ansary, T.M.; Komine, M.; Ohtsuki, M. Diversified Stimuli-Induced Inflammatory Pathways Cause Skin Pigmentation. Int. J. Mol. Sci. 2021, 22, 3970. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Sue O’Dorisio, M.; Leverich, W.M.; Kloepping, K.C.; Walsh, S.A.; Schultz, M.K. “Click”-Cyclized 68Ga-Labeled Peptides for Molecular Imaging and Therapy: Synthesis and Preliminary In Vitro and In Vivo Evaluation in a Melanoma Model System BT—Theranostics, Gallium-68, and Other Radionuclides; Baum, R.P., Rösch, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 149–175. [Google Scholar]
- Oliveira, M.C.; Correia, J.D.G. Biomedical applications of radioiodinated peptides. Eur. J. Med. Chem. 2019, 179, 56–77. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Z.; Lu, B.-Y.J.; Vazquez-Flores, G.; Yuen, J.; Daibes Figueroa, S.; Gallazzi, F.; Cutler, C.P.; Quinn, T.L.; Lacy, J. Copper-62 Labeled ReCCMSH Peptide Analogs for Melanoma PET Imaging. Curr. Radiopharm. 2012, 5, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Giblin, M.F.; Wang, N.; Hoffman, T.J.; Jurisson, S.S.; Quinn, T.P. Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proc. Natl. Acad. Sci. USA 1998, 95, 12814–12818. [Google Scholar] [CrossRef]
- Froidevaux, S.; Calame-Christe, M.; Tanner, H.; Eberle, A.N. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: Structural parameters affecting tumor uptake and kidney uptake. J. Nucl. Med. 2005, 46, 887–895. [Google Scholar] [PubMed]
- Nagy, G.; Dénes, N.; Kis, A.; Szabó, J.P.; Berényi, E.; Garai, I.; Bai, P.; Hajdu, I.; Szikra, D.; Trencsényi, G. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific 68Ga- and 44Sc-labeled DOTA-NAPamide in melanoma imaging. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 106, 336–344. [Google Scholar] [CrossRef]
- Bednarek, M.A.; Silva, M.V.; Arison, B.; MacNeil, T.; Kalyani, R.N.; Huang, R.R.; Weinberg, D.H. Structure-function studies on the cyclic peptide MT-II, lactam derivative of alpha-melanotropin. Peptides 1999, 20, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Gallazzi, F.; Guo, H.; Quinn, T.P. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjug. Chem. 2008, 19, 539–547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, H.; Yang, J.; Gallazzi, F.; Miao, Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide, resulting in enhanced melanoma uptake. J. Nucl. Med. 2010, 51, 418–426. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Xu, J.; Gonzalez, R.; Lindner, T.; Kratochwil, C.; Miao, Y. 68Ga-DOTA-GGNle-CycMSH(hex) targets the melanocortin-1 receptor for melanoma imaging. Sci. Transl. Med. 2018, 10, eaau4445. [Google Scholar] [CrossRef] [PubMed]
