Stem Cells for Cancer Therapy: Translating the Uncertainties and Possibilities of Stem Cell Properties into Opportunities for Effective Cancer Therapy
Abstract
1. Introduction
2. Mesenchymal Stroma/Stem Cells (MSC) and Cancer
2.1. MSC Homing and Paracrine Interaction with Cancer
2.2. The Double Life of MSC in Cancer Interaction Studies
2.2.1. MSC as a Tumor Enhancer
2.2.2. MSC as a Tumor Suppressor
2.2.3. Exosomal miRNA in Crosstalk between MSC and Cancer Cells
3. MSC and Its Derivatives as a Carrier for Anti-Cancer Agents
4. Challenges and Opportunities for MSC as a Cancer Therapeutic Agent
5. Application of Induced Pluripotent Stem Cells (iPSC) in Cancer Studies
5.1. iPSC Derived MSC (iMSC) and Cancer Therapy
5.2. iPSCs as a Cancer Model
5.3. Therapeutic Potentials of iPSC
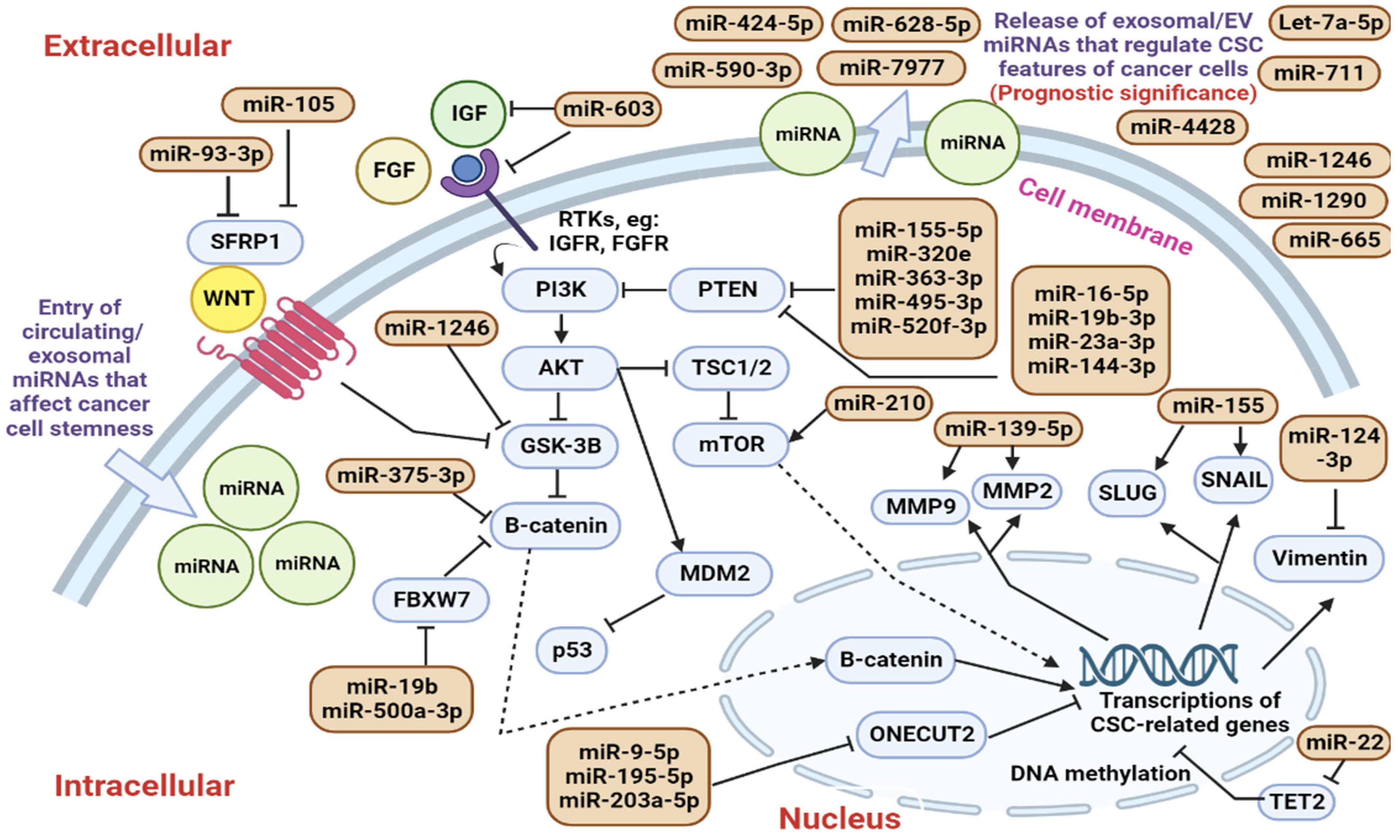
6. Cancer Stem Cells and Their Exosomal miRNA
6.1. Roles of Circulating CSC-Regulating miRNAs in Modulating Cancer Cell Stemness
6.2. Identification of Circulating CSC-Regulating miRNAs in Clinical Trials
6.3. Challengers and Future Direction
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular, T. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Kaur, G.; Gavins, F.; Wang, Y.; Boyer, C.; Alexander, J. Potential therapeutic roles of stem cells in ischemia-reperfusion injury. Stem Cell Res. 2019, 37, 101421. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, J.-W.; Jun, H.-S.; Roh, J.Y.; Lee, H.-Y.; Hong, I.-S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Ghaffari-Nazari, H. The known molecules involved in MSC homing and migration. J. Stem Cell Res. Med. 2018, 3, 1–4. [Google Scholar] [CrossRef][Green Version]
- Galland, S.; Stamenkovic, I. Mesenchymal stromal cells in cancer: A review of their immunomodulatory functions and dual effects on tumor progression. J. Pathol. 2019, 250, 555–572. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Zhu, L.; Lee, H.W.; Rajendran, R.L.; Baek, S.H.; Jeon, Y.H.; Jeong, S.Y.; Lee, S.-W.; et al. In Vivo Tracking of Chemokine Receptor CXCR4-Engineered Mesenchymal Stem Cell Migration by Optical Molecular Imaging. Stem Cells Int. 2017, 2017, 8085637. [Google Scholar] [CrossRef]
- Menon, L.G.; Picinich, S.; Koneru, R.; Gao, H.; Lin, S.Y.; Koneru, M.; Mayer-Kuckuk, P.; Glod, J.; Banerjee, D. Differential Gene Expression Associated with Migration of Mesenchymal Stem Cells to Conditioned Medium from Tumor Cells or Bone Marrow Cells. Stem Cells 2006, 25, 520–528. [Google Scholar] [CrossRef]
- Christodoulou, I.; Goulielmaki, M.; Devetzi, M.; Panagiotidis, M.; Koliakos, G.; Zoumpourlis, V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. Ther. 2018, 9, 336. [Google Scholar] [CrossRef]
- Chen, M.-S.; Lin, C.-Y.; Chiu, Y.-H.; Chen, C.-P.; Tsai, P.-J.; Wang, H.-S. IL-1β-Induced Matrix Metalloprotease-1 Promotes Mesenchymal Stem Cell Migration via PAR1 and G-Protein-Coupled Signaling Pathway. Stem Cells Int. 2018, 2018, 3524759. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.-Z.; Lin, Y.-H.; Su, L.-J.; Wu, M.-S.; Jeng, H.-Y.; Chang, H.-C.; Huang, Y.-H.; Ling, T.-Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-De-Souza, P.; Comito, G.; Pons-Segura, C.; Taddei, M.L.; Gori, V.; Becherucci, V.; Bambi, F.; Margheri, F.; Laurenzana, A.; Del Rosso, M.; et al. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-β1. Stem Cells 2016, 34, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Von Der Ohe, J.; Ungefroren, H. Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness. Cancers 2020, 12, 3716. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell. Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Altajer, A.H.; Rahman, H.S.; Saleh, M.M.; Bokov, D.O.; Abdelbasset, W.K.; Marofi, F.; Zamani, M.; Yaghoubi, Y.; Yazdanifar, M.; et al. Mesenchymal Stem/Stromal Cell-Based Delivery: A Rapidly Evolving Strategy for Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 686453. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-R.; Raymundo, B.; Kim, M.; Kim, C.-W. Mesenchymal stem cells co-cultured with colorectal cancer cells showed increased invasive and proliferative abilities due to its altered p53/TGF-β1 levels. Biosci. Biotechnol. Biochem. 2019, 84, 256–267. [Google Scholar] [CrossRef]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Wang, X.; Lu, F.; Gao, J. Adipose-derived mesenchymal stem cells exhibit tumor tropism and promote tumorsphere formation of breast cancer cells. Oncol. Rep. 2019, 41, 2126–2136. [Google Scholar] [CrossRef]
- Aoto, K.; Ito, K.; Aoki, S. Complex formation between platelet-derived growth factor receptor β and transforming growth factor β receptor regulates the differentiation of mesenchymal stem cells into cancer-associated fibroblasts. Oncotarget 2018, 9, 34090–34102. [Google Scholar] [CrossRef]
