Proenkephalin Levels and Its Determinants in Patients with End-Stage Kidney Disease Treated with Hemodialysis and Peritoneal Dialysis
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
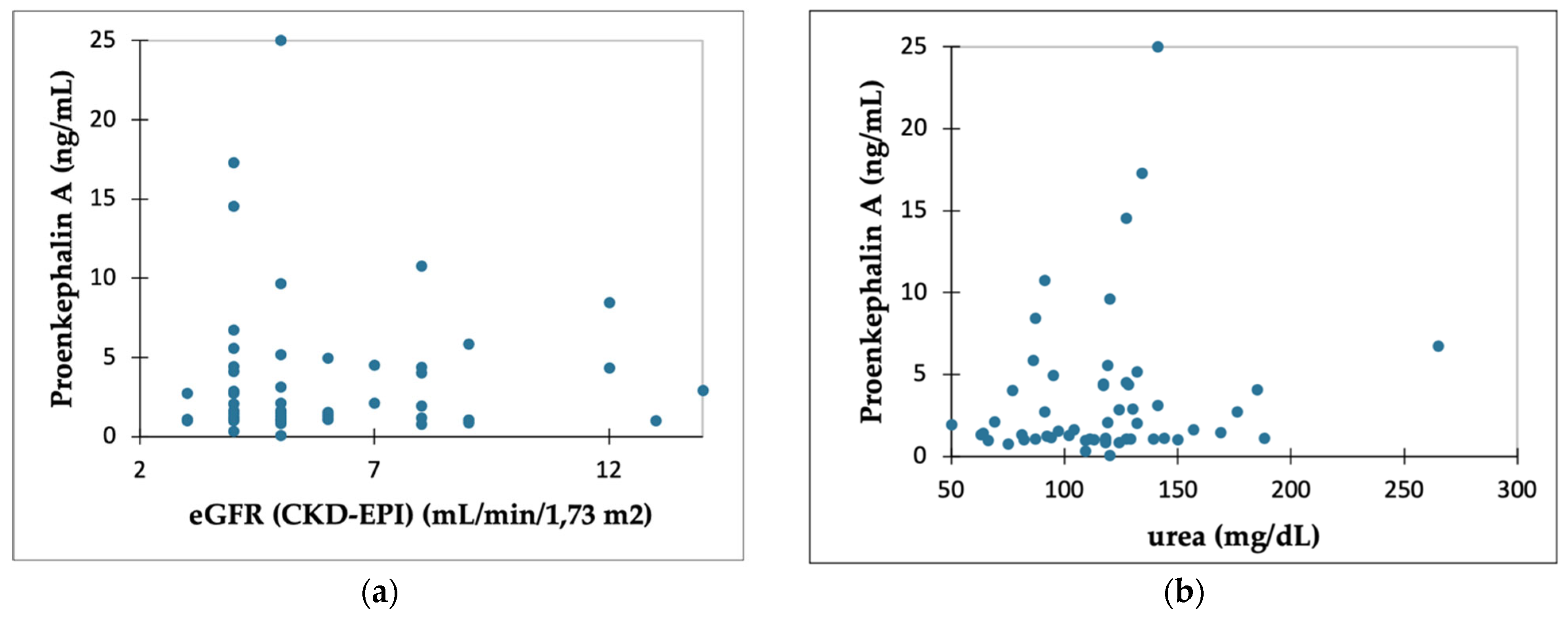
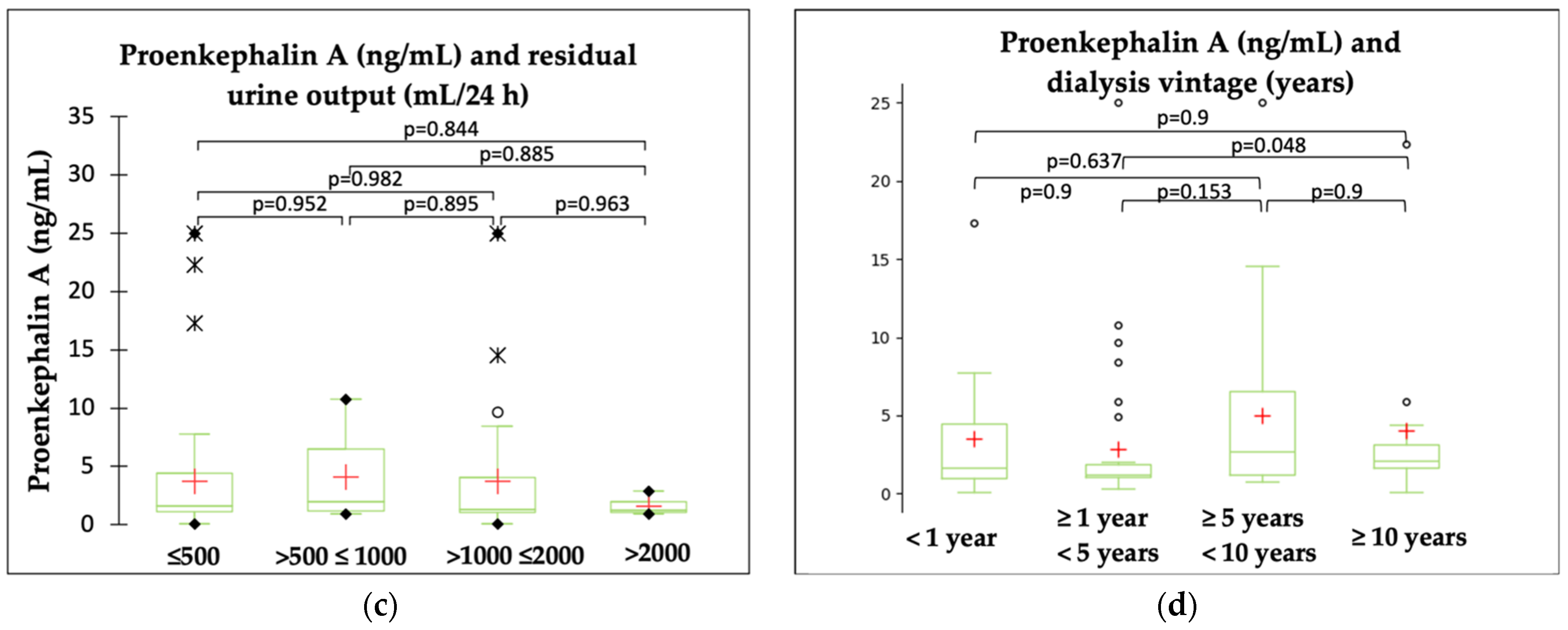
2.2. PENK A and Kidney Function Parameters
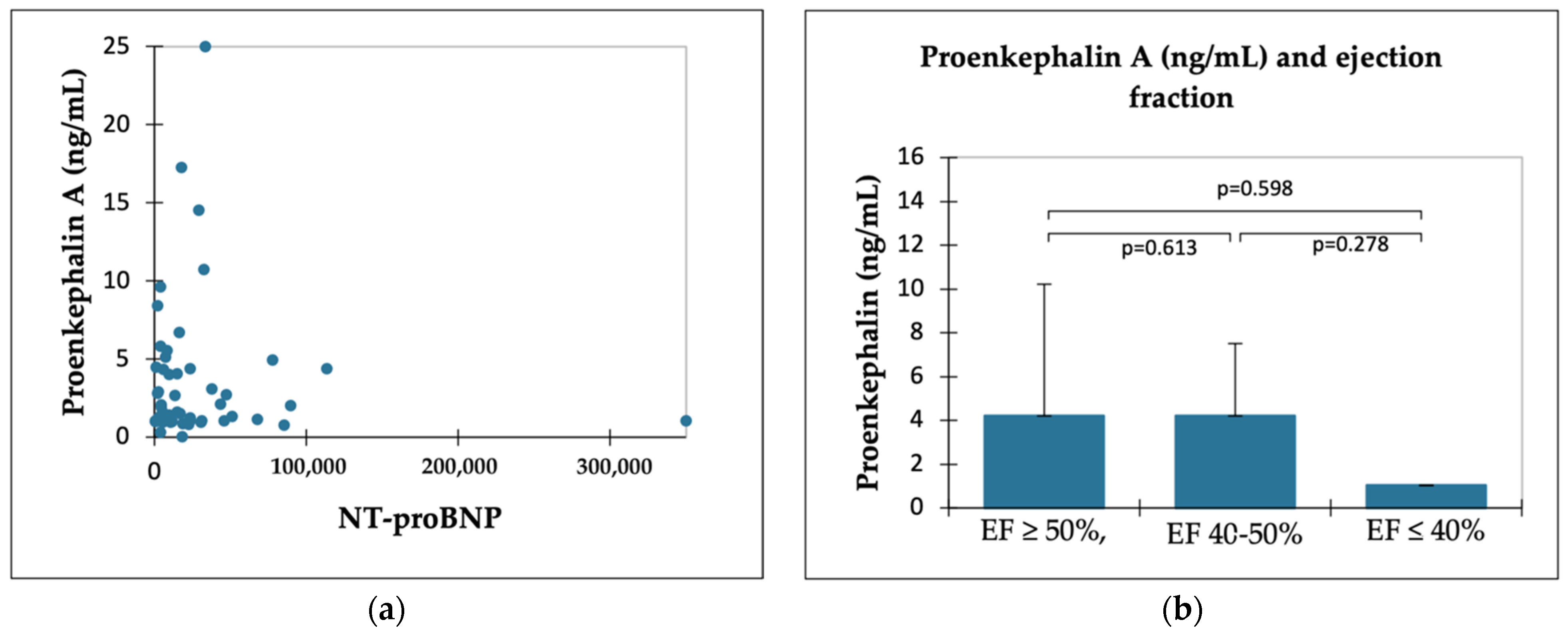
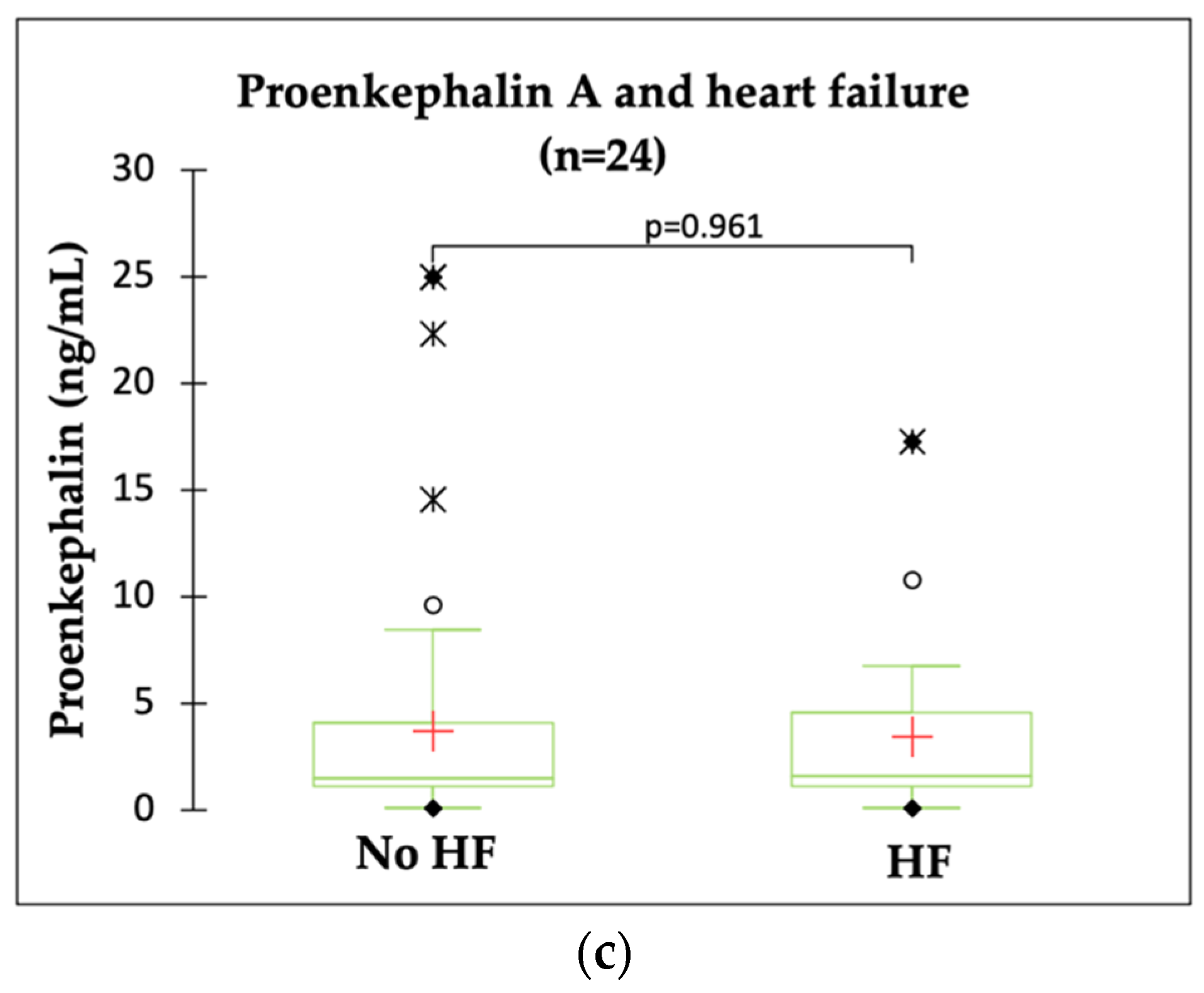
2.3. PENK A and HF Indicators
2.4. PENK A and Type of Dialysis Membrane
3. Discussion
3.1. PENK A as a Biomarker of Kidney Dysfunction
PENK A and Hemodialysis
3.2. PENK A in Patients with Heart Failure
3.3. Limitations of the Study
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Measurements
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khorashadi, M.; Beunders, R.; Pickkers, P.; Legrand, M. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron 2020, 144, 655–661. [Google Scholar] [CrossRef]
- Denning, G.M.; Ackermann, L.W.; Barna, T.J.; Armstrong, J.G.; Stoll, L.L.; Weintraub, N.L.; Dickson, E.W. Proenkephalin Expression and Enkephalin Release Are Widely Observed in Non-Neuronal Tissues. Peptides 2008, 29, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sezen, S.F.; Kenigs, V.A.; Kapusta, D.R. Renal Excretory Responses Produced by the Delta Opioid Agonist, BW373U86, in Conscious Rats. J. Pharmacol. Exp. Ther. 1998, 287, 238–245. [Google Scholar] [PubMed]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical Performance of an Immunoassay to Measure Proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Köhrle, J.; Bergmann, A. Proenkephalin A 119–159, a Stable Proenkephalin A Precursor Fragment Identified in Human Circulation. Peptides 2006, 27, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Breidthardt, T.; Jaeger, C.; Christ, A.; Klima, T.; Mosimann, T.; Twerenbold, R.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; Struck, J.; et al. Proenkephalin for the Early Detection of Acute Kidney Injury in Hospitalized Patients with Chronic Kidney Disease. Eur. J. Clin. Investig. 2018, 48, e12999. [Google Scholar] [CrossRef]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. Proenkephalin, Renal Dysfunction, and Prognosis in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 56–69. [Google Scholar] [CrossRef]
- Mossanen, J.; Pracht, J.; Jansen, T.; Buendgens, L.; Stoppe, C.; Goetzenich, A.; Struck, J.; Autschbach, R.; Marx, G.; Tacke, F. Elevated Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery. Int. J. Mol. Sci. 2017, 18, 1662. [Google Scholar] [CrossRef]
- Beunders, R.; van Groenendael, R.; Leijte, G.P.; Kox, M.; Pickkers, P. Proenkephalin Compared to Conventional Methods to Assess Kidney Function in Critically Ill Sepsis Patients. Shock 2020, 54, 308–314. [Google Scholar] [CrossRef]