- McQuade, P.; Miao, Y.; Yoo, J.; Quinn, T.P.; Welch, M.J.; Lewis, J.S. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of alpha-MSH. J. Med. Chem. 2005, 48, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Miao, Y.; Gallazzi, F.; Quinn, T.P.; Welch, M.J.; Vāvere, A.L.; Lewis, J.S. Gallium-68-labeled DOTA-rhenium-cyclized alpha-melanocyte-stimulating hormone analog for imaging of malignant melanoma. Nucl. Med. Biol. 2007, 34, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gallazzi, F.; Miao, Y. Gallium-67-labeled lactam bridge-cyclized alpha-MSH peptides with enhanced melanoma uptake and reduced renal uptake. Bioconjug. Chem. 2012, 23, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Miao, Y. Introduction of an 8-aminooctanoic acid linker enhances uptake of 99mTc-labeled lactam bridge-cyclized α-MSH peptide in melanoma. J. Nucl. Med. 2014, 55, 2057–2063. [Google Scholar] [CrossRef]
- Wei, W.; Ehlerding, E.B.; Lan, X.; Luo, Q.; Cai, W. PET and SPECT imaging of melanoma: The state of the art. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 132–150. [Google Scholar] [CrossRef]
- Guo, H.; Miao, Y. Cu-64-labeled lactam bridge-cyclized α-MSH peptides for PET imaging of melanoma. Mol. Pharm. 2012, 9, 2322–2330. [Google Scholar] [CrossRef]
- Demir, E.S.; Ozgenc, E.; Ekinci, M.; Gundogdu, E.A.; Özdemir, D.İ.; Asikoglu, M. Computational Study of Radiopharmaceuticals; Stefaniu, A., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 6, ISBN 978-1-78984-092-6. [Google Scholar]
- Ataeinia, B.; Heidari, P. Artificial Intelligence and the Future of Diagnostic and Therapeutic Radiopharmaceutical Development:: In Silico Smart Molecular Design. PET Clin. 2021, 16, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Caparrotti, F.; Troussier, I.; Ali, A.; Zilli, T. Localized Non-melanoma Skin Cancer: Risk Factors of Post-surgical Relapse and Role of Postoperative Radiotherapy. Curr. Treat. Options Oncol. 2020, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Nehal, K.S.; Bichakjian, C.K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018, 379, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Asgari, M.M.; Green, A.C.; Guhan, S.M.; Arron, S.T.; Proby, C.M.; Rollison, D.E.; Harwood, C.A.; Toland, A.E. Keratinocyte Carcinomas: Current Concepts and Future Research Priorities. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Fung, K.; Chan, A.