Genome-Wide Identification and Characterization of WRKY Transcription Factors in Betula platyphylla Suk. and Their Responses to Abiotic Stresses
Abstract
:1. Introduction
2. Results
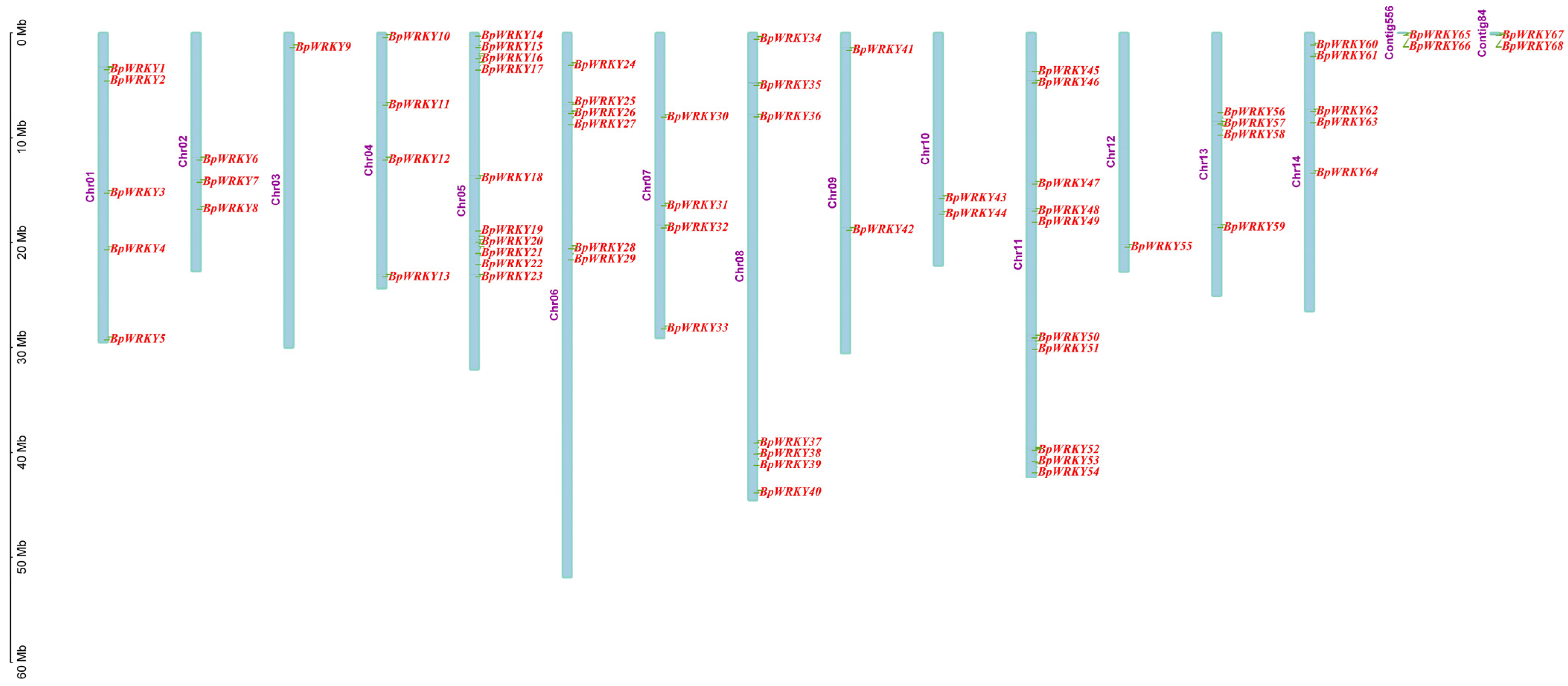
2.1. Identification and Characterization of WRKY Transcription Factors in Betula platyphylla
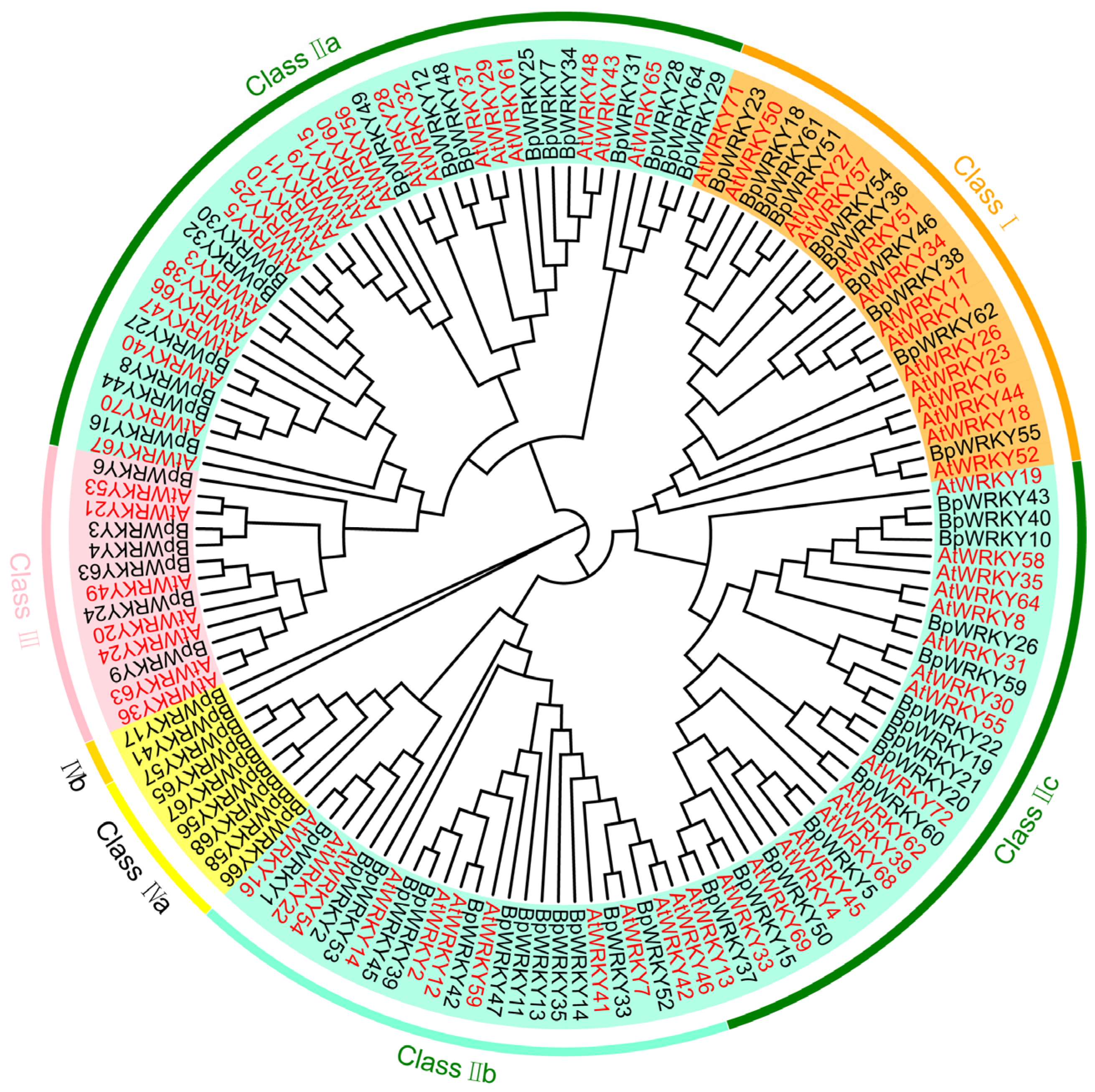
2.2. Phylogenetic Analysis of BpWRKYs
2.3. Gene Structure of BpWRKYs
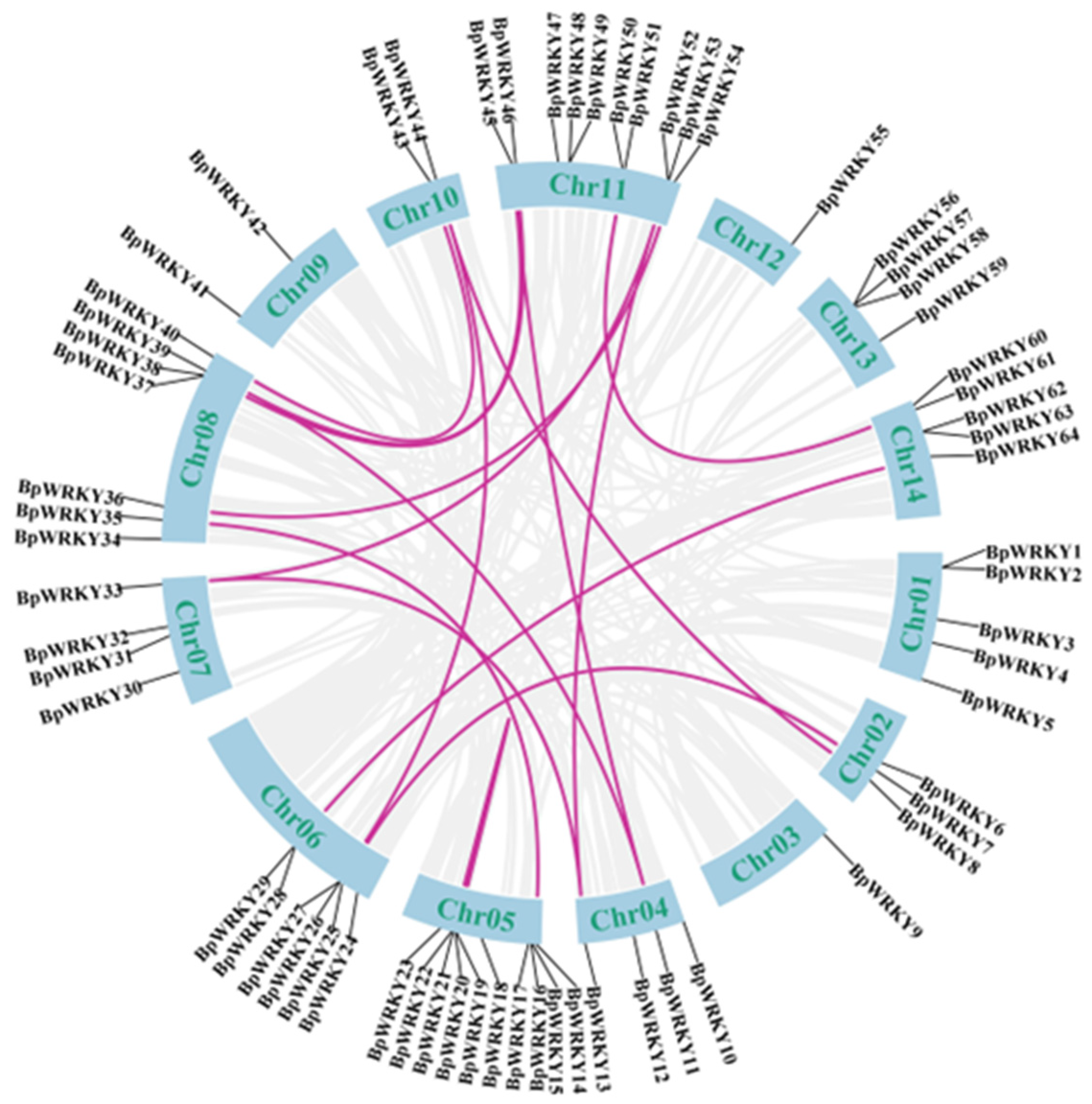
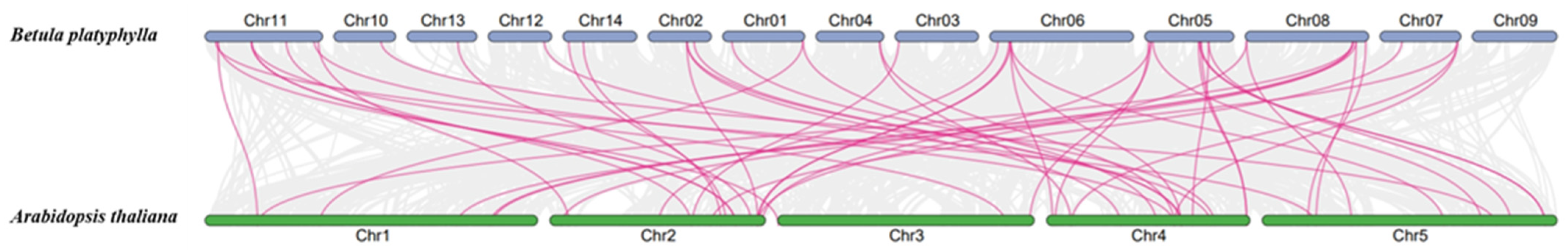
2.4. Synteny Analysis of BpWRKYs
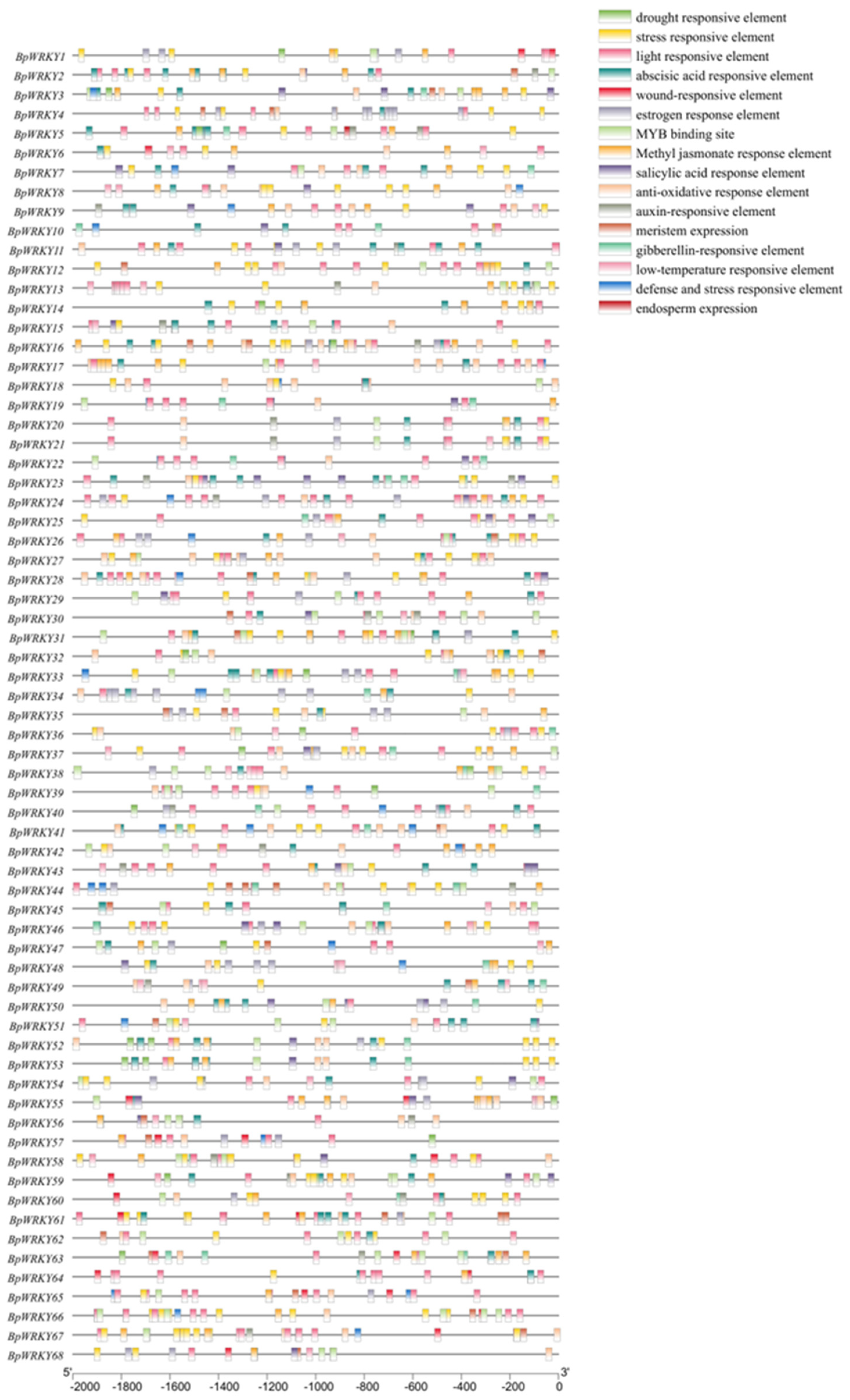
2.5. Analysis of Cis-Acting Elements in the Promoters of BpWRKYs
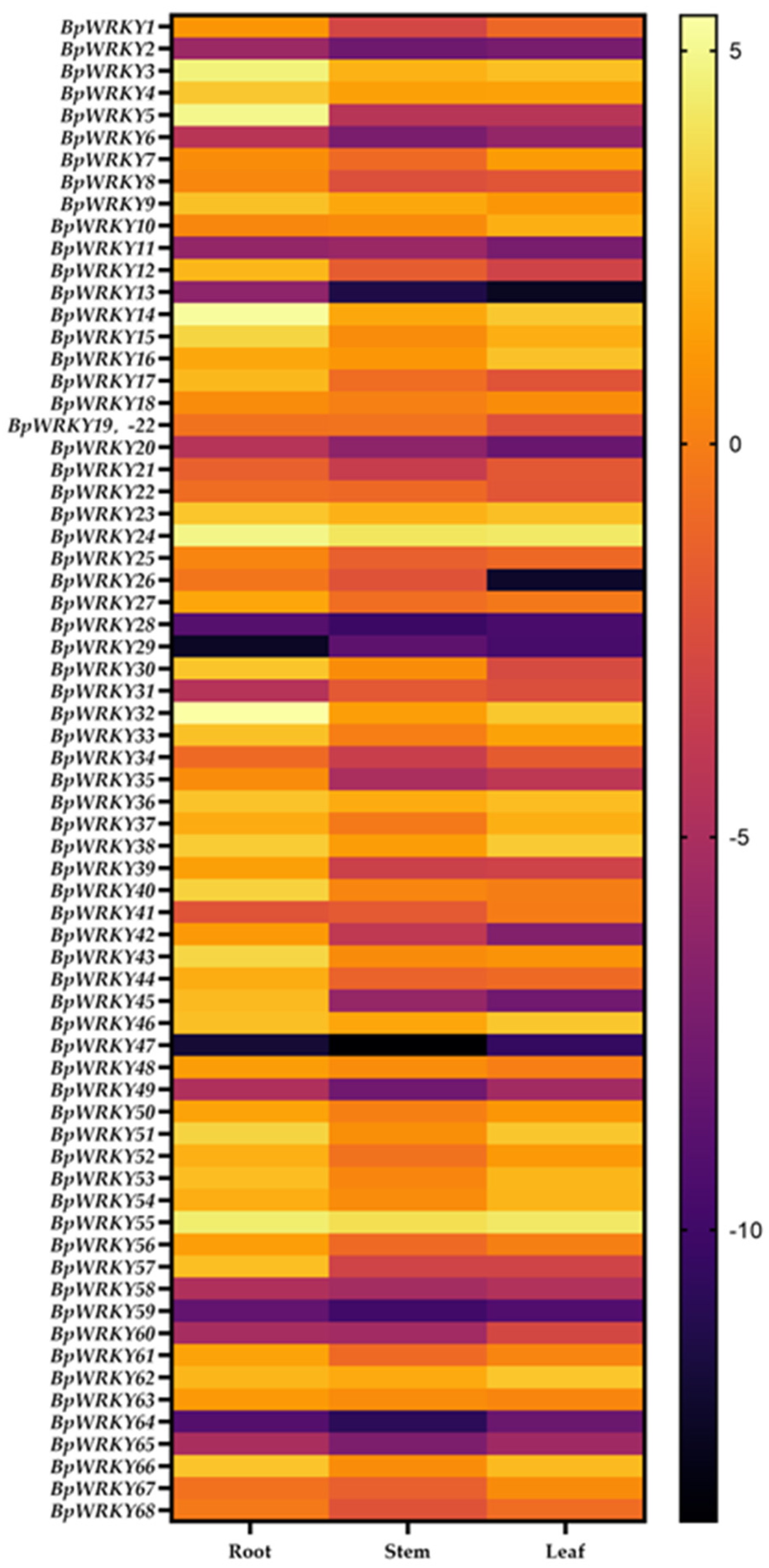
2.6. Expression Pattern Analysis of BpWRKYs
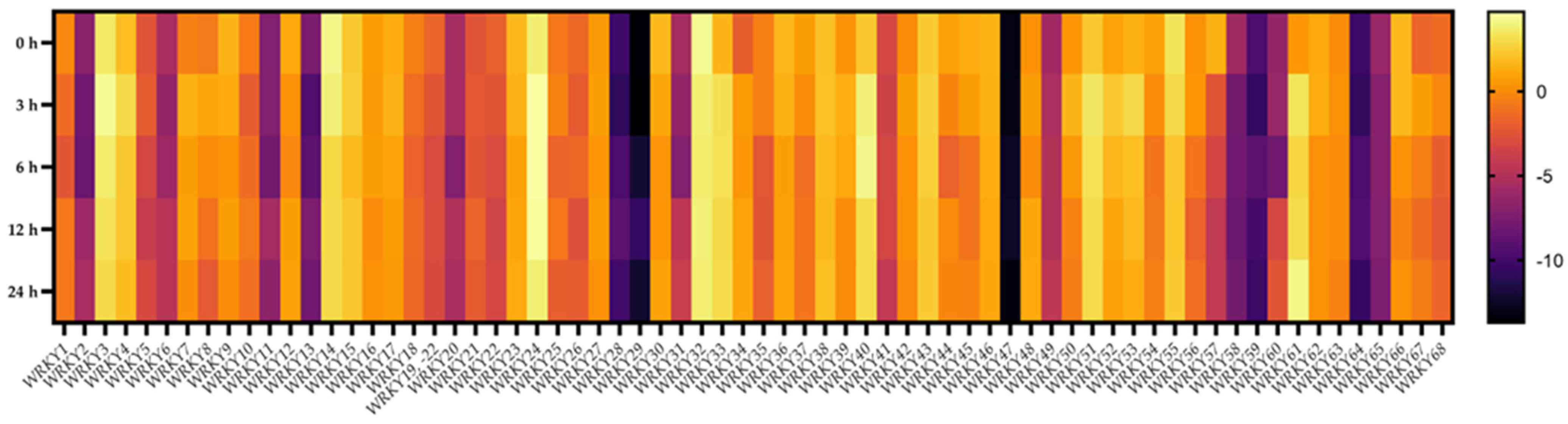
2.7. Transcriptional Responses of BpWRKYs upon ABA Treatment
2.8. Transcriptional Responses of BpWRKYs upon Salt Treatment
2.9. Transcriptional Responses of BpWRKYs upon Cold Treatment
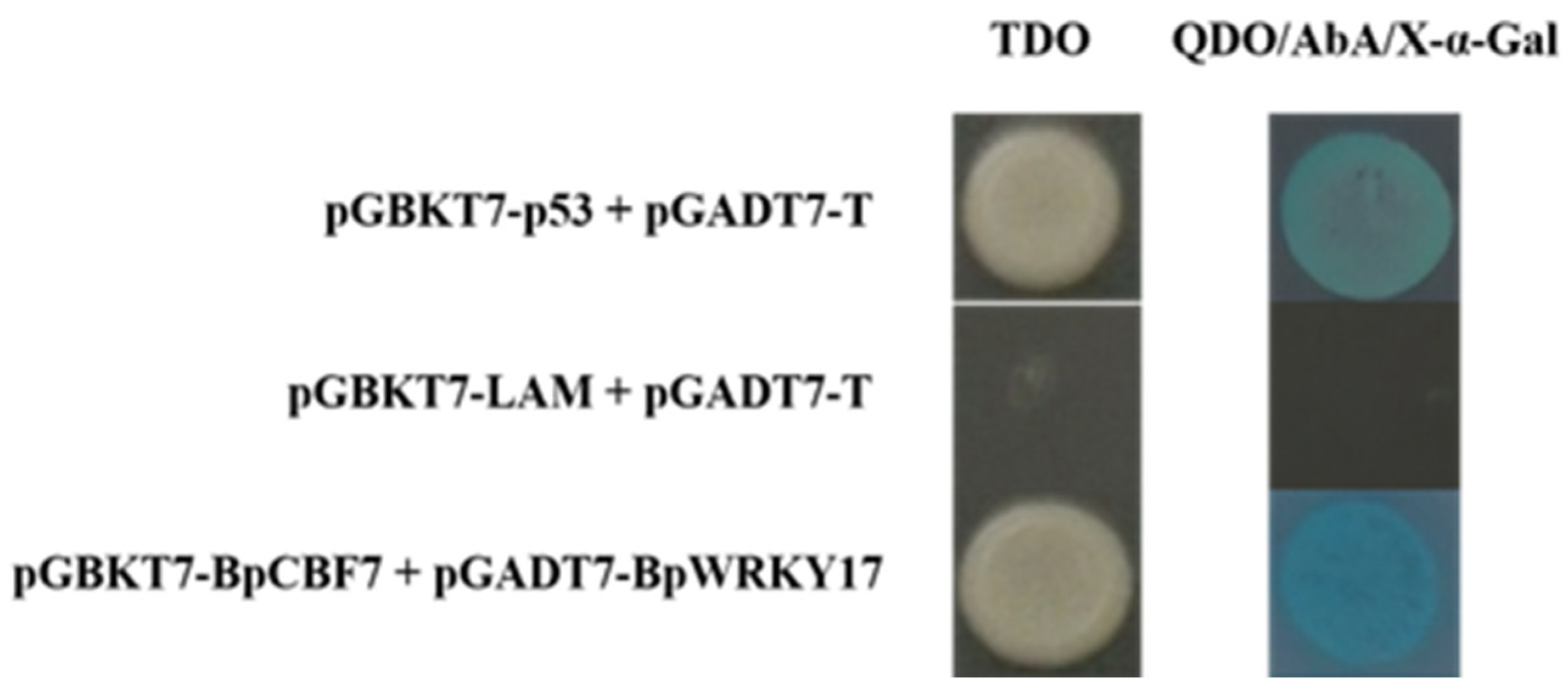
2.10. Interaction of BpWRKY17 with the Cold-Responsive TF BpCBF7
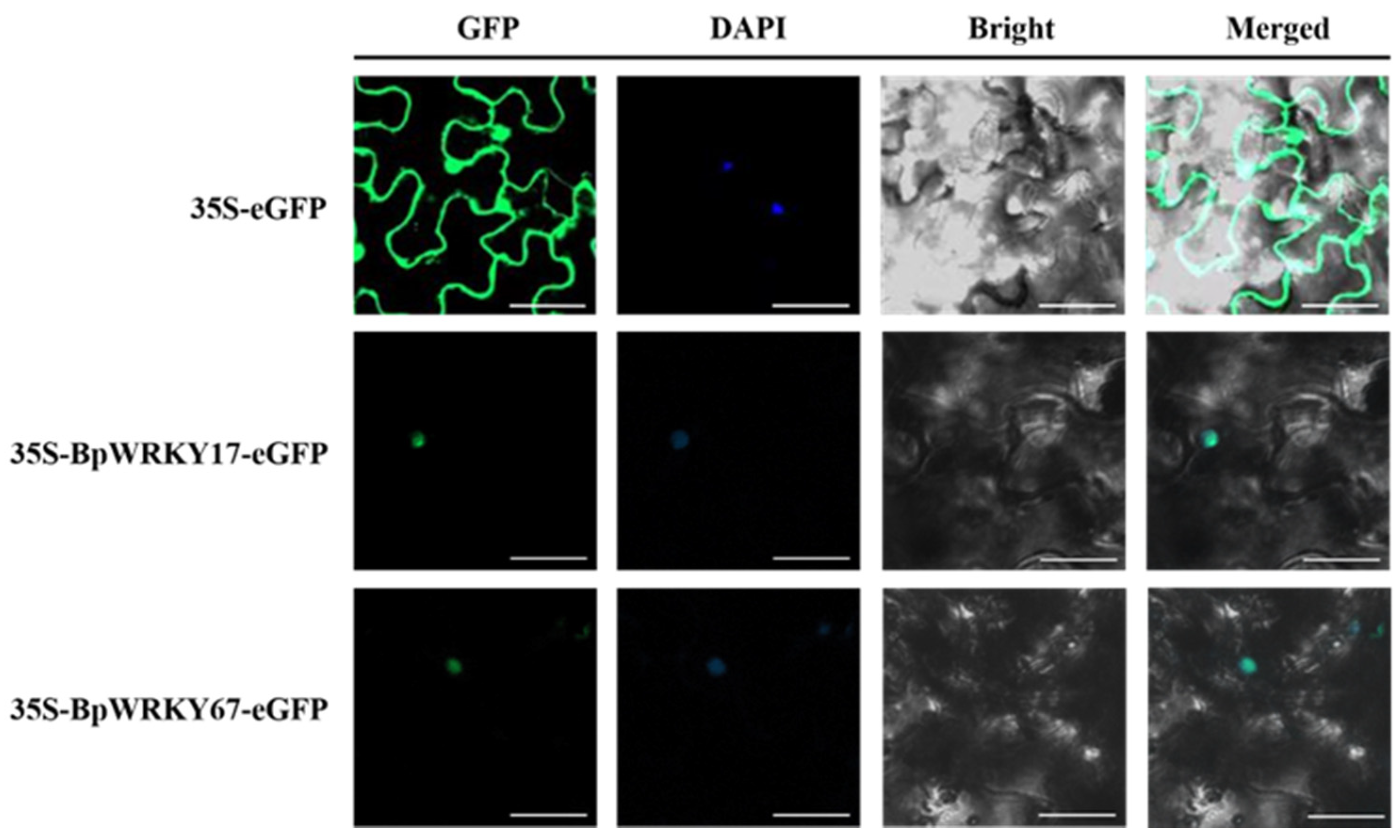
2.11. Subcellular Localization of BpWRKY17 and BpWRKY67
3. Discussion
4. Materials and Methods
4.1. Identification and Characterization of WRKY Transcription Factors in Betula platyphylla
4.2. Phylogenetic Analysis
4.3. Gene Structure Analysis of BpWRKYs
4.4. Gene Synteny Analyses of BpWRKYs
