Abstract
A series of novel derivatives of 18β-glycyrrhetinic acid (GA) were synthesized by introducing aromatic or heterocyclic structures to extend the side chain, thereby enhancing their interaction with amino acid residues in the active pocket of the target protein. These compounds were structurally characterized using 1H NMR, 13C NMR, and HRMS. The compounds were subsequently evaluated for their inhibitory effects on HIV-1 protease and cell viability in the human cancer cell lines K562 and HeLa and the mouse cancer cell line CT26. Towards HIV-1 protease, compounds 28 and 32, which featured the introduction of heterocyclic moieties at the C3 position of GA, exhibited the highest inhibition, with inhibition rates of 76% and 70.5%, respectively, at 1 mg/mL concentration. Further molecular docking suggests that a 3-substituted polar moiety would be likely to enhance the inhibitory activity against HIV-1 protease. As for the anti-proliferative activities of the GA derivatives, incorporation of a thiazole heterocycle at the C3- position in compound 29 significantly enhanced the effect against K562 cells with an IC50 value of 8.86 ± 0.93 µM. The introduction of electron-withdrawing substituents on the C3-substituted phenyl ring augmented the anti-proliferative activity against Hela and CT26 cells. Compound 13 exhibited the highest inhibitory activity against Hela cells with an IC50 value of 9.89 ± 0.86 µM, whereas compound 7 exerted the strongest inhibition against CT26 cells with an IC50 value of 4.54 ± 0.37 µM. These findings suggest that further modification of GA is a promising path for developing potent novel anti-HIV and anticancer therapeutics.
1. Introduction
Licorice (Glycyrrhiza glabra) is a medicinal plant widely used in traditional Chinese medicine (TCM). Its major active ingredient, glycyrrhetinic acid (GA), is a pentacyclic triterpenoid with a broad spectrum of pharmacological activities [1,2,3]. GA has been shown to possess anticancer [4,5,6,7], antiviral [8,9,10], antioxidant [11], antibacterial [12], and anti-inflammatory [13] functions. Especially, its anticancer and antiviral functions have attracted extensive research attention in recent years. Various types of modification of GA have been undertaken to improve its pharmacological activities and boost its application in complementary and alternative medicine (CAM).
Human immunodeficiency virus (HIV), which has emerged to be a sustained global epidemic, has infected approximately 79.3 million and killed 36.3 million people so far [14,15,16]. To combat HIV infection, antiretroviral therapy (ART), which is a cocktail combination of reverse transcriptase inhibitors [17], protease inhibitors [18], and/or integrase inhibitors [19,20], has been developed and shown to be effective. However, drug resistance arises rapidly towards these therapeutics, and the continuous search for novel anti-HIV agents is critical for improving patient survival and patients’s quality of life.
Besides HIV infection, cancer is another serious disease threatening human health. Globally, it is the second leading cause of death, only after cardiovascular diseases [21]. Despite the large number of anticancer therapies that have been developed, cancer heterogeneity [22] and the rapid development of resistance towards the therapeutics [23] have imposed a big challenge to therapeutic efficacy and patient survival. Thus, similar to HIV infection, identifying and advancing novel anticancer treatments is still of high importance.
As a valuable resource, natural products possess a series of desirable attributes for drug development, including biodegradability, wide availability, and a reduced likelihood of drug resistance [24,25,26]. In terms of licorice, its active ingredient, glycyrrhizic acid (GL), enhanced the level of HIV-1 p24 antigen in patients with hemophilia and AIDS [27]. GL is also able to stimulate interferon production, augment the activity of natural killer cells, and boost the count of CD4-positive T lymphocytes, which, in turn, would impede AIDS progression [28,29]. All these observations imply that GA, the primary hydrolytic metabolite of GL [30], is potentially capable of suppressing HIV-1 replication in vivo. Furthermore, GA was shown to exhibit selective cytotoxicity towards various cancer cells, including breast, ovarian, leukemia, liver, and gastric cancer cells [31,32,33], making it a promising candidate for designing and developing novel anticancer agents. GA can exert its cytotoxicity via diverse mechanisms, such as disruption of the actin cytoskeleton, suppression of the p38 MAPK-AP1 signaling axis, and DNA fragmentation-mediated apoptosis [34,35,36]. The inherent biological activities of natural compounds are usually moderate against specific targets [37]. Therefore, in this study, we have developed a series of 32 novel GA derivatives by introducing aromatic or heterocyclic structures to extend the side chains, thereby enhancing their interaction with the target proteins and improving their antiviral and anticancer activities.
2. Results and Discussion
2.1. Synthesis of GA Derivatives
The chemical synthesis of GA derivatives is presented in Scheme 1. Briefly, with GA as the starting substance, its 3-hydroxyl group was oxidized into a carbonyl group by Dess-Martin Periodinane, resulting in compound 1 (white solid, yield 99%). Compound 1 was esterized by reacting with CH3CH2Br, using K2CO3 and KI as catalysts, to form compound 2 (white solid, yield 64%). Compound 2 was subsequently treated with NH2OH·HCl and NaHCO3, converting the 3-carbonyl group into an oxime group to generate the intermediate compound 3 (white solid, yield 80%). Finally, compound 3 underwent nucleophilic substitution reactions with various halogenated hydrocarbons under the catalysis of NaH to form a series of GA derivatives (compounds 4–35). The structures of all compounds were characterized by 1H NMR, 13C NMR, and HRMS. The stereostructure of compound 12 was further confirmed by X-ray crystallography (Figure 1 and Tables S1–S7 of the Supporting Information). Crystallographic data for compound 12 has been deposited in the Cambridge Crystallographic Data Centre (deposition number: CCDC 2287206).
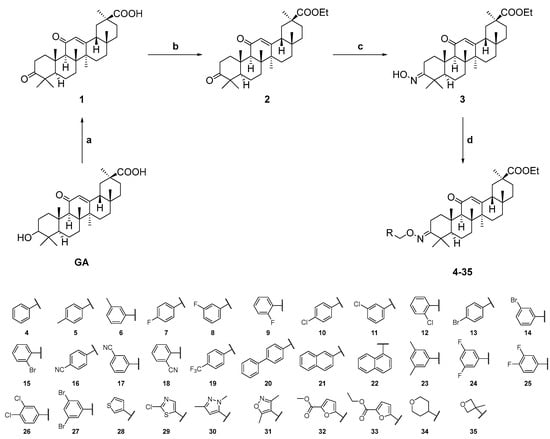
Scheme 1.
Synthesis of compounds 1–35. Reagents and conditions: a. Dess-Martin Periodinane, DCM, rt, 3 h. b. K2CO3, KI, DMF, C2H5Br, rt, 4 h. c. NH2OH·HCl, NaHCO3, C2H5OH, 90 °C, 14 h, 80%. d. Halogenated hydrocarbons, NaH, Anhydrous THF, rt.
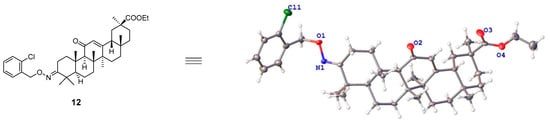
Figure 1.
Structure of compound 12 determined by X-ray crystallography.
2.2. In Vitro Inhibitory Activity against HIV-1 Protease
Compounds 4–35 were assayed for their inhibitory activities against HIV-1 protease. As shown in Table 1, the inhibitory effect was mild to moderate except for compounds 7, 28, and 32. Compound 28, which has a 3-thiophene group, displayed the highest activity with an inhibition rate of 76% at 1 mg/mL concentration. Compound 32, which has a 3-furan moiety, exhibited comparable activity to compound 28 with an inhibition rate of 70.5%. Compound 7 with a para-fluorophenyl at 3-position exhibited weaker activity than compounds 28 and 32 with an inhibition rate of 53.6%, but was the strongest among compounds with a 3-phenyl moiety with different substitutions.

Table 1.
In vitro inhibitory activity against HIV-1 protease.
2.3. Molecular Docking
To get a glimpse of potential interactions between the most active GA derivatives and HIV-1 protease, we docked compounds 28 and 32 into the active site of HIV-1 protease (PDB ID: 1QBS), respectively (Table 2). Compound 28 gave a docking score of −7.236 and a binding energy of −53.102 kcal/moL. It formed four hydrogen bonds with residues Ile 50(A), Ile 50(B), Arg 8(A), and Arg 8(B) of HIV-1 protease, with respective bond lengths of 1.89 Å, 2.18 Å, 2.45 Å, and 2.46 Å (Figure 2a). Compound 32 gave a lower docking score of -6.834 but a better binding energy of −64.549 kcal/moL. It formed four hydrogen bonds with residues Ile 50(A), Ile 50(B), Gly 48(A), and Gly 48(B) of HIV-1 protease, with respective bond lengths of 2.09 Å, 2.49 Å, 2.03 Å, and 2.14 Å (Figure 2b). It also formed a π-cation interaction with residue Arg 8(A). The polar interactions between the 3-substitue group of compounds 28 and 32 and HIV-1 protease imply that a 3-substitute hydrophobic group, such as a phenyl group, would be unfavorable for interactions with HIV-1 protease, which would render inhibitory activity lower.

Table 2.
Docking of compounds 28 and 32 into the active site of HIV-1 protease (PDB ID: 1QBS).