- Hollinger, A.; Wittebole, X.; François, B.; Pickkers, P.; Antonelli, M.; Gayat, E.; Chousterman, B.G.; Lascarrou, J.-B.; Dugernier, T.; Di Somma, S.; et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int. Rep. 2018, 3, 1424–1433. [Google Scholar] [CrossRef]
- Rosenqvist, M.; Bronton, K.; Hartmann, O.; Bergmann, A.; Struck, J.; Melander, O. Proenkephalin a 119–159 (PenKid)—A Novel Biomarker for Acute Kidney Injury in Sepsis: An Observational Study. BMC Emerg. Med. 2019, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hur, M.; Struck, J.; Bergmann, A.; Somma, S.D.; GREAT Network. Proenkephalin Predicts Organ Failure, Renal Replacement Therapy, and Mortality in Patients with Sepsis. Ann. Lab. Med. 2020, 40, 466–473. [Google Scholar] [CrossRef]
- Schulz, C.-A.; Christensson, A.; Ericson, U.; Almgren, P.; Hindy, G.; Nilsson, P.M.; Struck, J.; Bergmann, A.; Melander, O.; Orho-Melander, M. High Level of Fasting Plasma Proenkephalin-A Predicts Deterioration of Kidney Function and Incidence of CKD. J. Am. Soc. Nephrol. 2017, 28, 291–303. [Google Scholar] [CrossRef]
- Kieneker, L.M.; Hartmann, O.; Bergmann, A.; de Boer, R.A.; Gansevoort, R.T.; Joosten, M.M.; Struck, J.; Bakker, S.J.L. Proenkephalin and Risk of Developing Chronic Kidney Disease: The Prevention of Renal and Vascular End-Stage Disease Study. Biomarkers 2018, 23, 474–482. [Google Scholar] [CrossRef]