- Wu, X.-B.; Liu, Y.; Wang, G.-H.; Xu, X.; Cai, Y.; Wang, H.-Y.; Li, Y.-Q.; Meng, H.-F.; Dai, F.; Jin, J.-D. Mesenchymal stem cells promote colorectal cancer progression through AMPK/mTOR-mediated NF-κB activation. Sci. Rep. 2016, 6, 21420. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, N.; Barikrow, N.; Heshmati, M. Studying the suppressing effect of mesenchymal stem cells derived from amniotic membrane on colorectal cancer. J. Curr. Biomed. Rep. 2021, 2, 192–200. [Google Scholar] [CrossRef]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Scherzad, A.; Steber, M.; Gehrke, T.; Rak, K.; Froelich, K.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N.; Hackenberg, S. Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. Int. J. Oncol. 2015, 47, 391–397. [Google Scholar] [CrossRef]
- Lu, L.; Chen, G.; Yang, J.; Ma, Z.; Yang, Y.; Hu, Y.; Lu, Y.; Cao, Z.; Wang, Y.; Wang, X. Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed. Pharmacother. 2019, 112, 108625. [Google Scholar] [CrossRef]
- Ali, N.M.; Yeap, S.K.; Ho, W.Y.; Boo, L.; Ky, H.; Satharasinghe, D.A.; Tan, S.W.; Cheong, S.K.; Da Huang, H.; Lan, K.C.; et al. Adipose MSCs Suppress MCF7 and MDA-MB-231 Breast Cancer Metastasis and EMT Pathways Leading to Dormancy via Exosomal-miRNAs Following Co-Culture Interaction. Pharmaceuticals 2020, 14, 8. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Li, Y.; Zhang, X.; Gu, J.; Yan, Y.; Xu, X.; Wang, M.; Qian, H.; Xu, W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012, 315, 28–37. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Y.; Jiao, Z.; Wang, X.; Zhao, Y.; Li, Y.; Chen, H.; Yangbin, L.; Zhu, H.; Li, Y. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell. Physiol. Biochem. 2017, 42, 2242–2254. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, M.; Yang, F.; Tian, Y.; Cai, J.; Yang, H.; Fu, H.; Mao, F.; Zhu, W.; Qian, H.; et al. miR-155-5p inhibition promotes the transition of bone marrow mesenchymal stem cells to gastric cancer tissue derived MSC-like cells via NF-κB p65 activation. Oncotarget 2016, 7, 16567–16580. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W. Exosomes Derived from Mesenchymal Stem Cells Suppress Angiogenesis by Down-Regulating VEGF Expression in Breast Cancer Cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ji, J.; Chen, X.; Xu, W.; Chen, H.; Zhu, S.; Wu, J.; Wu, Y.; Sun, Y.; Sai, W.; et al. Human umbilical cord mesenchymal stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin. Transl. Oncol. 2021, 24, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, T.; Jin, Y.; Mai, W.; Zhou, J.; Zhao, C. MicroRNA-15a Carried by Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibits the Immune Evasion of Colorectal Cancer Cells by Regulating the KDM4B/HOXC4/PD-L1 Axis. Front. Cell Dev. Biol. 2021, 9, 629893. [Google Scholar] [CrossRef] [PubMed]
- Khazaei-Poul, Y.; Shojaei, S.; Koochaki, A.; Ghanbarian, H.; Mohammadi-Yeganeh, S. Evaluating the influence of Human Umbilical Cord Mesenchymal Stem Cells-derived exosomes loaded with miR-3182 on metastatic performance of Triple Negative Breast Cancer cells. Life Sci. 2021, 286, 120015. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhong, W. MiR-375 Enriched in Bone Marrow Mesenchymal Stem Cells (BMSC) Exosomes Inhibits Prostate Cancer Cell Migration and Invasion by Down-Regulating Trefoil Factor 3 (TFF3). J. Biomater. Tissue Eng. 2021, 11, 2407–2414. [Google Scholar] [CrossRef]
- Dalmizrak, A.; Dalmizrak, O. Mesenchymal stem cell-derived exosomes as new tools for delivery of miRNAs in the treatment of cancer. Front. Bioeng. Biotechnol. 2022, 10, 1747. [Google Scholar] [CrossRef]
- Ma, M.; Chen, S.; Liu, Z.; Xie, H.; Deng, H.; Shang, S.; Wang, X.; Xia, M.; Zuo, C. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. OncoTargets Ther. 2017, 10, 4161–4171. [Google Scholar] [CrossRef]
- Garnier, D.; Ratcliffe, E.; Briand, J.; Cartron, P.-F.; Oliver, L.; Vallette, F.M. The Activation of Mesenchymal Stem Cells by Glioblastoma Microvesicles Alters Their Exosomal Secretion of miR-100-5p, miR-9-5p and let-7d-5p. Biomedicines 2022, 10, 112. [Google Scholar] [CrossRef]
- Salah, R.A.; Nasr, M.A.; El-Derby, A.M.; Elkodous, M.A.; Mohamed, R.H.; El-Ekiaby, N.; Osama, A.; Elshenawy, S.E.; Hamad, M.H.M.; Magdeldin, S.; et al. Hepatocellular carcinoma cell line-microenvironment induced cancer-associated phenotype, genotype and functionality in mesenchymal stem cells. Life Sci. 2021, 288, 120168. [Google Scholar] [CrossRef]
- Shang, S.; Wang, J.; Chen, S.; Tian, R.; Zeng, H.; Wang, L.; Xia, M.; Zhu, H.; Zuo, C. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med. 2019, 8, 7728–7740. [Google Scholar] [CrossRef]