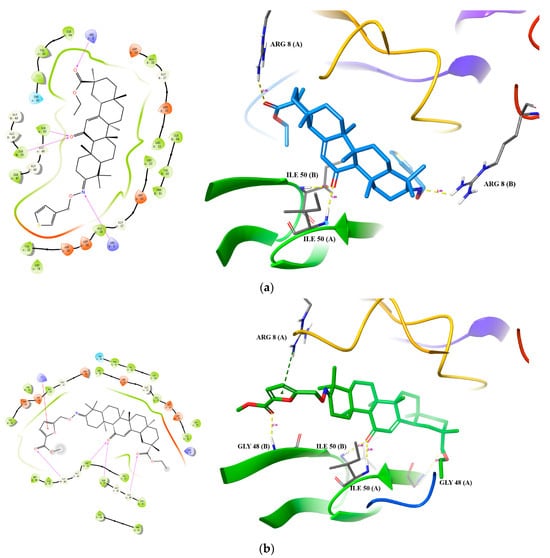
Figure 2.
Putative binding of compound 28 (a) and compound 32 (b) to HIV-1 protease. Carbon atoms were colored blue (a) or green (b), while oxygen, hydrogen, nitrogen, and sulfur atoms were colored red, grey, blue, and yellow, respectively. A green dotted line was used to show the π-cation interaction, and hydrogen bonds were shown as yellow dotted lines.
2.4. In Vitro effect on Viability of Cancer Cells
To identify whether the GA derivatives could be potentially used to treat human or animal tumors, their effects on the viability of human chronic myeloid leukemia cell lines K562, human cervical cancer cell lines Hela, and mouse colon cancer cell lines CT26 were evaluated using the MTT assay. The IC50 values of the compounds were summarized in Table 3. In human leukemia K562 cells, five compounds (4, 7, 8, 28, and 29) exhibited stronger inhibition on cell growth than the positive control cisplatin, with compound 29 having the best inhibitory activity (IC50 = 8.86 ± 0.93 µM). In human cervical cancer HeLa cells, four compounds (13, 24, 30, and 31) showed more potent activity than cisplatin, with compound 13 having the highest activity (IC50 = 9.89 ± 0.86 µM). As for the mouse colon CT26 cells, three compounds (7, 8, and 31) manifested a better inhibitory effect than cisplatin, with compound 7 showcasing the highest activity (IC50 = 4.54 ± 0.37 µM). Importantly, the IC50 values for the most effective compounds (7, 13, and 29) against normal NCM460 cells were all greater than 100 µM. These results suggest that GA derivatives with 3-substituted heterocyclic moieties possess inhibitory activities against human and animal cancer cells, and electron-withdrawing groups, particularly fluorine, on the 3-phenyl moiety render stronger effects.

Table 3.
In vitro effect of compounds on viability of cancer cells.
3. Materials and Methods
3.1. General Experimental Procedures
The progress of the chemical reactions was monitored by thin-layer chromatography (TLC) under UV light. Compound purification was carried out using flash chromatography on silica gel. Chemical yields refer to pure, isolated substances. HRMS spectra of the compounds were measured on a Waters Xevo G2-S QTOF mass spectrometer (Waters, Massachusetts, USA) using the electron spray ionization (ESI) method. 1H, 13C, and 19F NMR spectra were obtained using a Bruker DPX-400 MHz spectrometer (Bruker, Billerica, Massachusetts, USA). Chemical shifts were reported in ppm from CDCl3 or TMS, with solvent resonance as an internal standard. The following abbreviations were used to designate chemical shift multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, h = heptet, m = multiplet, and br = broad.
The HIV-1 protease kit (AS-71147) and HIV-1 protease (wild type, NC-001802) were purchased from AnaSpec (Fremont, CA, USA). Enzyme assays were conducted in 96- and 384-well plates using a Synergy II Microplate Reader (Biotec Co., Burlington, VT, USA).
3.2. Synthesis of the Compounds 1–35
Glycyrrhetinic acid (1 equiv) and Dess-Martin Periodinane (1 equiv) were dissolved in 20 mL of dichloromethane and stirred at room temperature for 3 h. Upon completion of the reaction (monitored by TLC), the reaction mixture was quenched with 20 mL of saturated sodium bicarbonate. The resulting mixture was then extracted three times with 20 mL of dichloromethane. The combined organic phases were dried using anhydrous sodium sulfate. After filtration, the solvent was evaporated to produce compound 1 (white solid) with a yield of 99%.
Compound 1 (1 equiv), K2CO3 (2 equiv), and KI (0.2 equiv) were dissolved in 20 mL of DMF. Then, C2H5Br (2 equiv) was added to the solution, and the mixture was stirred at room temperature for 4 h to complete the reaction (monitored by TLC). Upon completion, the reaction mixture was quenched with 20 mL of water and subsequently extracted three times with 20 mL of ethyl acetate. The organic phase was dried using anhydrous sodium sulfate and filtered. The solvent evaporated. The residue was purified by silica gel chromatography to give the white solid compound 2, with a yield of 74%.
Compound 2 (1 equiv.) was dissolved in EtOH (50 mL). Then, NH2OH·HCl (1.5 equiv.) and NaHCO3 (1.5 equiv.) were added to reflux for 4 h. Upon reaction completion, the reaction mixture was concentrated under vacuum, and the residue was extracted with ethyl acetate and H2O. The organic layer was washed with saturated aqueous NaCl, dried with MgSO4, and concentrated under vacuum to give the crude residue, which was subsequently purified by column chromatography on silica gel to produce compound 3 in 85% yield.
Compound 3 (1 equiv.) was dissolved in 2 mL of dry THF, followed by the addition of NaH (1.5 equiv.). After stirring in an ice bath for 30 min, the halogenated hydrocarbon (2 equiv.) was added. The reaction mixture was allowed to proceed at room temperature for 4 h. Upon reaction completion, the reaction was quenched with 1 mL of methanol. The solvent was then evaporated, and the target compounds 4–35 were purified by column chromatography.
3.3. Compound Characterization
Product 4 was a white solid in 83% yield, Mp: 156–158 °C; 1H NMR (400 MHz, CDCl3) δ 7.38–7.31 (m, 4H), 7.30–7.25 (m, 1H), 5.65 (s, 1H), 5.06 (s, 2H), 4.23–4.08 (m, 2H), 2.30–2.94 (m, 1H), 2.81–2.76 (m, 1H), 2.36 (s, 1H), 2.32–2.24 (m, 1H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.76–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.34–1.30 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.17 (s, 3H), 1.14 (s, 6H), 1.05–1.00 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 199.94, 176.47, 169.62, 166.37, 138.68, 128.55, 128.26, 128.18, 127.52, 75.35, 61.42, 60.42, 55.55, 48.49, 45.45, 43.94, 43.38, 41.18, 40.33, 39.10, 37.82, 37.03, 32.48, 31.92, 31.21, 28.69, 28.42, 27.55, 26.57, 26.51, 23.53, 23.42, 18.70, 18.34, 18.21, 15.77, 14.44. HRMS (ESI): Exact mass calcd for C39H55NNaO4 [M+Na]+: 624.4023, Found: 624.4014.
Product 5 is a white solid in 86% yield, Mp: 145–147 °C; 1H NMR (400 MHz, CDCl3) δ 7.26– 7.13 (m, 2H), 7.14 (d, J = 7.8 Hz, 2H), 5.65 (s, 1H), 5.01 (s, 2H), 4.23–4.08 (m, 2H), 2.99–2.93 (m, 1H), 2.80–2.76 (m, 1H), 2.36 (s, 1H), 2.33 (s, 3H), 2.30–2.22 (m, 1H), 2.13–1.96 (m, 2H), 1.94–1.89 (m, 1H), 1.86–1.78 (m, 1H), 1.82 (td, J = 13.7, 4.7 Hz, 1H), 1.78–1.57L (m, 4H), 1.50–1.38 (m, 3H), 1.34–1.30 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.17 (s, 3H), 1.14 (s, 6H), 1.06–1.00 (m, 3H), 1.06 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.94, 176.46, 169.58, 166.15, 137.18, 135.54, 128.96, 128.54, 128.33, 75.26, 61.42, 60.41, 55.56, 48.49, 45.44, 43.93, 43.37, 41.17, 40.32, 39.08, 37.81, 37.02, 32.49, 31.91, 31.21, 28.68, 28.40, 27.53, 26.56, 26.50, 23.55, 23.40, 21.29, 18.69, 18.32, 18.17, 15.75, 14.43. HRMS (ESI): Exact mass calcd for C40H57NNaO4 [M+Na]+: 638.4180, Found: 638.4176.
Product 6 was a white solid in 98% yield, Mp: 114–116 °C; 1H NMR (400 MHz, CDCl3) δ 7.26–7.20 (m, 1H), 7.18–7.14 (m, 2H), 7.10–7.08 m, 1H), 5.65 (s, 1H), 5.03 (s, 2H), 4.23–4.08 (m, 2H), 2.99–2.93 (m, 1H), 2.81–2.75 (m, 1H), 2.37–2.25 (m, 2H), 2.35 (s, 3H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.6, 4.6 Hz, 1H), 1.69–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.37–1.29 (m, 2H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.17 (s, 3H), 1.14 (m, 6H), 1.09–1.01 (m, 3H), 1.06 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.98, 176.50, 169.62, 166.32, 138.54, 137.86, 129.02, 128.60, 128.31, 128.22, 125.31, 75.45, 61.47, 60.45, 55.59, 48.53, 45.48, 43.97, 43.41, 41.22, 40.37, 39.15, 37.85, 37.06, 32.53, 31.95, 31.25, 28.72, 28.45, 27.60, 26.60, 26.55, 23.55, 23.45, 21.56, 18.73, 18.38, 18.25, 15.80, 14.47. Exact mass calcd for C40H57NNaO4 [M+Na]+: 638.4180, Found: 638.4168.