- Headrick, J.P.; See Hoe, L.E.; Du Toit, E.F.; Peart, J.N. Opioid Receptors and Cardioprotection—‘Opioidergic Conditioning’ of the Heart. Br. J. Pharmacol. 2015, 172, 2026–2050. [Google Scholar] [CrossRef]
- Siong Chan, D.C.; Cao, T.H.; Ng, L.L. Proenkephalin in Heart Failure. Heart Fail. Clin. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matsue, Y.; ter Maaten, J.M.; Struck, J.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Clinical Correlates and Prognostic Value of Proenkephalin in Acute and Chronic Heart Failure. J. Card. Fail. 2017, 23, 231–239. [Google Scholar] [CrossRef]
- Emmens, J.E.; ter Maaten, J.M.; Damman, K.; van Veldhuisen, D.J.; de Boer, R.A.; Struck, J.; Bergmann, A.; Sama, I.E.; Streng, K.W.; Anker, S.D.; et al. Proenkephalin, an Opioid System Surrogate, as a Novel Comprehensive Renal Marker in Heart Failure. Circ. Heart Fail. 2019, 12, e005544. [Google Scholar] [CrossRef] [PubMed]
- Short, S.A.P.; Wilkinson, K.; Schulte, J.; Renteria, M.A.; Cheung, K.L.; Nicoli, C.D.; Howard, V.J.; Cushman, M. Plasma Pro-Enkephalin A and Incident Cognitive Impairment: The Reasons for Geographic and Racial Differences in Stroke Cohort. J. Am. Heart Assoc. 2023, 12, e029081. [Google Scholar] [CrossRef]
- Smith, R.; Grossman, A.; Gaillard, R.; Clement-Jones, V.; Ratter, S.; Mallinson, J.; Lowry, P.J.; Besser, G.M.; Rees, L.H. Studies on Circulating Met-Enkephalin and Beta-Endorphin: Normal Subjects and Patients with Renal and Adrenal Disease. Clin. Endocrinol. 1981, 15, 291–300. [Google Scholar] [CrossRef] [PubMed]