-W. Incidence and mortality rates of keratinocyte carcinoma from 1998–2017: A population-based study of sex differences in Ontario, Canada. CMAJ J. L’association Med. Can. 2021, 193, E1516–E1524. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Gubarkova, E.V.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Zagaynova, E.V.; Gladkova, N.D.; Zaitsev, V.Y. Histological validation of in vivo assessment of cancer tissue inhomogeneity and automated morphological segmentation enabled by Optical Coherence Elastography. Sci. Rep. 2020, 10, 11781. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.A.; Caruntu, C.; Lixandru, D.; Tampa, M.; Georgescu, S.-R.; Constantin, M.-M.; Constantin, C.; Neagu, M.; Zurac, S.A.; Boda, D. In vivo confocal laser scanning microscopy imaging of skin inflammation: Clinical applications and research directions. Exp. Ther. Med. 2019, 17, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Masłowska, K.; Halik, P.K.; Tymecka, D.; Misicka, A.; Gniazdowska, E. The Role of VEGF Receptors as Molecular Target in Nuclear Medicine for Cancer Diagnosis and Combination Therapy. Cancers 2021, 13, 1072. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Aboian, M.S.; Zhu, X.; Marquez-Nostra, B. Clinical Evaluation of Nuclear Imaging Agents in Breast Cancer. Cancers 2022, 14, 2103. [Google Scholar] [CrossRef] [PubMed]
- Ghita, M.A.; Caruntu, C.; Rosca, A.E.; Kaleshi, H.; Caruntu, A.; Moraru, L.; Docea, A.O.; Zurac, S.; Boda, D.; Neagu, M.; et al. Reflectance confocal microscopy and dermoscopy for in vivo, non-invasive skin imaging of superficial basal cell carcinoma. Oncol. Lett. 2016, 11, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Huck, C.W.; Ishigaki, M.; Ishikawa, D.; Ikehata, A.; Shinzawa, H. Near-Infrared Spectroscopy in Biological Molecules and Tissues BT—Encyclopedia of Biophysics; Roberts, G., Watts, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–19. ISBN 978-3-642-35943-9. [Google Scholar]
- Sowa, A.R.; Jackson, I.M.; Desmond, T.J.; Alicea, J.; Mufarreh, A.J.; Pham, J.M.; Stauff, J.; Winton, W.P.; Fawaz, M.V.; Henderson, B.D.; et al. Futureproofing [18F]Fludeoxyglucose manufacture at an Academic Medical Center. EJNMMI Radiopharm. Chem. 2018, 3, 12. [Google Scholar] [CrossRef]
- Hadian, M.; Jabbari, A.; Mazaheri, E.; Norouzi, M. What is the impact of clinical guidelines on imaging costs? J. Educ. Health Promot. 2021, 10, 10. [Google Scholar] [CrossRef]