4.5. Analysis of Cis-Acting Elements in the Promoters of BpWRKYs
4.6. Plant Materials and Treatments
4.7. Data Processing and Expression Analysis
4.8. Yeast Two Hybrid (Y2H) Assay
4.9. Subcellular Localization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The Role of Stress-Responsive Transcription Factors in Modulating Abiotic Stress Tolerance in Plants. Agronomy 2020, 10, 788–810. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Oelmuller, R. WRKY transcription factors: Jack of many trades in plants. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Fang, L.; Sun, X.; Su, L.; Liang, Z.; Wang, N.; Londo, J.P.; Li, S.; Xin, H. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera. BMC Plant Biol. 2014, 14, 103–116. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5’ upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Rinerson, C.I.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Rushton, P.J. The evolution of WRKY transcription factors. BMC Plant Biol. 2015, 15, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yue, C.; Chen, D.; Zheng, Y.; Zhang, Q.; Yang, J.; Ye, N. Genome-wide identification of WRKY family genes and their response to abiotic stresses in tea plant (Camellia sinensis). Genes Genom. 2019, 41, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Cao, H.; Xiu, H.; Luo, T.; Li, J.; Chen, X.; Luo, J.; Luo, Z. Transcriptomics-based identification of WRKY genes and characterization of a salt and hormone-responsive PgWRKY1 gene in Panax ginseng. Acta Biochim. Biophys. Sin. 2016, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, R.; Jiang, S.Y.; Kumar, N.; Venkatesh, P.N.; Ramachandran, S. A comprehensive transcriptional profiling of the WRKY gene family in rice under various abiotic and phytohormone treatments. Plant Cell Physiol. 2008, 49, 865–879. [Google Scholar] [CrossRef]
- Wei, K.F.; Chen, J.; Chen, Y.F.; Wu, L.J.; Xie, D.X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012, 19, 153–164. [Google Scholar] [CrossRef]
- Fan, C.; Yao, H.; Qiu, Z.; Ma, H.; Zeng, B. Genome-wide analysis of Eucalyptus grandis WRKY genes family and their expression profiling in response to hormone and abiotic stress treatment. Gene 2018, 678, 38–48. [Google Scholar] [CrossRef]
- Lui, S.; Luo, C.; Zhu, L.; Sha, R.; Qu, S.; Cai, B.; Wang, S. Identification and expression analysis of WRKY transcription factor genes in response to fungal pathogen and hormone treatments in apple (Malus domestica). J. Plant Biol. 2017, 60, 215–230. [Google Scholar] [CrossRef]
- Ma, J.; Lu, J.; Xu, J.; Duan, B.; He, X.; Liu, J. Genome-wide Identification of WRKY Genes in the Desert Poplar Populus euphratica and Adaptive Evolution of the Genes in Response to Salt Stress. Evol. Bioinform. Online 2015, 11, 47–55. [Google Scholar] [CrossRef]