- Li, T.; Zhou, X.; Wang, J.; Liu, Z.; Han, S.; Wan, L.; Sun, X.; Chen, H. Adipose-derived mesenchymal stem cells and extracellular vesicles confer antitumor activity in preclinical treatment of breast cancer. Pharmacol. Res. 2020, 157, 104843. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-W.; Cho, K.-A.; Kim, J.; Lee, H.-J.; Kim, Y.-H.; Park, J.-W.; Woo, S.-Y. Extracellular vesicles from tonsil-derived mesenchymal stromal cells show anti-tumor effect via miR-199a-3p. Int. J. Mol. Med. 2021, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bliss, S.A.; Sinha, G.; Sandiford, O.A.; Williams, L.M.; Engelberth, D.J.; Guiro, K.; Isenalumhe, L.L.; Greco, S.J.; Ayer, S.; Bryan, M.; et al. Mesenchymal stem cell–derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 2016, 76, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Lee, S.; Lee, J.; Kim, M.; Kim, W.J.; Lee, H.W.; Lee, M.Y.; Kim, J.; Chang, W. Exosomes derived from microrna-584 transfected mesenchymal stem cells: Novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018, 51, 406–411. [Google Scholar] [CrossRef]
- Han, S.; Li, G.; Jia, M.; Zhao, Y.; He, C.; Huang, M.; Jiang, L.; Wu, M.; Yang, J.; Ji, X.; et al. Delivery of Anti-miRNA-221 for Colorectal Carcinoma Therapy Using Modified Cord Blood Mesenchymal Stem Cells-Derived Exosomes. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Sharifi, K.; Salehi, M.; Mohammadi-Yeganeh, S. Delivery of miR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem Cell Rev. Rep. 2021, 17, 1027–1038. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Rahmani, S.; Ostad, S.N.; Madjd, Z.; Dinarvand, R.; Atyabi, F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: A new approach for drug delivery. Life Sci. 2020, 266, 118871. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, Y.; Xu, X.; Qi, X.; Liu, J.; Pu, Y.; Zhang, S.; Gao, X.; Luo, X.; Li, M.; et al. MiR-30c-5p loss-induced PELI1 accumulation regulates cell proliferation and migration via activating PI3K/AKT pathway in papillary thyroid carcinoma. J. Transl. Med. 2022, 20, 20. [Google Scholar] [CrossRef]
- Egea, V.; Kessenbrock, K.; Lawson, D.; Bartelt, A.; Weber, C.; Ries, C. Let-7f miRNA regulates SDF-1α- and hypoxia-promoted migration of mesenchymal stem cells and attenuates mammary tumor growth upon exosomal release. Cell Death Dis. 2021, 12, 516. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, N.; Wei, Y.; Zhou, D.; Lin, R.; Wang, X.; Shi, B. Anticancer effects of miR-124 delivered by BM-MSC derived exosomes on cell proliferation, epithelial mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells. Aging 2020, 12, 19660–19676. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, J.; Li, Y.; Cao, W.; Wang, Y.; Ma, Z.; Li, F. Mesenchymal stem cells expressing interleukin-18 inhibit breast cancer in a mouse model. Oncol. Lett. 2018, 15, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhong, X.; Dai, Q.; Li, K.; Zhang, W.; Wang, J.; Zhao, Y.; Shen, J.; Xiao, Z.; Xing, H.; et al. Human Umbilical Cord MSC Delivered-Soluble TRAIL Inhibits the Proliferation and Promotes Apoptosis of B-ALL Cell In Vitro and In Vivo. Pharmaceuticals 2022, 15, 1391. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Z.; Xu, X.; Xu, Z.; Wang, S.; Huang, D.; Li, Y.; Mou, X.; Liu, F.; Xiang, C. Menstrual Blood-Derived Stem Cells as Delivery Vehicles for Oncolytic Adenovirus Virotherapy for Colorectal Cancer. Stem Cells Dev. 2019, 28, 882–896. [Google Scholar] [CrossRef]
- Moku, G.; Layek, B.; Trautman, L.; Putnam, S.; Panyam, J.; Prabha, S. Improving Payload Capacity and Anti-Tumor Efficacy of Mesenchymal Stem Cells Using TAT Peptide Functionalized Polymeric Nanoparticles. Cancers 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Wang, H.; Bivalacqua, T.J.; Partin, A.W.; Lim, S.J.; Chapman, C.; Abdallah, R.; Levy, O.; Bhowmick, N.A.; Karp, J.M.; et al. A Phase I Study to Assess the Safety and Cancer-Homing Ability of Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Men with Localized Prostate Cancer. Stem Cells Transl. Med. 2019, 8, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Von Einem, J.C.; Guenther, C.; Volk, H.-D.; Grütz, G.; Hirsch, D.; Salat, C.; Stoetzer, O.; Nelson, P.J.; Michl, M.; Modest, D.P.; et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int. J. Cancer 2019, 145, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- von Einem, J.C.; Peter, S.; Günther, C.; Volk, H.-D.; Grütz, G.; Salat, C.; Stoetzer, O.; Nelson, P.J.; Michl, M.; Modest, D.P.; et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells—TREAT-ME-1—A phase I, first in human, first in class trial. Oncotarget 2017, 8, 80156–80166. [Google Scholar] [CrossRef]