- Beyer, T.; Bailey, D.L.; Birk, U.J.; Buvat, I.; Catana, C.; Cheng, Z.; Fang, Q.; Giove, F.; Kuntner, C.; Laistler, E.; et al. Medical Physics and Imaging–A Timely Perspective. Front. Phys. 2021, 9, 634693. [Google Scholar] [CrossRef]
- Iftimia, N.; Sahu, A.; Cordova, M.; Maguluri, G.; Gill, M.; Alessi-Fox, C.; Gonzalez, S.; Navarrete-Dechent, C.; Marghoob, A.; Chen, C.-S.J.; et al. The potential utility of integrated reflectance confocal microscopy-optical coherence tomography for guiding triage and therapy of basal cell carcinomas. J. Cancer 2020, 11, 6019–6024. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hong, S.; Zhang, W.; Li, H. Artificial Intelligence in Skin Diseases: Fulfilling its Potentials to Meet the Real Needs in Dermatology Practice. Health Data Sci. 2022, 2022, 9791467. [Google Scholar] [CrossRef]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Academic Press: New York, NY, USA, 2020; pp. 25–60. [Google Scholar]
- Record, J.D.; Ziegelstein, R.C.; Christmas, C.; Rand, C.S.; Hanyok, L.A. Delivering Personalized Care at a Distance: How Telemedicine Can Foster Getting to Know the Patient as a Person. J. Pers. Med. 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]

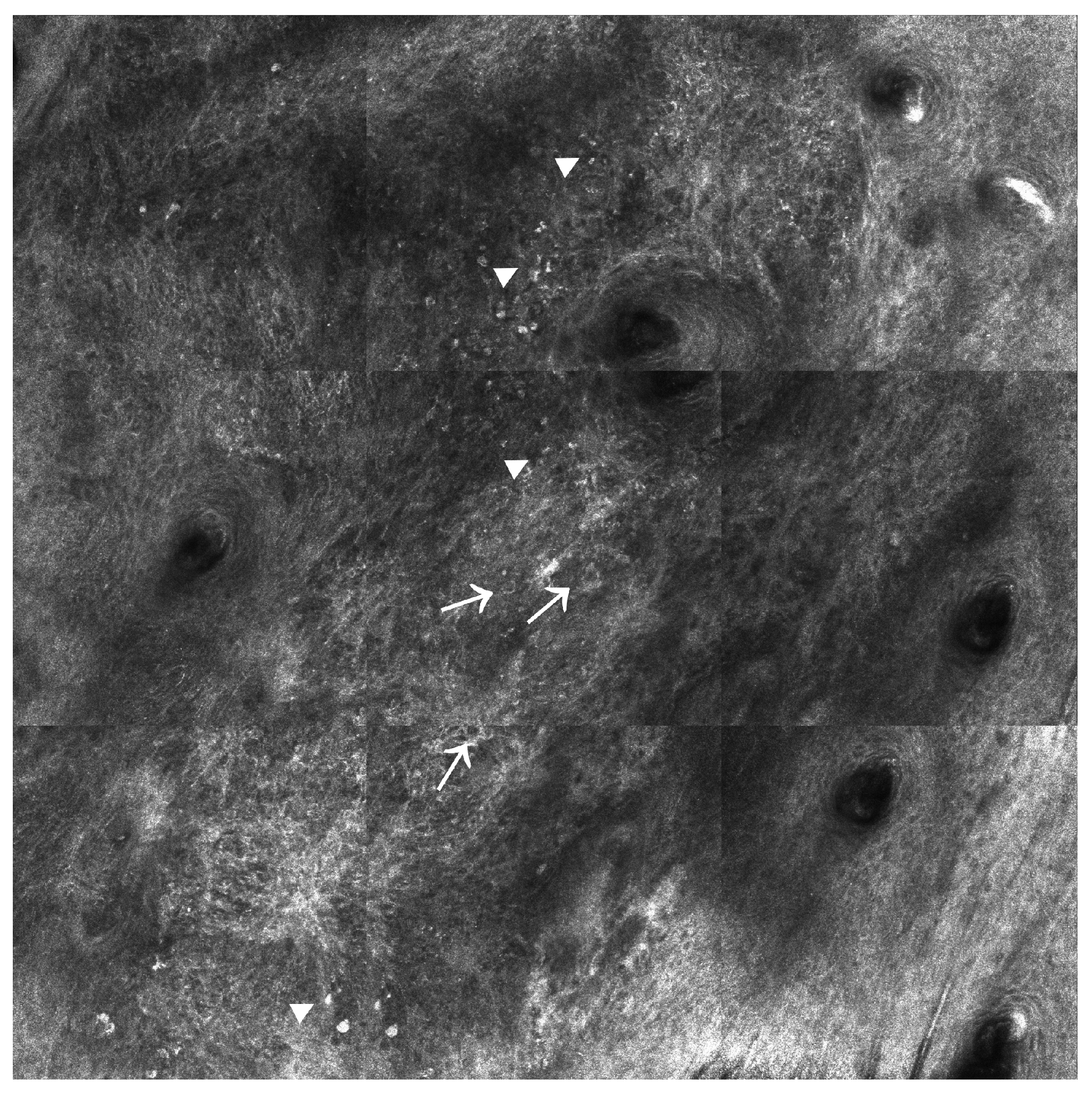
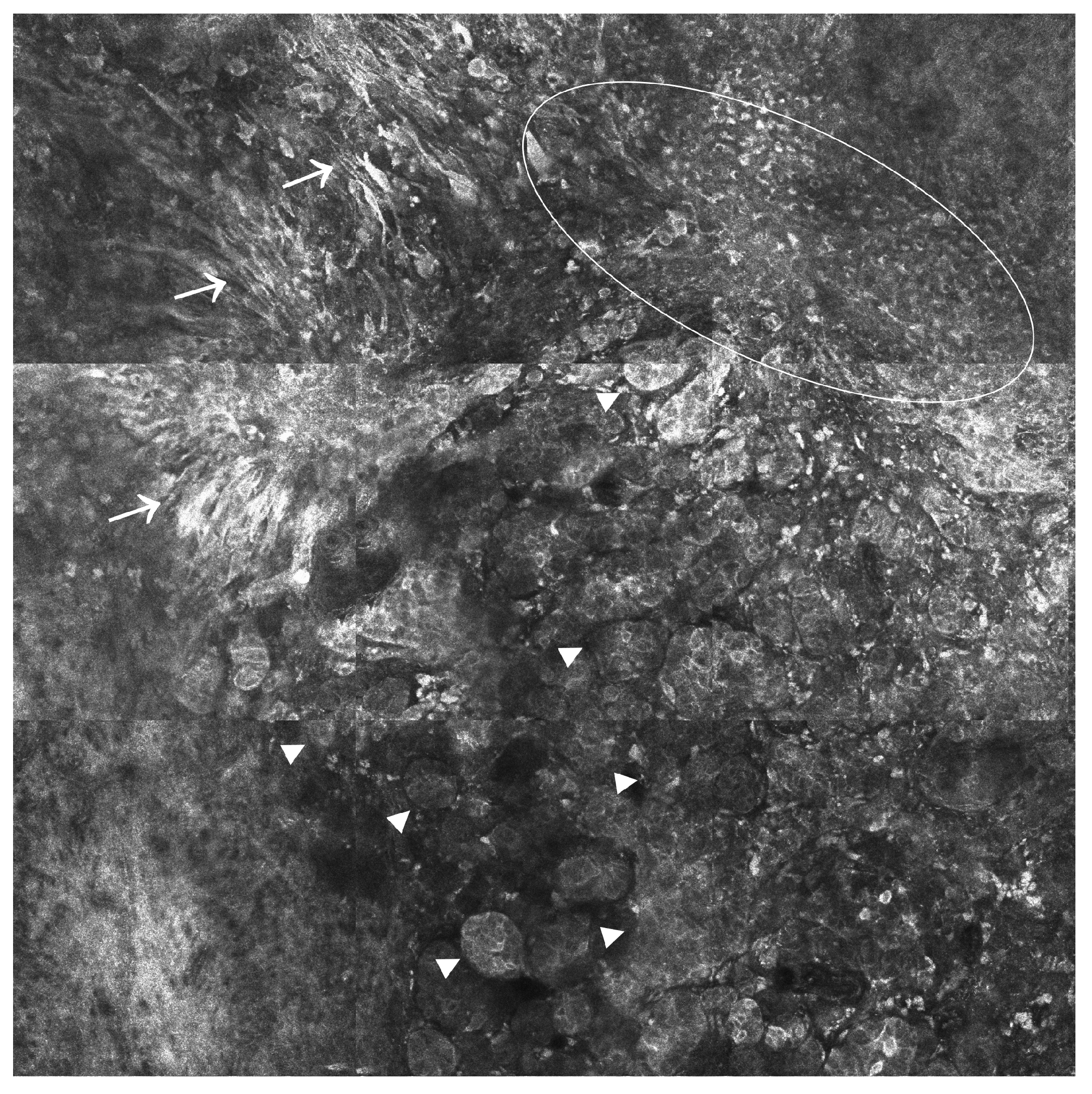
| Technique | Spectra | Contrast Agent Needed | Resolution | Penetration Depth | Advantages | Limitations |
|---|---|---|---|---|---|---|
| RCM | Near-infrared | No | 0.5–1 µm [34] | 200 µm [16] | High resolution; fast in real-time imaging of the tumor-associated vasculature and inflammation; tumor margins assessment before surgical excision [33] | Limited penetration, which may result in false-negative results for tumors below the papillary dermis; extensive training [16]; backscattered nuclear contrast [78] |
| FCM | Near-infrared | Yes | 0.4–1 µm [77] | 200 µm [16] | Examination of freshly excised or frozen specimens for diagnostic purposes; tumor margin assessment in Mohs surgery; increased resolution [77] | Difficulties in preserving tissue properties when preparing it for FCM examination [78] |
| MPM (TPEF) | Near-infrared | Sometimes | >300 μm [233] | 150–200 μm in the skin; 2 mm in the brain [234] | High resolution; suitable for ex vivo clinical investigations; enable the assessment of size variation of cell nuclei, blood vessel architecture, and inflammatory states [235] | Imaging resolution and depth dependable on the composition of the tissues of interest; varying indices of refraction distort the excitation focus, resulting in signal loss and a reduction in image resolution; local photodamage [234] |