- He, H.; Dong, Q.; Shao, Y.; Jiang, H.; Zhu, S.; Cheng, B.; Xiang, Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012, 31, 1199–1217. [Google Scholar] [CrossRef]
- Liu, X.; Bai, X.; Wang, X.; Chu, C. OsWRKY71, a rice transcription factor, is involved in rice defense response. J. Plant Physiol. 2007, 164, 969–979. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Q.; Yu, D. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci. 2012, 185, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Macdonald, H.; Huttly, A.K.; Lazarus, C.M.; Hooley, R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes. Plant Mol. Biol. 1995, 29, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zentgraf, U. A HECT E3 ubiquitin ligase negatively regulates Arabidopsis leaf senescence through degradation of the transcription factor WRKY53. Plant J. 2010, 63, 179–188. [Google Scholar] [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef]
- Sun, X.C.; Gao, Y.F.; Li, H.R.; Yang, S.Z.; Liu, Y.S. Over-expression of SlWRKY39 leads to enhanced resistance to multiple stress factors in tomato. J. Plant Biol. 2015, 58, 52–60. [Google Scholar] [CrossRef]
- Peng, Y.; Bartley, L.E.; Chen, X.; Dardick, C.; Chern, M.; Ruan, R.; Canlas, P.E.; Ronald, P.C. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant 2008, 1, 446–458. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 2009, 69, 91–105. [Google Scholar] [CrossRef]