Product 7 was a white solid in 95% yield, Mp: 149–151 °C; 1H NMR (400 MHz, CDCl3) δ 7.34–7.26 (m, 2H), 7.03–6.99 (m, 2H), 5.65 (s, 1H), 5.01 (s, 2H), 4.23–4.08 (m, 2H), 2.95–2.89 (m, 1H), 2.81–2.75 (m, 1H), 2.36 (s, 1H), 2.31–2.23 (m, 1H), 2.14–2.08 (m, 1H), 2.06–1.97 (m, 2H), 1.94–1.98 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.66–1.56 (m, 4H), 1.53–1.40 (m, 3H), 1.37–1.29 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.19 (s, 3H), 1.15 (d, J = 6.0 Hz, 9H), 1.06–0.99 (m, 3H), 1.04 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.99, 176.53, 169.70, 166.61, 162.41 (d, J = 244.9 Hz), 134.52 (d, J = 3.2 Hz), 130.08 (d, J = 8.0 Hz), 128.60, 115.12 (d, J = 21.1 Hz), 74.64, 61.47, 60.48, 55.59, 48.54, 45.49, 43.99, 43.43, 41.23, 40.41, 39.13, 37.86, 37.06, 32.52, 31.97, 31.26, 28.73, 28.46, 27.60, 26.62, 26.55, 23.56, 23.46, 18.74, 18.39, 18.26, 15.80, 14.48. 19F NMR (377 MHz, CDCl3) δ -115.23. Exact mass calcd for C39H54FNNaO4 [M+Na]+: 642.3929, Found: 642.3917.
Product 8 was a white solid in 99% yield, Mp: 118–120 °C; 1H NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 1H), 7.12–7.10 (m, 1H), 7.08–7.04 (m, 1H), 6.98–6.93 (m, 1H), 5.66 (s, 1H), 5.05 (s, 2H), 4.23–4.09 (m, 2H), 2.99–2.93 (m, 1H), 2.83–2.78 (m, 1H), 2.37 (s, 1H), 2.34–2.26 (m, 1H), 2.13–2.09 (m, 1H), 2.07–1.97 (m, 2H), 1.95–1.90 (m, 1H), 1.83 (td, J = 13.7, 4.6 Hz, 1H), 1.70–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.37–1.30 (m, 2H), 1.35 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.17–1.13 (m, 9H), 1.10–1.00 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.94, 176.50, 169.66, 166.76, 162.91 (d, J = 245.5 Hz), 141.56 (d, J = 7.2 Hz), 129.76 (d, J = 8.0 Hz), 128.59, 123.45 (d, J = 2.9 Hz), 114.85 (d, J = 21.7 Hz), 114.30 (d, J = 21.4 Hz), 74.52, 61.45, 60.46, 55.60, 48.54, 45.48, 43.98, 43.42, 41.22, 40.41, 39.12, 37.86, 37.06, 32.51, 31.96, 31.26, 28.72, 28.45, 27.58, 26.61, 26.55, 23.56, 23.45, 18.73, 18.38, 18.27, 15.80, 14.48. 19F NMR (377 MHz, CDCl3) δ -113.68. Exact mass calcd for C39H54FNNaO4 [M+Na]+: 642.3929, Found: 642.3911.
Product 9 was a white solid in 81% yield, Mp: 171–173 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (td, J = 7.5, 1.9 Hz, 1H), 7.28–7.22 (m, 1H), 7.10 (td, J = 7.5, 1.2 Hz, 1H), 7.05–7.00 (m, 1H), 5.66 (s, 1H), 5.13 (s, 2H), 4.23–4.08 (m, 2H), 2.98–2.92 (m, 1H), 2.82–2.76 (m, 1H), 2.37 (s, 1H), 2.33–2.25 (m, 1H), 2.13–2.09 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.7, 4.6 Hz, 1H), 1.76–1.52 (m, 4H), 1.49–1.38 (m, 3H), 1.37–1.29 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.15 (d, J = 6.3 Hz, 9H), 1.09–1.00 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.93, 176.47, 169.63, 166.66, 160.83 (d, J = 247.0 Hz), 130.52 (d, J = 4.6 Hz), 129.17 (d, J = 8.1 Hz), 128.56, 125.86 (d, J = 14.6 Hz), 123.85 (d, J = 3.6 Hz), 115.17 (d, J = 21.7 Hz), 68.75 (d, J = 4.0 Hz), 61.42, 60.42, 55.52, 48.50, 45.44, 43.94, 43.39, 41.19, 40.35, 39.10, 37.83, 37.02, 32.47, 31.93, 31.22, 28.69, 28.42, 27.55, 26.58, 26.51, 23.51, 23.42, 18.70, 18.34, 18.17, 15.77, 14.44. 19F NMR (377 MHz, CDCl3) δ -118.65. Exact mass calcd for C39H54FNNaO4 [M+Na]+: 642.3929, Found: 642.3911.
Product 10 was a white solid in 99% yield, Mp: 117–119 °C; 1H NMR (400 MHz, CDCl3) δ 7.31–7.26 (m, 4H), 5.66 (s, 1H), 5.01 (s, 2H), 4.23–4.08 (m, 2H), 2.96–2.90 (m, 1H), 2.82–2.76 (m, 1H), 2.36 (s, 1H), 2.32–2.23 (m, 1H), 2.14–2.09 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (dt, J = 13.7, 6.8 Hz, 1H), 1.74–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.35–1.30 (m, 2H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.14 (d, J = 2.8 Hz, 9H), 1.04–1.00 (m, 3H), 1.04 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.93, 176.48, 169.68, 166.65, 137.31, 133.25, 129.56, 128.55, 128.41, 74.48, 61.42, 60.43, 55.54, 48.50, 45.45, 43.95, 43.39, 41.19, 40.38, 39.09, 37.83, 37.02, 32.47, 31.93, 31.22, 28.70, 28.42, 27.56, 26.58, 26.51, 23.53, 23.43, 18.70, 18.35, 18.22, 15.78, 14.45. HRMS (ESI): Exact mass calcd for C39H54ClNNaO4 [M+Na]+: 658.3634, Found: 658.3615.
Product 11 was a white solid in 81% yield, Mp: 115–117 °C; 1H NMR (400 MHz, CDCl3) δ 7.34 (t, J = 3.60 Hz, 1H), 7.28–7.20 (m, 3H), 5.66 (s, 1H), 5.03 (s, 2H), 4.23–4.08 (m, 2H), 2.98–2.92 (m, 1H), 2.83–2.77 (m, 1H), 2.37 (s, 1H), 2.34–2.26 (m, 1H), 2.14–2.07 (m, 1H), 2.03–1.98 (m, 2H), 1.95–1.90 (m, 1H), 1.83 (td, J = 13.6, 4.6 Hz, 1H), 1.72–1.57 (m, 4H), 1.52–1.37 (m, 3H), 1.35–1.32 (m, 2H), 1.35 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.15 (d, J = 4.3 Hz, 9H), 1.05–1.00 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.93, 176.49, 169.67, 166.86, 140.96, 134.10, 129.57, 128.56, 128.19, 127.58, 126.10, 74.45, 61.43, 60.44, 55.58, 48.51, 45.46, 43.96, 43.40, 41.20, 40.42, 39.10, 37.84, 37.04, 32.48, 31.94, 31.23, 28.71, 28.43, 27.56, 26.59, 26.52, 23.51, 23.43, 18.71, 18.36, 18.27, 15.79, 14.46. Exact mass calcd for C39H54ClNNaO4 [M+Na]+: 658.3634, Found: 658.3614.
Product 12 was a white solid in 62% yield, Mp: 189–191 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (dd, J = 7.4, 1.9 Hz, 1H), 7.34 (dd, J = 7.5, 1.7 Hz, 1H), 7.27–7.18 (m, 2H), 5.66 (s, 1H), 5.18 (s, 2H), 4.23–4.09 (m, 2H), 3.03–2.97 (m, 1H), 2.85–2.79 (m, 1H), 2.39–2.30 (m, 2H), 2.14–2.09 (m, 1H), 2.07–1.97 (m, 2H), 1.95–1.90 (m, 1H), 1.83 (td, J = 13.7, 4.6 Hz, 1H), 1.70–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.35–1.30 (m, 2H), 1.35 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.16–1.14 (m, 9H), 1.09–1.00 (m, 3H), 1.06 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.96, 176.50, 169.68, 166.89, 136.67, 132.97, 129.39, 129.26, 128.59, 128.49, 126.61, 72.35, 61.46, 60.45, 55.54, 48.53, 45.47, 43.97, 43.42, 41.23, 40.38, 39.18, 37.85, 37.06, 32.49, 31.95, 31.25, 28.72, 28.45, 27.63, 26.61, 26.54, 23.53, 23.45, 18.72, 18.38, 18.29, 15.82, 14.47. Exact mass calcd for C39H54ClNNaO4 [M+Na]+: 658.3634, Found: 658.3620.