| OCT | Infrared | No | 2–20 µm [86] | 2–3 mm [16] | High resolution; ideal for cross-sectional imaging [16] | Limited penetrance depth; hard to distinguish SCC and AK from scar and inflammation [86]; false negative rates in thin melanomas and false positive rates in dysplastic naevi [108] |
| RCM and OCT | Infrared | No | 3–20 µm [236] | 200–1000 µm [236] | Comprehensive 3D sampling in vivo; increased diagnostic accuracy [78]; tumor margin assessment in BCCs [236] | Complexity of the data processing software [236] |
| HFUS | 20–30 MHz | Sometimes | 50–500 µm [16] | 6–7 mm [16] | Wide availability; enable the assessment of vascularization and therapeutic responses in BCCs; suitable for SLN visualization in tumors > 4.5 mm [130] | Low resolution; lack of functional contrast; inability to detect melanoma micrometastases [131] |
| TPI | Terahertz wave | No | 20–200 µm [17] | Frequency dependent, but less than 1 mm [17] | Allows a clear demarcation of healthy and pathological tissues based on their water content [134]; potential therapeutic applications in melanoma [30] | Limited availability; strong water absorption, which limits the penetration depth in fresh tissues; not fully validated for use in clinical settings [136] |
| MRI | Radio waves | Sometimes | 25–100 µm [17] | No limit [17] | High accuracy; good tissue contrast; asymptomatic metastases detection before the occurrence of neurological symptoms; assessment of intratumoral characteristics; enable adaptative treatment strategy changes when needed [153,169] | Increased acquisition time; variability in signal intensity amongst inter-patient and intra-patient acquisitions [237]; difficulty in interpretation; expensive [17] |
| NIR | Near-infrared | Yes | 1.2–20 µm [238] | ~760 µm [239] | SLNB identification [187]; visualization of fluorescence-labeled immune cells in vivo during immunotherapy; less hazardous to biological samples than the short-wavelength excitation light in visible fluorescence bioimaging [193] | The need for assay standardization; assessment of assay reproducibility in larger clinical cohorts [186] |
| SPECT/CT | - | Yes, radiotracers | 8–12 mm [240] | Limitless [240] | SN identification when the lymphatic drainage is unclear [221]; detection of the second lymph node level [219]; increased availability and half-life of SPECT radiopharmaceuticals; remarkable imaging abilities of the biological processes in vivo [226] | Limited resolution; increased workload for pathology departments and increased risk of cancellation of the SNB procedure on the day of surgery, which may negatively impact nodal relapse-free survival [224] |