- Ramirez, M.; Ruano, D.; Moreno, L.; Lassaletta, Á.; Sirvent, F.J.B.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-child trial of celyvir (autologous mesenchymal stem cells carrying the oncolytic virus ICOVIR-5) in patients with relapsed and refractory pediatric solid tumors. J. Clin. Oncol. 2018, 36, 10543. [Google Scholar] [CrossRef]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Nam, G.; Choi, Y.; Kim, G.B.; Kim, S.; A Kim, S.; Kim, I. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv. Mater. 2020, 32, e2002440. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, A.; Pattarozzi, A.; Corsaro, A.; Barbieri, F.; Daga, A.; Bosio, A.; Gatti, M.; Pisaturo, V.; Sirito, R.; Florio, T. Different Effects of Human Umbilical Cord Mesenchymal Stem Cells on Glioblastoma Stem Cells by Direct Cell Interaction or Via Released Soluble Factors. Front. Cell. Neurosci. 2017, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Kimura, K.; Nagano, M.; Takano, S.; Salazar, G.; Yamashita, T.; Ohneda, O. Umbilical Cord Blood-Derived Mesenchymal Stem Cells Inhibit, But Adipose Tissue-Derived Mesenchymal Stem Cells Promote, Glioblastoma Multiforme Proliferation. Stem Cells Dev. 2013, 22, 1370–1386. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cruz, C.M.; Shearer, J.J.; Neto, M.F.; Figueiredo, M.L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Sipos, F.; Műzes, G. Controversies in therapeutic application of mesenchymal stem cell-derived secretome. Biocell 2022, 46, 903–906. [Google Scholar] [CrossRef]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert Opin. Drug Deliv. 2021, 18, 1627–1642. [Google Scholar] [CrossRef]
- Vicinanza, C.; Lombardi, E.; Da Ros, F.; Marangon, M.; Durante, C.; Mazzucato, M.; Agostini, F. Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications. World J. Stem Cells 2022, 14, 54–75. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.-S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles 2018, 7, 1440131. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7, 533. [Google Scholar] [CrossRef]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2020, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Omole, A.E.; Fakoya, A.O.J. Ten years of progress and promise of induced pluripotent stem cells: Historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018, 6, e4370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Liao, J.; Chang, C.; Liu, Y.; Padhiar, A.A.; Zhou, Y.; Zhou, G. Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Hold Lower Heterogeneity and Great Promise in Biological Research and Clinical Applications. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, Y.; Zhang, C.; Huang, J.; Du, J.; Liao, J.; Bergholt, N.L.; Bünger, C.; Xu, F.; Lin, L.; et al. Feeder-free generation and transcriptome characterization of functional mesenchymal stromal cells from human pluripotent stem cells. Stem Cell Res. 2020, 48, 101990. [Google Scholar] [CrossRef]
- Rajasingh, S.; Sigamani, V.; Selvam, V.; Gurusamy, N.; Kirankumar, S.; Vasanthan, J.; Rajasingh, J. Comparative analysis of human induced pluripotent stem cell-derived mesenchymal stem cells and umbilical cord mesenchymal stem cells. J. Cell. Mol. Med. 2021, 25, 8904–8919. [Google Scholar] [CrossRef]
- Fernandez-Rebollo, E.; Franzen, J.; Goetzke, R.; Hollmann, J.; Ostrowska, A.; Oliverio, M.; Sieben, T.; Rath, B.; Kornfeld, J.-W.; Wagner, W. Senescence-Associated Metabolomic Phenotype in Primary and iPSC-Derived Mesenchymal Stromal Cells. Stem Cell Rep. 2020, 14, 201–209. [Google Scholar] [CrossRef]
- Zhao, Q.; Gregory, C.A.; Lee, R.H.; Reger, R.L.; Qin, L.; Hai, B.; Park, M.S.; Yoon, N.; Clough, B.; McNeill, E.; et al. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc. Natl. Acad. Sci. USA 2014, 112, 530–535. [Google Scholar] [CrossRef]
- Loh, J.-K.; Wang, M.-L.; Cheong, S.-K.; Tsai, F.-T.; Huang, S.-H.; Wu, J.-R.; Yang, Y.-P.; Chiou, S.-H.; Ong, A.H.-K. The Study of Cancer Cell in Stromal Environment through iPSC derived Mesenchymal Stem Cells. J. Chin. Med. Assoc. 2022, 85, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, F.; Wu, Y.; Wang, X.; Feng, M.; Li, Z.; Zhou, M.; Wang, Y.; Wu, L.; Liu, X.; et al. Enhanced Tumor Growth In-hibition by Mesenchymal Stem Cells Derived from IPSCs with Targeted Integration of Interleukin24 into RDNA Loci. Oncotarget 2017, 8, 40791. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, H.; Wang, P.; Zhou, M.; Li, G.; Hu, Z.; Hu, Q.; Zhao, J.; Liu, X.; Wu, L.; et al. Site-Specific Integration of TRAIL in iPSC-Derived Mesenchymal Stem Cells for Targeted Cancer Therapy. Stem Cells Transl. Med. 2022, 11, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Portier, L.; Desterke, C.; Chaker, D.; Oudrhiri, N.; Asgarova, A.; Dkhissi, F.; Turhan, A.; Bennaceur-Griscelli, A.; Griscelli, F. iPSC-Derived Hereditary Breast Cancer Model Reveals the BRCA1-Deleted Tumor Niche as a New Culprit in Disease Progression. Int. J. Mol. Sci. 2021, 22, 1227. [Google Scholar] [CrossRef]