- Ma, Q.; Xia, Z.; Cai, Z.; Li, L.; Cheng, Y.; Liu, J.; Nian, H. GmWRKY16 Enhances Drought and Salt Tolerance Through an ABA-Mediated Pathway in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 01979. [Google Scholar] [CrossRef]
- Wu, G.Q.; Li, Z.Q.; Cao, H.; Wang, J.L. Genome-wide identification and expression analysis of the WRKY genes in sugar beet (Beta vulgaris L.) under alkaline stress. PeerJ 2019, 7, e7817. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Li, M.; Zhao, D.; Yang, J.F.; Fu, J.D.; Xu, Z.S. Maize WRKY Transcription Factor ZmWRKY106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018, 19, 19103046. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, B.; Wang, N.; Zheng, Z.; Yang, L.; Zhong, S.; Fang, Q.; Xiao, Z.; Zhao, H. A WRKY Transcription Factor PmWRKY57 from Prunus mume Improves Cold Tolerance in Arabidopsis thaliana. Mol. Biotechnol. 2023, 65, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Mondini, L.; Nachit, M.; Porceddu, E.; Pagnotta, M. Identification of SNP Mutations in DREB1, HKT1, and WRKY1 Genes Involved in Drought and Salt Stress Tolerance in Durum Wheat (Triticum turgidum L. var durum). OMICS J. Integr. Biol. 2012, 16, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.; Li, X.; Yang, Y.; Liu, C.; Zhou, G.; Wan, H.; Cheng, Y. Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2–mediated stomatal closure. Environ. Exp. Bot. 2020, 171, 103960. [Google Scholar] [CrossRef]