- De Marco, V.; Possenti, R.; Vita, F.; Rapposelli, B.; D’Alagni, M.; Giorgio Roda, L. Enkephalin Binding Systems in Human Plasma II: Leu-Enkephalin Serum Albumin Interaction. Neurochem. Res. 1985, 10, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Ciccarelli, M.; Mallamaci, F.; Maggiore, Q.; Lotti, M.; Zucchelli, G.C. Plasma Met-Enkephalin and Leu-Enkephalin in Chronic Renal Failure. Nephrol. Dial. Transplant. 1987, 1, 219–222. [Google Scholar]
- Wala-Zielińska, K.; Świerczyńska-Mróz, K.; Krajewski, P.K.; Nowicka-Suszko, D.; Krajewska, M.; Szepietowski, J.C. Endogenous Opioid Imbalance as a Potential Factor Involved in the Pathogenesis of Chronic Kidney Disease-Associated Pruritus in Dialysis Patients. J. Clin. Med. 2023, 12, 2474. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Struck, J.; Hartmann, O.; Maisel, A.S.; Rehfeldt, M.; Magrini, L.; Melander, O.; Bergmann, A.; Di Somma, S. Diagnostic and Short-Term Prognostic Utility of Plasma pro-Enkephalin (pro-ENK) for Acute Kidney Injury in Patients Admitted with Sepsis in the Emergency Department. J. Nephrol. 2015, 28, 717–724. [Google Scholar] [CrossRef]
- Arbit, B.; Marston, N.; Shah, K.; Lee, E.L.; Aramin, H.; Clopton, P.; Maisel, A.S. Prognostic Usefulness of Proenkephalin in Stable Ambulatory Patients with Heart Failure. Am. J. Cardiol. 