Product 13 was a white solid in 78% yield, Mp: 158–160 °C; 1H NMR (400 MHz, CDCl3) δ 7.46–7.43 (m, 2H), 7.26–7.20 (m, 2H), 5.65 (s, 1H), 4.99 (s, 2H), 4.22–4.08 (m, 2H), 2.96–2.89 (m, 1H), 2.81–2.76 (m, 1H), 2.36 (s, 1H), 2.32–2.23 (m, 1H), 2.13–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.92–1.89 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.68–1.56 (m, 4H), 1.52–1.36 (m, 3H), 1.37–1.29 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.14 (s, 9H), 1.07–0.99 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.96, 176.50, 169.69, 166.70, 137.86, 131.39, 129.91, 128.58, 121.43, 74.52, 61.44, 60.46, 55.57, 48.53, 45.47, 43.97, 43.41, 41.21, 40.40, 39.10, 37.85, 37.04, 32.50, 31.95, 31.25, 28.72, 28.45, 27.57, 26.60, 26.54, 23.56, 23.46, 18.73, 18.37, 18.24, 15.79, 14.47. Exact mass calcd for C39H54BrNNaO4 [M+Na]+: 702.3128, Found: 602.3107.
Product 14 was a white solid in 98% yield, Mp: 111–113 °C; 1H NMR (400 MHz, CDCl3) δ 7.50 (t, J = 1.8 Hz, 1H), 7.39 (dt, J = 7.8, 1.6 Hz, 1H), 7.28–7.25 (m, 1H), 7.19 (t, J = 7.7 Hz, 1H), 5.66 (s, 1H), 5.02 (s, 2H), 4.23–4.09 (m, 2H), 2.98–2.91 (m, 1H), 2.83–2.77 (m, 1H), 2.37 (s, 1H), 2.35–2.25 (m, 1H), 2.14–2.08 (m, 1H), 2.04–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.6, 4.6 Hz, 1H), 1.67–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.37–1.30 (m, 2H), 1.35 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.15–1.14 (m, 9H), 1.10–0.99 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.97, 176.52, 169.69, 166.96, 141.26, 131.18, 130.53, 129.91, 128.60, 126.61, 122.37, 74.41, 61.46, 60.47, 55.62, 48.55, 45.49, 43.99, 43.43, 41.23, 40.46, 39.13, 37.86, 37.07, 32.52, 31.97, 31.27, 28.73, 28.46, 27.58, 26.62, 26.55, 23.52, 23.47, 18.74, 18.38, 18.30, 15.82, 14.48. Exact mass calcd for C39H54BrNNaO4 [M+Na]+: 702.3128, Found: 702.3108.
Product 15 was a white solid in 79% yield, Mp: 190–192 °C; 1H NMR (400 MHz, CDCl3) δ 7.52 (dd, J = 7.9, 1.2 Hz, 1H), 7.39 (dd, J = 7.7, 1.7 Hz, 1H), 7.29 (dd, J = 7.5, 1.2 Hz, 1H), 7.12 (td, J = 7.7, 1.8 Hz, 1H), 5.66 (s, 1H), 5.14 (s, 2H), 4.23–4.08 (m, 2H), 3.03–2.97 (m, 1H), 2.84–2.79 (m, 1H), 2.39–2.30 (m, 2H), 2.13–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.94–1.89 (m, 1H), 1.82 (td, J = 13.7, 4.6 Hz, 1H), 1.69–1.57 (m, 4H), 1.53–1.37 (m, 3H), 1.37–1.29 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.15–1.14 (m, 9H), 1.08–0.99 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.94, 176.49, 169.67, 166.95, 138.28, 132.51, 129.45, 128.75, 128.58, 127.21, 122.73, 74.59, 61.45, 60.45, 55.53, 48.52, 45.46, 43.96, 43.41, 41.22, 40.38, 39.19, 37.85, 37.05, 32.48, 31.95, 31.24, 28.72, 28.44, 27.64, 26.60, 26.53, 23.51, 23.45, 18.72, 18.38, 18.32, 15.82, 14.47. Exact mass calcd for C39H54BrNNaO4 [M+Na]+: 702.3128, Found: 702.3114.
Product 16 was a white solid in 88% yield, Mp: 153–155 °C; 1H NMR (400 MHz, CDCl3) δ 7.62–7.60 (m, 2H), 7.43–7.41 (m, 2H), 5.65 (s, 1H), 5.09 (s, 2H), 4.22–4.07 (m, 2H), 2.96–2.90 (m, 1H), 2.84–2.78 (m, 1H), 2.36–2.27 (m, 2H), 2.13–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.93–1.88 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.67–1.55 (m, 4H), 1.52–1.37 (m, 3H), 1.36–1.29 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.19 (s, 3H), 1.13 (s, 6H), 1.11 (s, 3H), 1.06–0.99 (m, 3H), 1.28 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.89, 176.48, 169.79, 167.20, 144.62, 132.14, 128.55, 128.27, 119.10, 111.15, 74.20, 61.42, 60.44, 55.50, 48.51, 45.44, 43.96, 43.41, 41.22, 40.41, 39.09, 37.83, 37.02, 32.44, 31.94, 31.23, 28.71, 28.43, 27.61, 26.59, 26.52, 23.50, 23.44, 18.70, 18.37, 18.29, 15.81, 14.46. Exact mass calcd for C40H54N2NaO4 [M+Na]+: 649.3976, Found: 649.3957.
Product 17 was a white solid in 74% yield, Mp: 133–135 °C; 1H NMR (400 MHz, CDCl3) δ 7.64–7.63 (m, 1H), 7.58–7.41 (m, 2H), 7.43 (t, J = 7.7 Hz, 1H), 5.65 (s, 1H), 5.06 (s, 2H), 4.22–4.07 (m, 2H), 2.95–2.88 (m, 1H), 2.82–2.77 (m, 1H), 2.36 (s, 1H), 2.34–2.25 (m, 1H), 2.12–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.93–1.88 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.69–1.56 (m, 4H), 1.52–1.37 (m, 3H), 1.34 (s, 3H), 1.36–1.28 (m, 2H), 1.25 (t, J = 7.1 Hz, 3H), 1.19 (s, 3H), 1.13 (s, 9H), 1.09–0.98 (m, 3H), 1.03 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.89, 176.49, 169.73, 167.21, 140.58, 132.37, 131.61, 131.15, 129.10, 128.55, 119.06, 112.32, 73.98, 61.40, 60.44, 55.52, 48.51, 45.45, 43.95, 43.40, 41.20, 40.44, 39.06, 37.83, 37.01, 32.45, 31.94, 31.23, 28.70, 28.43, 27.59, 26.59, 26.52, 23.53, 23.44, 18.70, 18.36, 18.28, 15.78, 14.46. Exact mass calcd for C40H54N2NaO4 [M+Na]+: 649.3976, Found: 649.3965.
Product 18 was a white solid in 88% yield, Mp: 169–171 °C; 1H NMR (400 MHz, CDCl3) δ 7.64 (dd, J = 7.8, 1.2 Hz, 1H), 7.59–7.51 (m, 2H), 7.36 (td, J = 7.4, 1.7 Hz, 1H), 5.65 (s, 1H), 5.24 (s, 2H), 4.23–4.08 (m, 2H), 2.98–2.92 (m, 1H), 2.82–2.76 (m, 1H), 2.37 (s, 1H), 2.35–2.28 (m, 1H), 2.13–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.94–1.89 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.66–1.56 (m, 4H), 1.51–1.36 (m, 3H), 1.36–1.30 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.18 (s, 3H), 1.13 (d, J = 1.5 Hz, 9H), 1.07–1.00 (m, 3H), 1.03 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.96, 176.53, 169.73, 167.33, 142.66, 132.79, 132.72, 129.23, 128.58, 127.94, 117.58, 111.77, 72.72, 61.44, 60.48, 55.48, 48.54, 45.48, 43.98, 43.43, 41.23, 40.43, 39.12, 37.86, 37.04, 32.48, 31.97, 31.26, 28.73, 28.46, 27.61, 26.62, 26.55, 23.50, 23.46, 18.72, 18.38, 18.27, 15.82, 14.48. Exact mass calcd for C40H54N2NaO4 [M+Na]+: 649.3976, Found: 649.3961.
Product 19 was a white solid in 99% yield, Mp: 148–150 °C; 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 8.1 Hz, 2H), 7.45 (d, J = 8.0 Hz, 2H), 5.66 (s, 1H), 5.10 (s, 2H), 4.22–4.08 (m, 2H), 2.98–2.92 (m, 1H), 2.84–2.78 (m, 1H), 2.37–2.27 (m, 2H), 2.13–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.94–1.99 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.70–1.56 (m, 4H), 1.52–1.38 (m, 3H), 1.37–1.30 (m, 2H), 1.37 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.14 (d, J = 1.2 Hz, 9H), 1.06–1.00 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 199.94, 176.50, 169.73, 166.93, 143.05, 129.62 (q, J = 32.3 Hz), 128.59, 128.07, 125.23 (q, J = 3.9 Hz), 121.68 (q, J = 272.0 Hz), 74.44, 61.45, 60.46, 55.57, 48.54, 45.47, 43.98, 43.42, 41.23, 40.42, 39.12, 37.86, 37.05, 32.49, 31.96, 31.25, 28.72, 28.45, 27.59, 26.61, 26.54, 23.54, 23.44, 18.73, 18.38, 18.28, 15.80, 14.47. 19F NMR (377 MHz, CDCl3) δ-62.41. Exact mass calcd for C40H54F3NNaO4 [M+Na]+: 692.3897, Found: 692.3880.