- Zhao, Q.; Hai, B.; Kelly, J.; Wu, S.; Liu, F. Extracellular vesicle mimics made from iPS cell-derived mesenchymal stem cells improve the treatment of metastatic prostate cancer. Stem Cell Res. Ther. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Bertolino, G.M.; Maumus, M.; Jorgensen, C.; Noël, D. Recent Advances in Extracellular Vesicle-Based Therapies Using Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells. Biomedicines 2022, 10, 2281. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef]
- Crespo, M.; Vilar, E.; Tsai, S.-Y.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.C.S.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, A.; Tu, J.; Liu, M.; Gingold, J.; Zhao, R.; Lee, D.-F. Modeling Osteosarcoma Using Li-Fraumeni Syndrome Patient-derived Induced Pluripotent Stem Cells. J. Vis. Exp. 2018, 2018, e57664. [Google Scholar] [CrossRef]
- Kitani, T.; Ong, S.-G.; Lam, C.K.; Rhee, J.-W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L.; et al. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients With Breast Cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef]
- Hoerster, K.; Uhrberg, M.; Wiek, C.; Horn, P.A.; Hanenberg, H.; Heinrichs, S. HLA Class I Knockout Converts Allogeneic Primary NK Cells Into Suitable Effectors for “Off-the-Shelf” Immunotherapy. Front. Immunol. 2021, 11, 586168. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Zaman, W.S.W.K.; Nurul, A.A.; Nordin, F. Stem Cells and Cancer Stem Cells: The Jekyll and Hyde Scenario and Their Implications in Stem Cell Therapy. Biomedicines 2021, 9, 1245. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zhang, C.; Liu, P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. J. Cell. Mol. Med. 2020, 24, 8930–8941. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Nguyen, H.P.T.; Jones, J.J.; Stylli, S.S.; Whitehead, C.A.; Paradiso, L.; Luwor, R.B.; Areeb, Z.; Hanssen, E.; Cho, E.; et al. Extracellular Vesicles Secreted by Glioma Stem Cells Are Involved in Radiation Resistance and Glioma Progression. Int. J. Mol. Sci. 2022, 23, 2770. [Google Scholar] [CrossRef]
- Shen, M.; Dong, C.; Ruan, X.; Yan, W.; Cao, M.; Pizzo, D.; Wu, X.; Yang, L.; Liu, L.; Ren, X.; et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019, 79, 3608–3621. [Google Scholar] [CrossRef]
- Sun, T.; Yin, Y.; Jin, H.; Liu, H.; Tian, W. Exosomal microRNA -19b targets FBXW7 to promote colorectal cancer stem cell stemness and induce resistance to radiotherapy. Kaohsiung J. Med. Sci. 2021, 38, 108–119. [Google Scholar] [CrossRef]
- Santos, J.C.; da Silva Lima, N.; Sarian, L.O.; Matheu, A.; Ribeiro, M.L.; Derchain, S.F.M. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci. Rep. 2018, 8, 829. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xiao, S.; Li, Y.; Chen, Q. miR-125a/b inhibits tumor-associated macrophages mediated in cancer stem cells of hepatocellular carcinoma by targeting CD90. J. Cell. Biochem. 2018, 120, 3046–3055. [Google Scholar] [CrossRef]
- Chai, S.; Ng, K.-Y.; Tong, M.; Lau, E.Y.; Lee, T.K.; Chan, K.W.; Yuan, Y.-F.; Cheung, T.-T.; Cheung, S.-T.; Wang, X.-Q.; et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology 2016, 64, 2062–2076. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Hu, H.; Tu, L.; Sun, Z.; Liu, Y.; Luo, F. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J. Cell. Mol. Med. 2020, 24, 6324–6339. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Hong, X.; Cazacu, S.; Goldstein, H.; Rand, D.; Xiang, C.; Jiang, W.; Ben-Asher, H.W.; Attia, M.; Brodie, A.; et al. miR-504 modulates the stemness and mesenchymal transition of glioma stem cells and their interaction with microglia via delivery by extracellular vesicles. Cell Death Dis. 2020, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.A.; Andahur, E.I.; Valenzuela, R.; Castellón, E.A.; Fullá, J.A.; Ramos, C.G.; Triviño, J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2015, 7, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Xu, B.; Akers, J.; Nguyen, T.; Ma, J.; Dhawan, S.; Ning, J.; Mao, Y.; Hua, W.; Kokkoli, E.; et al. Radiation-induced extracellular vesicle (EV) release of miR-603 promotes IGF1-mediated stem cell state in glioblastomas. eBioMedicine 2020, 55, 102736. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, N.; Cui, J.; Wu, H.; Xiong, J.; Peng, T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell. Oncol. 2019, 43, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Tsang, J.Y.; Tse, G.M. Tumor Microenvironment in Breast Cancer—Updates on Therapeutic Implications and Pathologic Assessment. Cancers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zou, C.; Zhu, Y.; Luo, Y.; Chen, L.; Lei, Y.; Tang, K.; Sun, Y.; Zhang, W.; Li, S.; et al. HIF-1ɑ-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and Notch signaling. Theranostics 2020, 10, 2553–2570. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Liang, J.-L.; Kuo, Y.-L.; Lee, H.-H.; Calkins, M.J.; Chang, H.-T.; Lin, F.-C.; Chen, Y.-C.; Hsu, T.-I.; Hsiao, M.; et al. miR-105/93-3p promotes chemoresistance and circulating miR-105/93-3p acts as a diagnostic biomarker for triple negative breast cancer. Breast Cancer Res. 2017, 19, 133. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Li, J.; Shao, J.; Fang, L. MiR-130a-3p inhibits migration and invasion by regulating RAB5B in human breast cancer stem cell-like cells. Biochem. Biophys. Res. Commun. 2018, 501, 486–493. [Google Scholar] [CrossRef]
- Rezaei, R.; Baghaei, K.; Amani, D.; Piccin, A.; Hashemi, S.M.; Aghdaei, H.A.; Zali, M.R. Exosome-mediated delivery of functionally active miRNA-375-3p mimic regulate epithelial mesenchymal transition (EMT) of colon cancer cells. Life Sci. 2021, 269, 119035. [Google Scholar] [CrossRef] [PubMed]
- Tűzesi, Á.; Kling, T.; Wenger, A.; Lunavat, T.R.; Jang, S.C.; Rydenhag, B.; Lötvall, J.; Pollard, S.M.; Danielsson, A.; Carén, H. Pediatric brain tumor cells release exosomes with a miRNA repertoire that differs from exosomes secreted by normal cells. Oncotarget 2017, 8, 90164–90175. [Google Scholar] [CrossRef] [PubMed]
- Peddareddigari, V.G.; Wang, D.; DuBois, R.N. The Tumor Microenvironment in Colorectal Carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Liao, T.T.; Lin, C.C.; Yuan, L.T.E.; Lan, H.Y.; Lin, H.H.; Teng, H.W.; Chang, H.C.; Lin, C.H.; Yang, C.Y.; et al. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int. J. Cancer 2019, 145, 2209–2224. [Google Scholar] [CrossRef]
- Du, Q.; Ye, X.; Lu, S.-R.; Li, H.; Liu, H.-Y.; Zhai, Q.; Yu, B. Exosomal miR-30a and miR-222 derived from colon cancer mesenchymal stem cells promote the tumorigenicity of colon cancer through targeting MIA3. J. Gastrointest. Oncol. 2021, 12, 52–68. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Sun, C.-Y.; Tian, S.; Yu, C.; Chen, M.-Y.; Zhang, H. Tumor suppressor Fbxw7 antagonizes WNT signaling by targeting β-catenin for degradation in pancreatic cancer. Tumor Biol. 2016, 37, 13893–13902. [Google Scholar] [CrossRef]
- Patel, S.; Alam, A.; Pant, R.; Chattopadhyay, S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019, 10, 2872. [Google Scholar] [CrossRef]
- Sun, Z.-P.; Li, A.-Q.; Jia, W.-H.; Ye, S.; Van Eps, G.; Yu, J.-M.; Yang, W.-J. MicroRNA expression profiling in exosomes derived from gastric cancer stem-like cells. Oncotarget 2017, 8, 93839–93855. [Google Scholar] [CrossRef]
- Tamai, S.; Ichinose, T.; Tsutsui, T.; Tanaka, S.; Garaeva, F.; Sabit, H.; Nakada, M. Tumor Microenvironment in Glioma Invasion. Brain Sci. 2022, 12, 505. [Google Scholar] [CrossRef]
- Yin, J.; Ge, X.; Shi, Z.; Yu, C.; Lu, C.; Wei, Y.; Zeng, A.; Wang, X.; Yan, W.; Zhang, J.; et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p. Theranostics 2021, 11, 1763–1779. [Google Scholar] [CrossRef]
- Mendes, J.M.F.; Valverde, L.D.F.; Vidal, M.T.A.; Paredes, B.D.; Coelho, P.; Allahdadi, K.J.; Della Coletta, R.; Souza, B.S.D.F.; Rocha, C.A.G. Effects of IGF-1 on Proliferation, Angiogenesis, Tumor Stem Cell Populations and Activation of AKT and Hedgehog Pathways in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 6487. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, G.; Zhao, D.; Wang, J.; Bai, Y.; Peng, Q.; Wang, H.; Fang, R.; Chen, G.; Wang, Z.; et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: Role of remote MiR-19b-3p. Mol. Cancer 2019, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Tang, F.; Huang, Y.; He, C.; Chen, C.; Zhao, J.; Wu, W.; He, Z. The tumour microenvironment and metabolism in renal cell carcinoma targeted or immune therapy. J. Cell. Physiol. 2020, 236, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Sas, Z.; Cendrowicz, E.; Weinhäuser, I.; Rygiel, T.P. Tumor Microenvironment of Hepatocellular Carcinoma: Challenges and Opportunities for New Treatment Options. Int. J. Mol. Sci. 2022, 23, 3778. [Google Scholar] [CrossRef]
- Yu, J.; Liu, D.; Sun, X.; Yang, K.; Yao, J.; Cheng, C.; Wang, C.; Zheng, J. CDX2 inhibits the proliferation and tumor formation of colon cancer cells by suppressing Wnt/β-catenin signaling via transactivation of GSK-3β and Axin2 expression. Cell Death Dis. 2019, 10, 26. [Google Scholar] [CrossRef]
- Do, H.; Luong, A.B.; Bonazza, D.; Bottin, C.; Doan, T.P.; Tran, L.D.; Truong, N.H.; Tell, G.; Pham, H.L.; Tiribelli, C.; et al. Differential capacity of CD90+ cells in autophagy activation following chemotherapy in hepatocellular carcinoma. Ann. Hepatol. 2020, 19, 645–652. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Shoff, M.; Booker, T.; Leavitt, B.; Harmon, D.; Kingsley, K.; Howard, K.M. Differential exosome miRNA expression in oral cancer stem cells. ExRNA 2020, 2, 3. [Google Scholar] [CrossRef]