- Wu, F.Z. Proteomic Analysis of Low Temperature Stress Responses and Chloroplast RNA Binding Proteins in Betula platyphylla. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2008. [Google Scholar]
- Zhang, X.; Yu, J.J.; Wang, R.Q.; Liu, W.X.; Chen, S.; Wang, Y.R.; Yu, Y.; Qu, G.Z.; Chen, S. Genome-Wide Identification and Expression Profiles of C-Repeat Binding Factor Transcription Factors in Betula platyphylla under Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 10573. [Google Scholar] [CrossRef]
- Du, Z.; You, S.; Zhao, X.; Xiong, L.; Li, J. Genome-Wide Identification of WRKY Genes and Their Responses to Chilling Stress in Kandelia obovata. Front. Genet. 2022, 13, 875316. [Google Scholar] [CrossRef]
- Qu, R.; Cao, Y.; Tang, X.; Sun, L.; Wei, L.; Wang, K. Identification and expression analysis of the WRKY gene family in Isatis indigotica. Gene 2021, 783, 145561. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, C.M.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Tremblay, M.F.; Nadeau, P.; Lalonde, M. Effect of ABA on freezing resistance of Betula papyrifera and Alnus incana woody plant cell suspensions. Tree Physiol. 1992, 10, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, K.; Yang, C. Functional roles of the birch BpRAV1 transcription factor in salt and osmotic stress response. Plant Sci. 2022, 315, 111131. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, F.N. Transcriptome, Physiological, and Biochemical Analysis of Betula platyphylla S. in Response to Cold and Heat Stresses. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2022. [Google Scholar]