2016, 117, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Coca, S.G.; Shannon, R.P.; Kimmel, S.E.; Cappola, T.P. Influence of Renal Dysfunction Phenotype on Mortality in the Setting of Cardiac Dysfunction: Analysis of Three Randomized Controlled Trials. Eur. J. Heart Fail. 2011, 13, 1224–1230. [Google Scholar] [CrossRef]
- United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health: Bethesda, MD, USA; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2022. [Google Scholar]

| Overall (n = 88) | HD (n = 66) | PD (n = 22) | p Value | |
| Age (years) | 65 (51–72) | |||
| Female, n (%) | 41 (47) | 26 (39) | 15 (68) | |
| eGFR (CKD-EPI) (creatinine-based) | 5 (4–7) | 5 (4–7) | 5 (4–8) | 0.856 |
| Plasma PENK A (ng/mL) | 1.492 (1.071–4.380) | 1.368 (1.068–4.030) n = 65 * | 1.706 (1.211–5.858) n = 21 * | 0.305 |
| Dialysis vintage, n (%) | ||||
| <1 year | 19 (22) | 17 (26) | 2 (9) | |
| ≥1 and <5 years | 35 (40) | 27 (41) | 8 (36) | |
| ≥5 and <10 years | 20 (23) | 13 (20) | 7 (32) | |
| ≥10 years | 14 (16) | 9 (14) | 5 (23) | |
| Residual diuresis (mL/24 h), n (%) | ||||
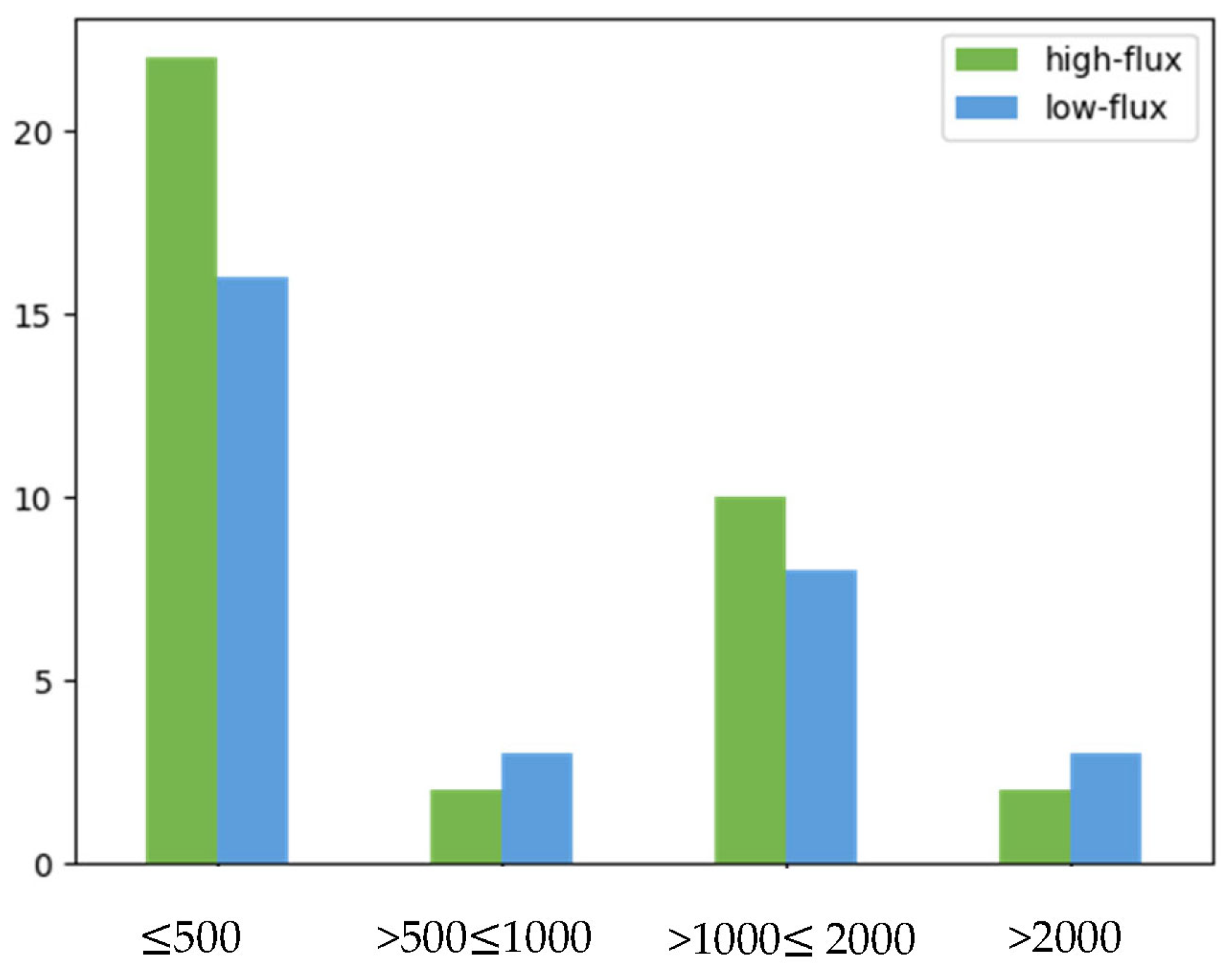
| ≤500 | 49 (56) | 38 (58) | 11 (50) | |
| >500 and ≤1000 | 7 (8) | 5 (8) | 2 (9) | |
| >1000 and ≤2000 | 26 (30) | 18 (27) | 8 (36) | |
| >2000 | 6 (7) | 5 (8) | 1 (5) | |
| Heart failure, n (%) | 24 (27) | 23 (35) | 1 (5) | |
| Hypertension, n (%) | 77 (88) | 60 (90) | 17 (77) | |