- Svanberg, R.; Janum, S.; Patten, P.E.; Ramsay, A.G.; Niemann, C.U. Targeting the tumor microenvironment in chronic lymphocytic leukemia. Haematologica 2021, 106, 2312–2324. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, D.; Lim, S.W.; Ryu, K.J.; Choi, M.E.; Yoon, S.E.; Kim, K.; Park, C.; Kim, S.J. Exosomal miR-1305 in the oncogenic activity of hypoxic multiple myeloma cells: A biomarker for predicting prognosis. J. Cancer 2021, 12, 2825–2834. [Google Scholar] [CrossRef]
- Tian, C.; You, M.J.; Yu, Y.; Zhu, L.; Zheng, G.; Zhang, Y. MicroRNA-9 promotes proliferation of leukemia cells in adult CD34-positive acute myeloid leukemia with normal karyotype by downregulation of Hes1. Tumor Biol. 2015, 37, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Li, L.; Ma, X.; Chen, Y.; Zhou, H.; Luo, Y.; Xiao, Y.; Liu, L. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 2018, 32, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Ito, K.; Ala, U.; Kats, L.; Webster, K.; Sun, S.; Manova-Todorova, K.; Teruya-Feldstein, J.; Avigan, D.E.; Delwel, R.; et al. The Oncogenic MicroRNA miR-22 Targets the TET2 Tumor Suppressor to Promote Hematopoietic Stem Cell Self-Renewal and Transformation. Cell Stem Cell 2013, 13, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shen, Z. Exosomal miRNAs as biomarkers for diagnostic and prognostic in lung cancer. Cancer Med. 2020, 9, 6909–6922. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, J.; Li, H.; Wang, C.; Fletcher, C.; Li, J.; Zhan, Y.; Du, L.; Wang, F.; Jiang, Y. Progress in the research of nanomaterial-based exosome bioanalysis and exosome-based nanomaterials tumor therapy. Biomaterials 2021, 274, 120873. [Google Scholar] [CrossRef]
| Exosomal miRNA | MSC | Cancer Type | Signaling Pathway | Function | Ref. |
|---|---|---|---|---|---|
| mrR-21 miR-34a | Bone marrow | Breast cancer | Activation of (ERK1/2) pathway | Promote tumor growth | [27] |
| miR-221 | Bone marrow | Osteosarcoma (MG63) and gastric cancer (SGC7901) cells | Activation of hedgehog signaling pathway. | Promote tumor growth | [28] |
| miR-193a-3p miR-210-3p miR-5100 | Bone marrow grown under hypoxic condition | Lung cancer cells and an in vivo mouse syngeneic tumor model | STAT3-induced EMT | Promote cancer cell invasion and EMT. | [29] |
| miR-221 | Bone marrow | Gastric cancer BGC-823 and SGC-7901 cells | ND | Proliferation, migration, invasion, and adhesion to the matrix of GC BGC-823 and SGC-7901 cells were significantly enhanced | [37] |
| miR-100-5p miR-9-5p let-7d-5p | Bone marrow | Glioblastoma | Activation of MSCs into (CAFs)-like cells | Promote tumor growth via a decrease in anti-tumoral miR-100-5p, miR-9-5p, and let-7d-5p | [38] |
| miR-17-5p miR-615-5p | Human adipose MSCs | Hepatocellular carcinoma cell line (Huh-7 cells) | Generation of cancer-associated phenotype of some CAF-like characteristics | Promote tumor growth via upregulation of miR-17-5p and 615-5p | [39] |
| miR-16 | Bone marrow | Mouse breast cancer cell line (4T1) | Down-regulation of expressed VEGF in tumor cells | Suppress tumor growth via inhibition of angiogenesis | [31] |
| miRNA-1231 | Bone marrow | Pancreatic cancer | ND | Suppress tumor growth | [40] |
| miRNA-16-5p | Adipose-derived mesenchymal stem cells | Breast cancer | ND | Suppress tumor growth | [41] |
| miRNA-128-3p | Human umbilical cord mesenchymal stem cell- | Pancreatic ductal cell carcinoma | Inhibiting galectin-3 | Suppress pancreatic ductal cell carcinoma | [32] |
| miR-15a | Adipose-derived mesenchymal stem cells | Colorectal cancer | Restriction of immune evasion of CRC via the KDM4B/HOXC4/PD-L1 axis | Suppress tumor growth | [33] |
| miR-199a-3p | T-MScs | HepG2 cells. | Potentially targeting CD151, integrin α3 and 6 | Inhibit tumor growth and HepG2 cell migration | [42] |
| miR-375 | Enriched in bone marrow mesenchymal stem cells (BMSC) | Prostate cancer cell | Down-regulating trefoil factor 3 (TFF3) | Inhibit migration and invasion | [35] |
| Exosomal miRNA | MSC | Cancer Type | Delivery Method | Function/Target | Ref. |
|---|---|---|---|---|---|
| miR 222/223 | ND | Immunodeficient mouse model of dormant breast cancer | MSC transfected with antagomiR 222/223 | ND | [43] |
| microRNA-584 | Human MSC (Origin ND) | U87 human glioma cells | Exosomes derived from microRNA-584 transfected mesenchymal stem cells | Suppression of the expression of CYP2J2; reduced the levels of phosphorylated AKT and MAPK | [44] |
| miRNA-221 | Human cord blood mesenchymal stromal | Colorectal carcinoma | Cell-derived exosomes were used in the delivery of anti-miRNA oligonucleotides | Anti-tumor efficacy | [45] |