- Giricz, O.; Lauer-Fields, J.L.; Fields, G.B. The normalization of gene expression data in melanoma: Investigating the use of glyceraldehyde 3-phosphate dehydrogenase and 18S ribosomal RNA as internal reference genes for quantitative real-time PCR. Anal. Biochem. 2008, 380, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Duplicated Gene Pairs | Ka | Ks | Ka/Ks | Effective Length * (bp) |
|---|---|---|---|---|
| BpWRKY19/BpWRKY22 | 0 | 0 | -- | 501 |
| BpWRKY20/BpWRKY21 | 0.064 | 0.100 | 0.636 | 366 |
| BpWRKY7/BpWRKY25 | 0.407 | 1.767 | 0.230 | 963 |
| BpWRKY13/BpWRKY33 | 0.404 | 2.425 | 0.167 | 1260 |
| BpWRKY11/BpWRKY39 | 0.335 | 1.266 | 0.265 | 1335 |
| BpWRKY14/BpWRKY35 | 0.424 | 1.507 | 0.282 | 1227 |
| BpWRKY40/BpWRKY43 | 0.294 | 1.287 | 0.229 | 510 |
| BpWRKY8/BpWRKY44 | 0.458 | 3.314 | 0.138 | 699 |
| BpWRKY27/BpWRKY44 | 0.363 | 2.688 | 0.135 | 678 |
| BpWRKY11/BpWRKY45 | 0.370 | 1.534 | 0.241 | 1362 |
| BpWRKY39/BpWRKY45 | 0.392 | 1.401 | 0.280 | 1533 |
| BpWRKY38/BpWRKY46 | 0.260 | 1.086 | 0.239 | 1425 |
| BpWRKY36/BpWRKY54 | 0.399 | 1.103 | 0.362 | 1080 |
| BpWRKY13/BpWRKY52 | 0.385 | 2.101 | 0.183 | 1299 |
| BpWRKY33/BpWRKY52 | 0.267 | 2.081 | 0.128 | 1578 |
| BpWRKY51/BpWRKY61 | 0.307 | 1.643 | 0.187 | 1614 |