| Coronary artery disease, n (%) | 22 (25) | 22 (33) | 0 | |
| Diabetes, n (%) | 31 (35) | 26 (40) | 5 (23) | |
| NT-proBNP (ng/L) | 14,100 (52,000–30,828) | 16,164 (7196–32,586) n = 40 | 8270 (3784– 21,231) n = 16 | 0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grycuk, W.; Jakubowska, Z.; Małyszko, J. Proenkephalin Levels and Its Determinants in Patients with End-Stage Kidney Disease Treated with Hemodialysis and Peritoneal Dialysis. Int. J. Mol. Sci. 2023, 24, 15015. https://doi.org/10.3390/ijms241915015
Grycuk W, Jakubowska Z, Małyszko J. Proenkephalin Levels and Its Determinants in Patients with End-Stage Kidney Disease Treated with Hemodialysis and Peritoneal Dialysis. International Journal of Molecular Sciences. 2023; 24(19):15015. https://doi.org/10.3390/ijms241915015
Chicago/Turabian StyleGrycuk, Wiktoria, Zuzanna Jakubowska, and Jolanta Małyszko. 2023. "Proenkephalin Levels and Its Determinants in Patients with End-Stage Kidney Disease Treated with Hemodialysis and Peritoneal Dialysis" International Journal of Molecular Sciences 24, no. 19: 15015. https://doi.org/10.3390/ijms241915015
APA StyleGrycuk, W., Jakubowska, Z., & Małyszko, J. (2023). Proenkephalin Levels and Its Determinants in Patients with End-Stage Kidney Disease Treated with Hemodialysis and Peritoneal Dialysis. International Journal of Molecular Sciences, 24(19), 15015. https://doi.org/10.3390/ijms241915015