| miR-381-3p Mimic | Adipose-derived mesenchymal stem cells | MDA-MB-231 cells | miR-381 loaded ADMSC-exosomes | Downregulation of expressed related genes and proteins; inhibited proliferation, migration, and invasion capacity | [46] |
| miR-34a | Dental pulp MSCs (DPSCs) | Breast carcinoma cells. | miR-34a loaded modified dental pulp MSCs (DPSCs) exosomes | Repression of tumor proliferation | [47] |
| miR-30c-5p | Human umbilical cord mesenchymal stem cells | Papillary thyroid carcinoma (PTC) | miR-30c-5p containing extracellular vesicles | Tumor-suppressive miRNA targeted PELI1 to inhibit PTC cell proliferation and migration via activating PI3K/AKT pathway | [48] |
| Let-7f miRNA | Bone marrow-derived human mesenchymal stem cells | 4T1 breast tumor model | Let-7f miRNA containing extracellular vesicles | Regulates SDF-1α- and hypoxia-promoted migration of mesenchymal stem cells | [49] |
| MiR-199a- | Adipose tissue-derived mesenchymal stem | Hepatocellular carcinoma | miR-199a-modified exosomes | Improve chemosensitivity through mTOR | [50] |
| miR-124 | BM-MSC | miR-124 derived exosomes | Anti-tumor effects on cell proliferation, epithelial–mesenchymal transition, and chemotherapy sensitivity | [51] |
| Trial ID No. | Trial Title | Cancer Type | Study Location | Study Type (Sample Size, n) | Study Duration (Status) |
|---|---|---|---|---|---|
| NCT01298414 | Pediatric Myeloid Leukemia-Specific miRNA Expression Profiles Induced by the Leukemic Stem Cell Niche | Acute myeloid leukemia | Feinstein Institute for Medical Research, United States of America | Observational, retrospective (20) | February 2011–May 2016 (Completed) |
| NCT01231386 | MIRNA Profiling of Breast Cancer in Patients Undergoing Neoadjuvant or Adjuvant Treatment for Locally Advanced and Inflammatory Breast Cancer | Breast cancer | City of Hope Medical Center, United States of America | Observational (199) | November 2009–May 2019 (Completed) |
| NCT01577511 | Invasiveness and Chemoresistance of Cancer Stem Cells in Colon Cancer: Molecular Characterization and Implications for Therapeutic Strategies | Colorectal cancer | Nîmes University Hospital, France | Observational, prospective (60) | June 2012–October 2017 (Completed) |
| NCT02052908 | A Phase Ib Biomarker Trial of Naproxen in Patients at Risk for DNA Mismatch Repair Deficient Colorectal Cancer | Colorectal cancer | Brigham and Women’s Hospital; University of Michigan Comprehensive Cancer Center; M D Anderson Cancer Center; Huntsman Cancer Institute/University of Utah, United States of America | Interventional, randomized, double-blinded (81) | January 2014–January 2021 (Completed) |
| NCT05328089 | Vacuolar ATPase and Drug Resistance of High-Grade Gliomas: a Study to Investigate Possible Therapeutic Roles for Proton Pump Inhibitors | Glioblastoma multiforme | University of Milano Bicocca, Italy | Observational, prospective (20) | January 2020–present (Recruiting) |
| NCT01216787 | A Pilot Trial to Evaluate the Molecular Effects of RO4929097 as Neoadjuvant Therapy for Resectable Stage IIIB, IIIC, or IV Melanoma | Melanoma | Montefiore Medical Center; New York University Langone Medical Center, United States of America | Interventional, single group assignment (0) | September 2010–November 2011 (Terminated) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldoghachi, A.F.; Chong, Z.X.; Yeap, S.K.; Cheong, S.K.; Ho, W.Y.; Ong, A.H.K. Stem Cells for Cancer Therapy: Translating the Uncertainties and Possibilities of Stem Cell Properties into Opportunities for Effective Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1012. https://doi.org/10.3390/ijms24021012
Aldoghachi AF, Chong ZX, Yeap SK, Cheong SK, Ho WY, Ong AHK. Stem Cells for Cancer Therapy: Translating the Uncertainties and Possibilities of Stem Cell Properties into Opportunities for Effective Cancer Therapy. International Journal of Molecular Sciences. 2023; 24(2):1012. https://doi.org/10.3390/ijms24021012
Chicago/Turabian StyleAldoghachi, Ahmed Faris, Zhi Xiong Chong, Swee Keong Yeap, Soon Keng Cheong, Wan Yong Ho, and Alan Han Kiat Ong. 2023. "Stem Cells for Cancer Therapy: Translating the Uncertainties and Possibilities of Stem Cell Properties into Opportunities for Effective Cancer Therapy" International Journal of Molecular Sciences 24, no. 2: 1012. https://doi.org/10.3390/ijms24021012
APA StyleAldoghachi, A. F., Chong, Z. X., Yeap, S. K., Cheong, S. K., Ho, W. Y., & Ong, A. H. K. (2023). Stem Cells for Cancer Therapy: Translating the Uncertainties and Possibilities of Stem Cell Properties into Opportunities for Effective Cancer Therapy. International Journal of Molecular Sciences, 24(2), 1012. https://doi.org/10.3390/ijms24021012