Product 20 was a white solid in 63% yield, Mp: 120–122 °C; 1H NMR (400 MHz, CDCl3) δ 7.61–7.55 (m, 4H), 7.45–7.01 (m, 4H), 7.35–7.31 (m, 1H), 5.66 (s, 1H), 5.11 (s, 2H), 4.23–4.09 (m, 2H), 3.02–2.96 (m, 1H), 2.83–2.77 (m, 1H), 2.36 (s, 1H), 2.34–2.26 (m, 1H), 2.14–2.08 (m, 1H), 2.06–1.97 (m, 2H), 1.94–1.98 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.70–1.58 (m, 4H), 1.53–1.37 (m, 3H), 1.35–1.30 (m, 2H),1.32 (s, 3H) 1.27 (t, J = 7.1 Hz, 3H), 1.22 (s, 3H), 1.19 (s, 3H), 1.15 (s, 6H), 1.07–0.99 (m, 3H), 1.07 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.97, 176.49, 169.62, 166.61, 141.12, 140.48, 137.75, 128.85, 128.73, 128.57, 127.31, 127.21, 127.06, 75.09, 61.45, 60.45, 55.63, 48.51, 45.47, 43.96, 43.39, 41.20, 40.42, 39.14, 37.84, 37.06, 32.51, 31.94, 31.24, 28.71, 28.45, 27.56, 26.59, 26.53, 23.57, 23.41, 18.73, 18.36, 18.28, 15.80, 14.47. Exact mass calcd for C45H59NNaO4 [M+Na]+: 700.4336, Found: 700.4331.
Product 21 was a white solid in 99% yield, Mp: 127–129 °C; 1H NMR (400 MHz, CDCl3) δ 7.84–7.80 (m, 4H), 7.52–7.48 (m, 1H), 7.47–7.43 (m, 2H), 5.66 (s, 1H), 5.23 (s, 2H), 4.23–4.09 (m, 2H), 3.04–2.98 (m, 1H), 2.85–2.79 (m, 1H), 2.36–2.28 (m, 2H), 2.14–2.09 (m, 1H), 2.06–1.97 (m, 2H), 1.96–1.90 (m, 1H), 1.82 (td, J = 13.7, 4.7 Hz, 1H), 1.74–1.57 (m, 4H), 1.53–1.37 (m, 3H), 1.38–1.30 (m, 2H), 1.33 (s, 3H), 1.27 (t, J = 7.1 Hz, 3H), 1.22 (s, 3H), 1.18 (s, 3H), 1.14 (d, J = 3.4 Hz, 6H), 1.11–0.98 (m, 3H), 1.07 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.94, 176.46, 169.62, 166.45, 136.28, 133.37, 132.98, 128.54, 128.03, 127.90, 127.74, 126.87, 126.38, 125.99, 125.79, 75.43, 61.42, 60.41, 55.55, 48.49, 45.43, 43.93, 43.36, 41.18, 40.37, 39.13, 37.82, 37.03, 32.46, 31.91, 31.21, 28.68, 28.41, 27.56, 26.55, 26.50, 23.54, 23.41, 18.68, 18.33, 18.24, 15.78, 14.44. Exact mass calcd for C43H57NNaO4 [M+Na]+: 674.4180, Found: 674.4157.
Product 22 was a white solid in 99% yield, Mp: 178–150 °C; 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (m, 1H), 7.87–7.85 (m, 1H), 7.82–7.79 (m, 1H), 7.54–7.49 (m, 3H), 7.47–7.42 (m, 1H), 5.65 (s, 1H), 5.53 (d, J = 2.0 Hz, 2H), 4.23–4.08 (m, 2H), 2.98–2.91 (m, 1H), 2.76–2.70 (m, 1H), 2.35 (s, 1H), 2.31–2.23 (m, 1H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.82 (td, J = 13.7, 4.6 Hz, 1H), 1.68–1.57 (m, 4H), 1.52–1.37 (m, 3H), 1.34–1.29 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.19 (d, J = 2.4 Hz, 6H), 1.14 (d, J = 2.8 Hz, 6H), 1.07–0.96 (m, 3H), 1.07 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.94, 176.49, 169.60, 166.62, 134.19, 133.77, 132.00, 128.58, 128.55, 128.51, 126.79, 126.12, 125.75, 125.38, 124.42, 73.84, 61.45, 60.45, 55.56, 48.53, 45.46, 43.97, 43.40, 41.21, 40.39, 39.19, 37.85, 37.05, 32.51, 31.95, 31.25, 28.72, 28.45, 27.61, 26.59, 26.54, 23.56, 23.45, 18.72, 18.37, 18.22, 15.80, 14.47. Exact mass calcd for C43H57NNaO4 [M+Na]+: 674.4180, Found: 674.4167.
Product 23 was a white solid in 98% yield, Mp: 139–141 °C; 1H NMR (400 MHz, CDCl3) δ 6.98 (s, 2H), 6.91 (s, 1H), 5.65 (s, 1H), 4.99 (s, 2H), 4.21–4.10 (m, 2H), 2.99–2.93 (m, 1H), 2.81–2.75 (m, 1H), 2.37 (s, 1H),2.33–2.25 (m, 1H), 2.31 (s, 6H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.6, 4.6 Hz, 1H), 1.71–1.57 (m, 4H), 1.53–1.38 (m, 3H), 1.37–1.29 (m, 2H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.18 (s, 3H), 1.14 (d, J = 1.5 Hz, 6H), 1.09–1.00 (m, 3H), 1.06 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.98, 176.51, 169.62, 166.25, 138.39, 137.79, 129.23, 128.60, 126.13, 75.50, 61.49, 60.45, 55.59, 48.54, 45.48, 43.97, 43.41, 41.21, 40.36, 39.17, 37.85, 37.06, 32.53, 31.96, 31.25, 28.72, 28.45, 27.62, 26.61, 26.54, 23.53, 23.44, 21.42, 18.73, 18.38, 18.25, 15.81, 14.47. Exact mass calcd for C41H59NNaO4 [M+Na]+: 652.4336, Found: 652.4318.
Product 24 was a white solid in 82% yield, Mp: 137–139 °C; 1H NMR (400 MHz, CDCl3) δ 6.87–6.82 (m, 2H), 6.69 (tt, J = 9.0, 2.4 Hz, 1H), 5.66 (s, 1H), 5.02 (s, 2H), 4.23–4.08 (m, 2H), 2.98–2.92 (m, 1H), 2.85–2.79 (m, 1H), 2.37 (s, 1H), 2.35–2.26 (m, 1H), 2.13–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.7, 4.6 Hz, 1H), 1.69–1.56 (m, 4H), 1.53–1.37 (m, 3H), 1.37–1.29 (m, 2H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.14 (d, J = 2.4 Hz, 9H), 1.07–1.00 (m, 3H), 1.05 (s, 3H) 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 198.93, 175.52, 168.71, 166.11, 162.09 (d, J = 248.9 Hz),161.97 (d, J = 247.8 Hz), 142.22 (t, J = 8.7 Hz), 127.59, 109.37 (d, J = 24.9 Hz), 109.37 (d, J = 11.7 Hz), 101.68 (t, J = 25.4 Hz), 72.98, 60.44, 59.47, 54.60, 47.54, 44.48, 42.98, 42.43, 40.23, 39.44, 38.10, 36.86, 36.06, 31.50, 30.96, 30.26, 27.73, 27.45, 26.57, 25.62, 25.55, 22.55, 22.45, 17.74, 17.38, 17.30, 14.79, 13.48. 19F NMR (377 MHz, CDCl3) δ -110.38. Exact mass calcd for C39H53F2NNaO4 [M+Na]+: 660.3835, Found: 660.3819.
Product 25 was a white solid in 83% yield, Mp: 131–133 °C; 1H NMR (400 MHz, CDCl3) δ 7.19–7.12 (m, 1H), 7.10–7.02 (m, 2H), 5.64 (s, 1H), 4.97 (s, 2H), 4.22–4.07 (m, 2H), 2.94–2.88 (m, 1H), 2.82–2.76 (m, 1H), 2.36 (s, 1H), 2.32–2.23 (m, 1H), 2.12–2.07 (m, 1H), 2.06–1.96 (m, 2H), 1.93–1.88 (m, 1H), 1.82 (td, J = 13.6, 4.5 Hz, 1H), 1.76–1.56 (m, 4H), 1.52–1.37 (m, 3H), 1.36–1.28 (m, 2H), 1.34 (s, 3H), 1.25 (t, J = 7.1, 1.1 Hz, 3H), 1.19 (s, 3H), 1.13 (s, 9H), 1.07–0.99 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 199.89, 176.47, 169.70, 166.85, 150.21 (dd, J = 248.5, 12.9 Hz), 149.80 (dd, J = 248.2, 12.5 Hz), 135.98 (dd, J = 5.3, 4.2 Hz), 128.54, 124.00 (dd, J = 6.5, 3.6 Hz), 117.01 (dd, J = 17.3, 12.3 Hz), 74.00, 61.41, 60.42, 55.54, 48.50, 45.43, 43.94, 43.38, 41.19, 40.39, 39.06, 37.82, 37.01, 32.45, 31.92, 31.21, 28.69, 28.41, 27.56, 26.57, 26.50, 23.52, 23.40, 18.69, 18.35, 18.23, 15.76, 14.44. 19F NMR (377 MHz, CDCl3): δ-138.34 (d, J = 20.8 Hz, 1F), -139.99 (d, J = 20.8 Hz, 1F). Exact mass calcd for C39H53F2NNaO4 [M+Na]+: 660.3835, Found: 660.3833.