| PET/CT | - | Yes, radiotracers | 4–6 mm [240] | Limitless [240] | Useful in the initial staging of advanced cutaneous melanomas (AJCC stages III and IV) and assessment of disease recurrence, highly accurate in detecting the lymph node, soft tissue, and visceral metastases [241] | Limited role in staging early melanoma (AJCC stages I and II) [241]; expensive technique; prone to imaging pitfalls related to patient preparation, inflammatory conditions, and lesion characteristics [242]; cumulative exposure to ionizing radiation [21] |
| Technique(s) | Suitable for the Identification of SN | Suitable for Skin Cancer Subtyping | Suitable for Patient Monitoring Following Therapy | Suitable for the Evaluation of CM Pseudo Progression |
|---|---|---|---|---|
| RCM | No | Yes | Yes | Not available |
| FCM | Yes | No | Not available | Yes |
| MPM | No | Yes | Not available | Not available |
| OCT | No | Yes | Yes | Not available |
| RCM/OCT | No | Not available | Yes | Not available |
| HFUS | Yes | Yes | Yes | Not available |
| TPI | No | No | Yes | Not available |
| MRI | Not available | Yes | Yes | Yes |
| NIR | Yes | No | Yes | Yes |
| SPECT/CT | Yes | Not available | Not available | Not available |
| PET/CT | Yes | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobre, E.-G.; Surcel, M.; Constantin, C.; Ilie, M.A.; Caruntu, A.; Caruntu, C.; Neagu, M. Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts. Int. J. Mol. Sci. 2023, 24, 1079. https://doi.org/10.3390/ijms24021079
Dobre E-G, Surcel M, Constantin C, Ilie MA, Caruntu A, Caruntu C, Neagu M. Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts. International Journal of Molecular Sciences. 2023; 24(2):1079. https://doi.org/10.3390/ijms24021079
Chicago/Turabian StyleDobre, Elena-Georgiana, Mihaela Surcel, Carolina Constantin, Mihaela Adriana Ilie, Ana Caruntu, Constantin Caruntu, and Monica Neagu. 2023. "Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts" International Journal of Molecular Sciences 24, no. 2: 1079. https://doi.org/10.3390/ijms24021079
APA StyleDobre, E.-G., Surcel, M., Constantin, C., Ilie, M. A., Caruntu, A., Caruntu, C., & Neagu, M. (2023). Skin Cancer Pathobiology at a Glance: A Focus on Imaging Techniques and Their Potential for Improved Diagnosis and Surveillance in Clinical Cohorts. International Journal of Molecular Sciences, 24(2), 1079. https://doi.org/10.3390/ijms24021079