| BpWRKY29/BpWRKY64 | 0.012 | 0.081 | 0.150 | 864 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhang, X.; Cao, J.; Bai, H.; Wang, R.; Wang, C.; Xu, Z.; Li, C.; Liu, G. Genome-Wide Identification and Characterization of WRKY Transcription Factors in Betula platyphylla Suk. and Their Responses to Abiotic Stresses. Int. J. Mol. Sci. 2023, 24, 15000. https://doi.org/10.3390/ijms241915000
Yu J, Zhang X, Cao J, Bai H, Wang R, Wang C, Xu Z, Li C, Liu G. Genome-Wide Identification and Characterization of WRKY Transcription Factors in Betula platyphylla Suk. and Their Responses to Abiotic Stresses. International Journal of Molecular Sciences. 2023; 24(19):15000. https://doi.org/10.3390/ijms241915000
Chicago/Turabian StyleYu, Jiajie, Xiang Zhang, Jiayu Cao, Heming Bai, Ruiqi Wang, Chao Wang, Zhiru Xu, Chunming Li, and Guanjun Liu. 2023. "Genome-Wide Identification and Characterization of WRKY Transcription Factors in Betula platyphylla Suk. and Their Responses to Abiotic Stresses" International Journal of Molecular Sciences 24, no. 19: 15000. https://doi.org/10.3390/ijms241915000
APA StyleYu, J., Zhang, X., Cao, J., Bai, H., Wang, R., Wang, C., Xu, Z., Li, C., & Liu, G. (2023). Genome-Wide Identification and Characterization of WRKY Transcription Factors in Betula platyphylla Suk. and Their Responses to Abiotic Stresses. International Journal of Molecular Sciences, 24(19), 15000. https://doi.org/10.3390/ijms241915000