Product 26 is a white solid in 69% yield, Mp: 162–164 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 2.0 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.17 (dd, J = 8.2, 2.0 Hz, 1H), 5.65 (s, 1H), 4.99 (s, 2H), 4.22–4.08 (m, 2H), 2.95–2.89 (m, 1H), 2.83–2.77 (m, 1H), 2.36 (s, 1H), 2.33–2.24 (m, 1H), 2.13–2.08 (m, 1H), 2.05–1.96 (m, 2H), 1.94–1.89 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.66–1.56 (m, 4H), 1.52–1.36 (m, 3H), 1.34–1.28 (m, 2H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.14 (s, 9H), 1.05–0.99 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.95, 176.52, 169.74, 167.11, 139.29, 132.27, 131.36, 130.28, 130.08, 128.57, 127.39, 73.79, 61.44, 60.47, 55.60, 48.53, 45.48, 43.98, 43.42, 41.22, 40.47, 39.11, 37.85, 37.05, 32.49, 31.96, 31.25, 28.72, 28.45, 27.57, 26.61, 26.54, 23.52, 23.46, 18.73, 18.37, 18.29, 15.81, 14.47. HRMS (ESI): Exact mass calcd for C39H53Cl2NNaO4 [M+Na]+: 692.3244, Found: 692.3221.
Product 27 was a white solid in 95% yield, Mp: 142–144 °C; 1H NMR (400 MHz, CDCl3) δ 7.55 (t, J = 1.8 Hz, 1H), 7.41 (d, J = 1.8 Hz, 2H), 5.66 (s, 1H), 4.98 (s, 2H), 4.23–4.08 (m, 2H), 2.96–2.90 (m, 1H), 2.84–2.79 (m, 1H), 2.38 (s, 1H), 2.34–2.26 (m, 1H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.95–1.89 (m, 1H), 1.83 (td, J = 13.7, 4.6 Hz, 1H), 1.67–1.56 (m, 4H), 1.52–1.38 (m, 3H), 1.37–1.30 (m, 2H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.15–1.12 (m, 9H), 1.08–0.99 (m, 3H), 1.05 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.94, 176.53, 169.73, 167.41, 143.09, 132.96, 129.73, 128.60, 122.79, 73.60, 61.46, 60.48, 55.64, 48.55, 45.49, 43.99, 43.44, 41.23, 40.54, 39.13, 37.86, 37.08, 32.50, 31.97, 31.27, 28.73, 28.47, 27.58, 26.63, 26.56, 23.49, 23.48, 18.74, 18.38, 18.35, 15.84, 14.49. Exact mass calcd for C39H53Br2NNaO4 [M+Na]+: 780.2234, Found: 780.2198.
Product 28 was a white solid in 84% yield, Mp: 147–149 °C; 1H NMR (400 MHz, CDCl3) δ 7.28–7.25 (m, 1H), 7.23–7.22 m, 1H), 7.12–7.10 (m, 1H), 5.65 (s, 1H), 5.05 (s, 2H), 4.23–4.08 (m, 2H), 2.97–2.91 (m, 1H), 2.81–2.75 (m, 1H), 2.37 (s, 1H), 2.31–2.22 (m, 1H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.6, 4.8 Hz, 1H), 1.70–1.57 (m, 4H), 1.54–1.38 (m, 3H), 1.35–1,30 (m, 2H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.18 (s, 3H), 1.14 (s, 6H), 1.06–1.01 (m, 3H), 1.06 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.95, 176.48, 169.63, 166.36, 139.73, 128.57, 127.96, 125.50, 123.14, 70.48, 61.43, 60.44, 55.57, 48.51, 45.46, 43.95, 43.40, 41.19, 40.36, 39.10, 37.83, 37.03, 32.50, 31.94, 31.23, 28.70, 28.43, 27.58, 26.59, 26.52, 23.57, 23.44, 18.71, 18.35, 18.18, 15.77, 14.45. HRMS (ESI): Exact mass calcd for C37H53NNaO3S [M+Na]+: 630.3588, Found: 630.3571.
Product 29 is a white solid in 53% yield, Mp: 131–133 °C; 1H NMR (400 MHz, CDCl3) δ 7.44 (t, J = 2 Hz, 1H), 5.65 (s, 1H), 5.09 (d, J = 0.8 Hz, 2H), 4.23–4.08 (m, 2H), 2.89–2.77 (m, 2H), 2.36 (s, 1H), 2.28–2.20 (m, 2H), 2.13–2.08 (m, 1H), 2.07–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.7, 4.6 Hz, 1H), 1.60–1.58L (m, 7H), 1.51–1.38 (m, 4H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.21 (d, J = 2.6 Hz, 6H), 1.14 (d, J = 3.9 Hz, 6H), 1.08 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.95, 176.54, 169.81, 167.84, 152.82, 139.76, 137.96, 128.58, 67.14, 61.42, 60.49, 55.60, 48.55, 45.49, 44.00, 43.45, 41.23, 40.66, 39.02, 37.86, 37.05, 32.51, 31.97, 31.27, 28.74, 28.46, 27.61, 26.63, 26.55, 23.59, 23.48, 18.75, 18.37, 18.33, 15.81, 14.48. HRMS (ESI): Exact mass calcd for C36H51ClN2NaO4S [M+Na]+: 665.3150, Found: 665.3135.
Product 30 was a white solid in 85% yield, Mp: 174–176 °C; 1H NMR (400 MHz, CDCl3) δ 6.01 (s, 1H), 5.64 (s, 1H), 4.96 (s, 2H), 4.20–4.09 (m, 2H), 3.82 (s, 3H), 2.90–2.84 (m, 1H), 2.80–2.74 (m, 1H), 2.35 (s, 1H), 2.22 (s, 3H), 2.13–2.11 (m, 1H), 2.09–1.96 (m, 2H), 1.93–1.88 (m, 1H), 1.86–1.76 (m, 2H), 1.68–1.56 (m, 3H), 1.49–1.35 (m, 4H), 1.35–1.29 (m, 2H), 1.35 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H), 1.19 (s, 3H), 1.13 (d, J = 3.3 Hz, 9H), 1.04–0.97 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H).13C NMR (100 MHz, CDCl3) δ 199.91, 176.46, 169.71, 167.85, 166.64, 160.23, 128.50, 111.84, 63.64, 61.40, 60.41, 55.54, 48.48, 45.43, 43.93, 43.39, 41.17, 40.36, 39.12, 37.81, 37.03, 32.45, 31.91, 31.20, 28.68, 28.40, 27.38, 26.56, 26.49, 23.48, 23.44, 18.69, 18.30, 17.95, 15.76, 14.43, 11.25, 10.28. Exact mass calcd for C38H57N3NaO4 [M+Na]+: 642.4241, Found: 642.4234.
Product 31 was a white solid in 90% yield, Mp: 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 5.63 (s, 1H), 4.76 (s, 2H), 4.21–4.06 (m, 2H), 2.86–2.80 (m, 1H), 2.78–2.73 (m, 1H), 2.38 (s, 3H), 2.34 (s, 1H), 2.26 (s, 3H), 2.22–1.13 (m, 1H), 2.11–2.06 (m, 1H), 2.02–1.95 (m, 2H), 1.92–1.87 (m, 1H), 1.83–1.79 (m, 2H), 1.68–1.54 (m, 3H), 1.50–1.36 (m, 3H), 1.33–1.28 (m, 2H), 1.33 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H), 1.17 (s, 3H), 1.11 (d, J = 5.9 Hz, 9H), 1.02–0.94 (m, 3H), 1.01 (s, 3H), 0.79 (s, 3H).13C NMR (100 MHz, CDCl3) δ 199.91, 176.46, 169.71, 167.85, 166.64, 160.23, 128.50, 111.84, 63.64, 61.40, 60.41, 55.54, 48.48, 45.43, 43.93, 43.39, 41.17, 40.36, 39.12, 37.81, 37.03, 32.45, 31.91, 31.20, 28.68, 28.40, 27.38, 26.56, 26.49, 23.48, 23.44, 18.69, 18.30, 17.95, 15.76, 14.43, 11.25, 10.28. Exact mass calcd for C38H56N2NaO5 [M+Na]+: 643.4081, Found: 643.4071.
Product 32 was a white solid in 54% yield, Mp: 159–161 °C; 1H NMR (400 MHz, CDCl3) δ 7.14 (d, J = 3.5 Hz, 1H), 6.42 (d, J = 3.5 Hz, 1H), 5.65 (s, 1H), 5.02 (s, 2H), 4.21–4.10 (m, 2H), 3.89 (s, 3H), 2.93–2.87 (m, 1H), 2.81–2.75 (m, 1H), 2.36 (s, 1H), 2.30–2.21 (m, 1H), 2.13–2.08 (m, 1H), 2.06–1.97 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.6, 4.6 Hz, 1H), 1.60–1.56 (m, 6H), 1.47–1.37 (m, 3H), 1.34 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.20 (s, 3H), 1.14 (d, J = 3.2 Hz, 9H), 1.07–0.99 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.95, 176.53, 169.69, 167.18, 159.37, 157.02, 144.02, 128.60, 119.08, 111.02, 67.33, 61.43, 60.49, 55.55, 52.03, 48.56, 45.49, 43.99, 43.43, 41.24, 40.44, 39.05, 37.87, 37.04, 32.52, 31.98, 31.27, 28.74, 28.47, 27.56, 26.63, 26.56, 23.59, 23.47, 18.75, 18.38, 18.22, 15.79, 14.49. Exact mass calcd for C39H55NNaO7 [M+Na]+: 672.3871, Found: 672.3844.
Product 33 was a white solid in 60% yield, Mp: 134–136 °C; 1H NMR (400 MHz, CDCl3) δ 7.12 (d, J = 3.5 Hz, 1H), 6.40 (d, J = 3.4 Hz, 1H), 5.64 (s, 1H), 5.02 (s, 2H), 4.35 (q, J = 7.1 Hz, 2H), 4.22–4.08 (m, 2H), 2.93–2,87 (m, 1H), 2.80–2,74 (m, 1H), 2.36 (s, 1H), 2.29–2.20 (m, 1H), 2.12–2.07 (m, 1H), 2.06–1.96 (m, 2H), 1.94–1.88 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.65–1.56 (m, 3H), 1.49–1.39 (m, 2H), 1.38–1.34 (m, 7H), 1.28–1.24 (m, 6H), 1.19 (s, 3H), 1.14 (d, J = 4.7 Hz, 9H), 1.07–0.99 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H).13C NMR (100 MHz, CDCl3) δ 199.94, 176.51, 169.67, 167.11, 158.99, 156.86, 144.31, 128.58, 118.81, 110.95, 61.41, 61.02, 60.46, 55.54, 48.54, 45.47, 43.97, 43.42, 41.22, 40.43, 39.03, 37.85, 37.02, 32.50, 31.96, 31.25, 29.83, 28.72, 28.45, 27.54, 26.61, 26.54, 23.58, 23.45, 18.73, 18.36, 18.20, 15.77, 14.50, 14.47. Exact mass calcd for C40H57NNaO7 [M+Na]+: 686.4027, Found: 686.4010.
Product 34 is a white solid in 48% yield, Mp: 164–166 °C; 1H NMR (400 MHz, CDCl3) δ 5.65 (s, 1H), 4.23–4.08 (m, 2H), 3.99–3.95 (m, 2H), 3.89–3.85 (m, 2H), 3.43–3.36 (m, 2H), 2.93–2.87 (m, 1H), 2.82–2.76 (m, 1H), 2.38 (s, 1H), 2.30–2.22 (m, 1H), 2.13–2.08 (m, 1H), 2.06–1.95 (m, 2H), 1.94–1.89 (m, 1H), 1.83 (td, J = 13.6, 4.7 Hz, 1H), 1.63–1.60 (m, 8H), 1.44–1.36 (m, 3H), 1.35–1.30 (m, 3H), 1.35 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H), 1.22 (s, 3H), 1.16 (s, 3H), 1.14 (d, J = 3.7 Hz, 6H), 1.10–1.00 (m, 3H), 1.06 (s, 3H), 0.81 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 200.04, 176.55, 169.73, 165.71, 128.60, 78.02, 67.92, 61.49, 60.49, 55.61, 48.55, 45.51, 44.00, 43.44, 41.24, 40.26, 39.20, 37.87, 37.10, 35.19, 32.53, 31.97, 31.27, 30.02, 28.74, 28.47, 27.65, 26.63, 26.56, 23.60, 23.47, 18.75, 18.42, 18.01, 15.82, 14.48. HRMS (ESI): Exact mass calcd for C38H59NNaO5 [M+Na]+: 632.4285, Found: 632.4273.
Product 35 was a white solid in 89% yield, Mp: 185–187 °C; 1H NMR (400 MHz, CDCl3) δ 5.65 (s, 1H), 4.63–4.60 (m, 2H), 4.32 (dd, J = 5.8, 1.2 Hz, 2H), 4.22–4.08 (m, 4H), 2.93–2.86 (m, 1H), 2.82–2.76 (m, 1H), 2.37 (s, 1H), 2.30–2.22 (m, 1H), 2.12–2.08 (m, 1H), 2.06–1.96 (m, 2H), 1.93–1.88 (m, 1H), 1.82 (td, J = 13.6, 4.6 Hz, 1H), 1.71–1.56 (m, 4H), 1.52–1.37 (m, 3H), 1.34 (s, 3H), 1.33–1.30 (m, 2H), 1.33 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H), 1.21 (s, 3H), 1.13 (d, J = 3.0 Hz, 9H), 1.05–0.99 (m, 3H), 1.04 (s, 3H), 0.80 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 199.93, 176.50, 169.69, 166.20, 128.58, 80.74, 80.66, 77.83, 61.43, 60.45, 55.55, 48.52, 45.47, 43.97, 43.42, 41.22, 40.37, 40.23, 39.12, 37.84, 37.07, 32.49, 31.95, 31.24, 28.72, 28.44, 27.66, 26.61, 26.54, 23.54, 23.45, 21.65, 18.72, 18.40, 17.97, 15.79, 14.46. Exact mass calcd for C37H57NNaO5 [M+Na]+: 618.4129, Found: 618.4118.
3.4. Inhibitory Effects on HIV-1 Protease
The enzyme activity of HIV-1 protease was assessed using a FRET assay, following the guidelines provided by the manufacturer. Briefly, HIV-1 protease activity was analyzed using 384-well microplates with a well volume of 120 µL. Recombinant HIV-1 protease (8 µL) was mixed with 2 µL of test compound (0.01~1.0 mg/mL). The reactions were initiated by adding 10 µL of HIV protease substrate (diluted 50 times with assay buffer). Fluorescence measurement was conducted using an EX/Em = 490/520 nm filter module on a Synergy II microplate reader. The inhibition rate was subsequently calculated using Microsoft Office Excel 2019 and SPSS software (version 19), followed by statistical analysis.
3.5. Cell Culture
Human cancer cell lines K562 and Hela and mouse cancer cell lines CT26 Cells were cultured aseptically in RPMI-1640 media with 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin at 37 °C with 5% CO2. All cells used in the experiment were in the logarithmic growth stage.
3.6. MTT Assay
A total of 5 × 103 logarithmically growing cells were inoculated into each well of a 96-well plate and incubated for 24 h at 37 °C with 5% CO2. Subsequently, various concentrations of compounds were added to each well and incubated for an additional 48 h. Next, 10 µL of MTT reagent (5 mg/mL) was added to each well to react with the mitochondria of viable cells for approximately 4 h. Finally, the medium was discarded, and the blue crystals were completely dissolved in 150 µL of DMSO. Absorbance at a wavelength of 490 nm was measured using a microplate reader. The concentration of compounds that resulted in a 50% inhibition of cell growth (IC50) was calculated using IBM SPSS Statistics (version 19). The IC50 value for each compound was determined in a minimum of three independent experiments.
3.7. Docking Studies
Molecular docking was conducted using the ligand docking module within the Schrödinger Suite (Schrödinger, LLC, New York City, NY, USA). The crystal structures of HIV-1 protease (PDB ID: 1QBS) were selected for docking. Prior to docking, protein preparation was performed using the protein preparation wizard, which involved assigning bond orders, adding hydrogen atoms, and eliminating water molecules. Subsequently, the protein structure was energy-minimized through restrained minimization employing the OPLS3 force field. The docking grid was generated at the orthosteric site, which was identified by selecting the original co-crystalized ligand using the receptor grid generation module. For the small-molecule compounds, 3D structures were generated and subjected to energy minimization using the LigPrep module. The ligand docking module was employed to dock the compound molecules onto the orthosteric site of HIV-1 protease, with the choice of Glide XP for docking precision.
4. Conclusions
In this study, a total of 32 derivatives of glycyrrhetinic acid were designed and synthesized. All compounds were characterized by 1H NMR, 13C NMR, and HRMS. Towards HIV-1 protease, compounds 28 and 32 exhibited potent inhibitory activities, with corresponding inhibition rates of 76% and 70.5%, respectively, at 1 mg/mL concentration. Further molecular docking implies that a 3-substituted polar group would provide GA derivatives with better inhibition of HIV-1 protease. We also evaluated the compounds for their inhibitory effects on the cell viability of the human cancer cell lines K562 and Hela and the mouse cancer cell line CT26 using the MTT assay. The results demonstrated that the introduction of the thiazole heterocycle, as seen in compound 29, significantly improved its anti-proliferative activity against K562 cells with an IC50 value of 8.86 ± 0.93 µM. The introduction of an electron-withdrawing substituent on the benzene ring enhanced anti-proliferative activity against Hela and CT26 cells. Compound 13 exhibited the highest inhibitory activity against Hela cells with the IC50 value of 9.89 ± 0.86 µM, whereas compound 7 demonstrated the most potent inhibition against CT26 cells with the IC50 value of 4.54 ± 0.37 µM. In conclusion, our current study shows that further structural modification of glycyrrhetinic acid is a very promising strategy for developing novel anti-HIV and anticancer therapeutics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241915012/s1.
Author Contributions
Conceptualization, B.-W.P.; methodology, L.-L.Z. and Y.S.; software, B.-W.P. and Z.-C.D.; formal analysis, Y.W. and L.-L.Z.; data curation, Y.W. and T.-T.F.; writing—original draft preparation, B.-W.P.; writing—review and editing, J.Y. and Y.Z.; supervision, Y.W. and B.-W.P.; project administration, Y.Z. and Y.W.; funding acquisition, Y.Z. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guizhou Provincial Basic Research Program (Natural Science) (ZK [2023] general 404 and ZK [2022] general 480), the Innovation Group Project of Guizhou Province (Qian Jiao He KY [2021]018), the 2023 Natural Science Research Project of Guizhou Provincial Department of Education (Qian Jiao Ji [2023] 69), the Key Disciplines of Traditional Chinese Medicine and Ethnic Medicine in Guizhou Province during the 14th Five-Year Plan (QZYYZDXK(JS)-2021-03), and the projects of Guizhou province (Qian Jiao He KY Zi [2022]253, gzwkj2022-232, and gzwkj2022-467).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biondi, D.M.; Rocco, C.; Ruberto, G. Dihydrostilbene derivatives from Glycyrrhiza glabra leaves. J. Nat. Prod. 2005, 68, 1099–1102. [Google Scholar] [CrossRef]
- El-Refai, A.-M.H.; Sallam, L.A.; El-Menoufy, H.A.; Amin, H.A.S. Physiological and chemical studies on the bioconversion of glycyrrhizin by Aspergillus niger NRRL 595. Malays. J. Microbiol. 2012, 8, 75–82. [Google Scholar]
- Nomura, T.; Fukai, T.; Akiyama, T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl. Chem. 2002, 74, 1199–1206. [Google Scholar] [CrossRef]
- Sheng, L.X.; Huang, J.Y.; Liu, C.M.; Zhang, J.Z.; Cheng, K.G. Synthesis of oleanolic acid/ursolic acid/glycyrrhetinic acid-hydrogen sulfide donor hybrids and their antitumor activity. Med. Chem. Res. 2019, 28, 1212–1222. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, Y.J.; Lee, M.S.; Han, E.S.; Lee, S.J. 18β-Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti-cancer drug toxicity. Life Sci. 2008, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Noshita, T.; Yu, T.; Kidachi, Y.; Kamiie, K.; Umetsu, H.; Ryoyama, K. Novel effects of glycyrrhetinic acid on the central nervous system tumorigenic progenitor cells: Induction of actin disruption and tumor cell-selective toxicity. Eur. J. Med. Chem. 2010, 45, 2943–2948. [Google Scholar] [CrossRef]
- Moustafa, G.O.; Shalaby, A.; Naglah, A.M.; Mounier, M.M.; El-Sayed, H.; Anwar, M.M.; Nossier, E.S. Synthesis, Characterization, In Vitro Anticancer Potentiality, and Antimicrobial Activities of Novel Peptide-Glycyrrhetinic-Acid-Based Derivatives. Molecules 2021, 26, 4573–4594. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, M.; Yu, X.; Jin, H.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019, 166, 328–338. [Google Scholar] [CrossRef]
- Ikeda, T.; Yokomizo, K.; Okawa, M.; Tsuchihashi, R.; Kinjo, J.; Nohara, T.; Uyeda, M. Anti-herpes virus type 1 activity of oleanane-type triterpenoids. Biol. Pharm. Bull. 2005, 28, 1779–1781. [Google Scholar] [CrossRef]
- Hoever, G.; Baltina, L.; Michaelis, M.; Kondratenko, R.; Baltina, L.; Tolstikov, G.A.; Doerr, H.W.; Cinatl, J. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005, 48, 1256–1259. [Google Scholar] [CrossRef]
- Yin, M.C.; Chan, K.C. Nonenzymatic Antioxidative and Antiglycative Effects of Oleanolic Acid and Ursolic Acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.B.; Zhao, Z.M.; Ma, Y.; Li, A.P.; Zhang, Z.J.; Hu, Y.M.; Zhou, Y.; Wang, R.; Luo, X.F.; Zhang, B.Q.; et al. Antifungal activity and preliminary mechanism of pristimerin against Sclerotinia sclerotiorum. Ind. Crops Prod. 2022, 185, 115124. [Google Scholar] [CrossRef]
- Zhou, J.X.; Wink, M. Evidence for Anti-Inflammatory Activity of Isoliquiritigenin, 18b-Glycyrrhetinic Acid, Ursolic Acid, and the Traditional Chinese Medicine Plants Glycyrrhiza glabra and Eriobotrya japonica, at the Molecular Level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, N.; Tamamura, H. Peptide-derived mid-sized anti-HIV agents. In Amino Acids, Peptides and Proteins 41; Ryadnov, M., Hudecz, F., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 1–29. [Google Scholar]
- Tamamura, H.; Kobayakawa, T.; Ohashi, N. Mid-size drugs based on peptides and peptidomimetics: A new drug category. In Springer Briefs in Pharmaceutical Science & Drug Development; Springer: Singapore, 2018; pp. 1–100. [Google Scholar]
- Maeda, K.; Das, D.; Kobayakawa, T.; Tamamura, H.; Takeuchi, H. Discovery and development of anti-HIV therapeutic agents: Progress towards improved HIV medication. Curr. Top. Med. Chem. 2019, 19, 1621–1649. [Google Scholar] [CrossRef]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; Clair, M.H.S.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3′-Azido-3′-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human Tlymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Dawson, Z.L.; Mitsuya, H. Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV. Bioorg. Med. Chem. 2007, 15, 7576–7580. [Google Scholar] [CrossRef]
- Cahn, P.; Sued, O. Raltegravir: A new antiretroviral class for salvage therapy. Lancet 2007, 369, 1235–1236. [Google Scholar] [CrossRef]
- Grinsztejn, B.; Nguyen, B.-Y.; Katlama, C.; Gatell, J.M.; Lazzarin, A.; Vittecoq, D.; Gonzalez, C.J.; Chen, J.; Harvey, C.M.; Isaacs, R.D. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: A phase II randomised controlled trial. Lancet 2007, 369, 1261–1269. [Google Scholar] [CrossRef]
- Feldman, D.B.; Corn, B.W. Hope and cancer. Curr. Opin. Psychol. 2023, 49, 101506. [Google Scholar] [CrossRef]
- Satapathy, S.; Patro, C.S.; Patro, G.; Panda, M.; Patra, A. Cancer Therapy. J. Pharm. Negat. Results 2023, 14, 3643–3649. [Google Scholar] [CrossRef]
- Schlemmer, H.-P. The Cancer Epidemic. Die Radiol. 2023, 63, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Mohamed, E.H.; Alghamdi, Y.S.; Mostafa Abdel-Hafez, S.; Soliman, M.M.; Alotaibi, S.H.; Hassan, M.Y.; Hany, N.A.D.; Amer, H.H. Susceptibility Assessment of Multidrug Resistant Bacteria to Natural Products. Dose-Response 2020, 18, 155932582093618. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef]
- Hattori, T.; Ikematsu, S.; Koito, A.; Matsushita, S.; Maeda, Y.; Hada, M.; Fujimaki, M.; Takatsuki, K. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antivir. Res. 1989, 11, 255–261. [Google Scholar] [CrossRef]
- Mori, K.; Sakai, H.; Suzuki, S.; Akutsu, Y.; Ishikawa, M.; Imaizumi, M.; Tada, K.; Aihara, M.; Sawada, Y.; Yokoyama, M.; et al. Effects of glycyrrhizin (SNMC: Stronger Neo-Minophagen C) in hemophilia patients with HIV-1 infection. Tohoku J. Exp. Med. 1990, 162, 183–193. [Google Scholar] [CrossRef][Green Version]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology 2002, 70, 229–236. [Google Scholar] [CrossRef]
- Takahashi, K.; Yoshino, T.; Maki, Y.; Ishiuchi, K.; Namiki, T.; Ogawa-Ochiai, K.; Minamizawa, K.; Makino, T.; Nakamura, T.; Mimura, M.; et al. Identification of glycyrrhizin metabolites in humans and of a potential biomarker of liquoriceinduced pseudoaldosteronism: A multi-centre cross-sectional study. Arch. Toxicol. 2019, 93, 3111–3119. [Google Scholar] [CrossRef]
- Csuk, R.; Schwarz, S.; Kluge, R.; Ströhl, D. Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid. Eur. J. Med. Chem. 2010, 45, 5718–5723. [Google Scholar] [CrossRef]
- Schwarz, S.; Siewert, B.; Xavier, N.M.; Jesus, A.R.; Pauter, A.P.; Csuk, R. A “natural” approach: Synthesis and cytoxicity of monodesmosidic glycyrrhetinic acid glycosides. Eur. J. Med. Chem. 2014, 72, 78–83. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Qiu, R.; Chen, Z.; Chen, Z.; Chen, W. 18 β-glycyrrhetinic acid exhibits potent antitumor effects against colo-rectal cancer via inhibition of cell proliferation and migration. Int. J. Oncol. 2017, 51, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Huang, S.; Su, S.-B. Glycyrrhetinic acid potently suppresses breast cancer invasion and metastasis by impairing the p38 MAPK-AP1 signaling axis. Expert Opin. Ther. Targets 2015, 19, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, V.; Fakhari, S.; Mirzaie, S.; Rahmani, M.; Farhadifar, F.; Pirzadeh, S.; Jalili, A. Glycyrrhetinic Acid Inhibits Cell Growth and Induces Apoptosis in Ovarian Cancer A2780 Cells. Adv. Pharm. Bull. 2014, 4, 437–441. [Google Scholar] [PubMed]
- Pirzadeh, S.; Fakhari, S.; Jalili, A.; Mirzai, S.; Ghaderi, B.; Haghshenas, V. Glycyrrhetinic acid induces apoptosis in leukemic HL60 cells through upregulating of CD95/CD178. Int. J. Mol. Cell. Med. 2014, 3, 272–278. [Google Scholar]
- Logashenko, E.B.; Salomatina, O.V.; Markov, A.V.; Korchagina, D.V.; Salakhutdinov, N.F.; Tolstikov, G.A.; Vlassov, V.V.; Zenkova, M.A. Synthesis and proapoptotic activity of novel glycyrrhetinic acid derivatives. ChemBioChem 2011, 12, 784–794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).