GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments
Abstract
:1. The G Protein-Coupled Estrogen Receptor
2. GPER/GPR30 Signaling in Normal Tissues
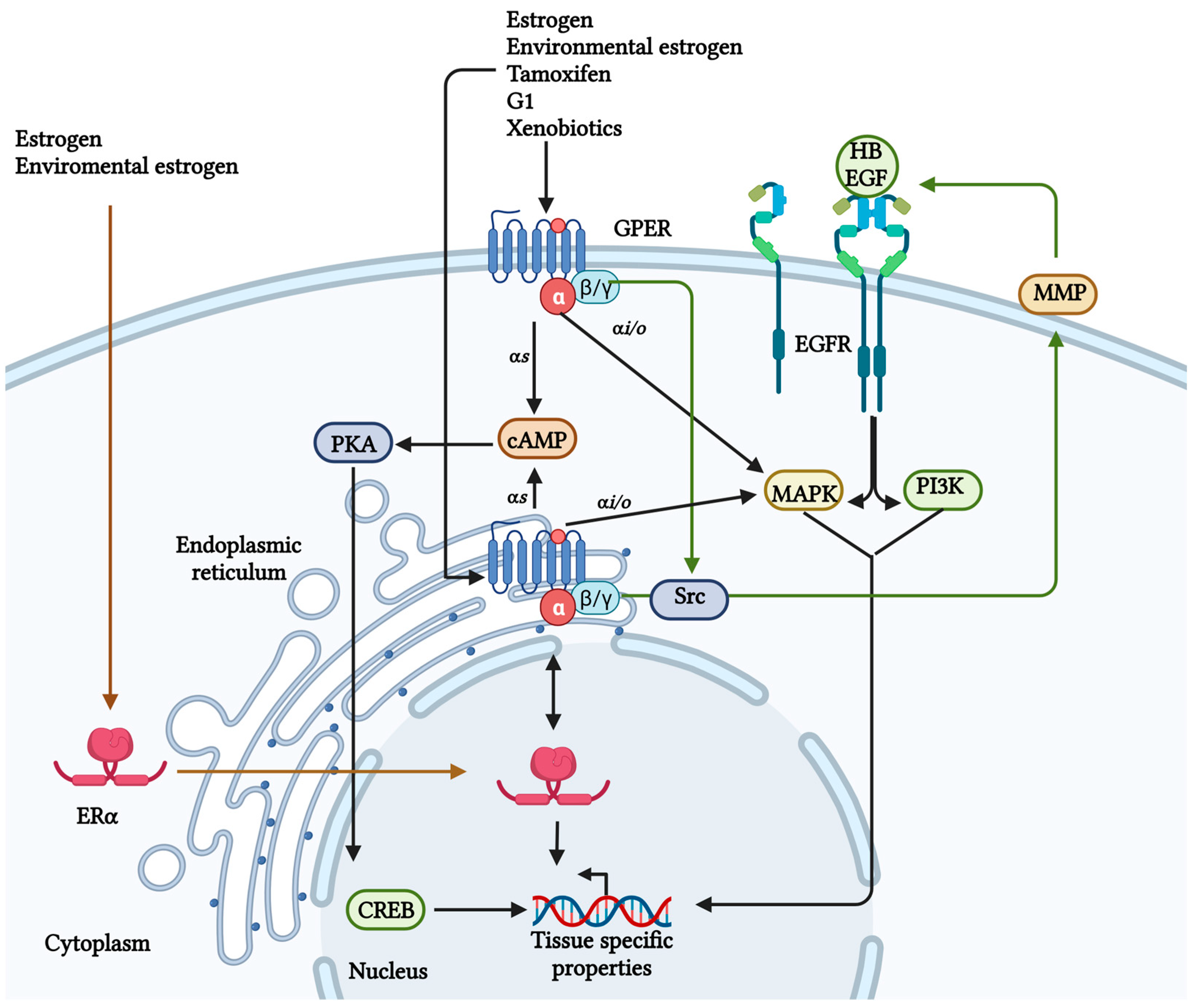
3. GPER Expression in Cancer
| Anti-Tumorigenic Role of GPER | |||
|---|---|---|---|
| Cancer Type | Receptor State | Activity | Mechanism |
| Hepatic | Overexpression | Anti-tumoral | Through GPER in hepatocarcinoma [43] |
| Prostate | Low expression | Anti-tumoral | G2 cell-cycle arrest [45] |
| Ovarian | Overexpression | Anti-tumoral | Epigenetic regulation, such as the H34me3 mark [42] |
| Pancreatic | Activation by tamoxifen | Inhibits the proliferation, migration, and invasion process | Regulates the tumorigenic environment, decreasing the anti-inflammatory macrophage phenotype M2 [47] |
| Leydig cell tumors | Low expression | Anti-tumoral | Induces apoptosis [63] |
| Pro-Tumorigenic Role of GPER Receptor | |||
| Cancer Type | Receptor State | Activity | Mechanism |
| Lung | Overexpression | Pro-tumoral, poor prognosis | The expression of GPER on the cell surface is related to cell survival [49] |
| Cervical | Overexpression | Pro-tumoral | Induction of tumor-promoting claudin-1 [50] |
| Prostate | Stimulation by estrogen | Increased stromal components and prostatic fibrosis accelerate the clinical progression | GPER activates EGFR/ERK and P13k/AKT HIF1α pathways [51] |
| ER-positive breast tumor | Overexpression | Poor prognosis | ERK1/2 pathway [52] |
| Triple-negative breast cancer | GPER interacts with FAK | Increases the migration, adhesion, and invasion | Increasing focal adhesion points [52] |
| Glioblastoma | Activation of GPER | Promotes tumor growth | Induces the epidermal growth factor receptor, triggering the ERK pathway [53] |
| Testicular germ neoplasms | Overexpression | Promotes proliferation | GPER activates the ERK 1/2 pathway [63] |
4. Epithelial–Mesenchymal Transition and Metastasis Development
5. Tumor Microenvironment Favors EMT through GPER
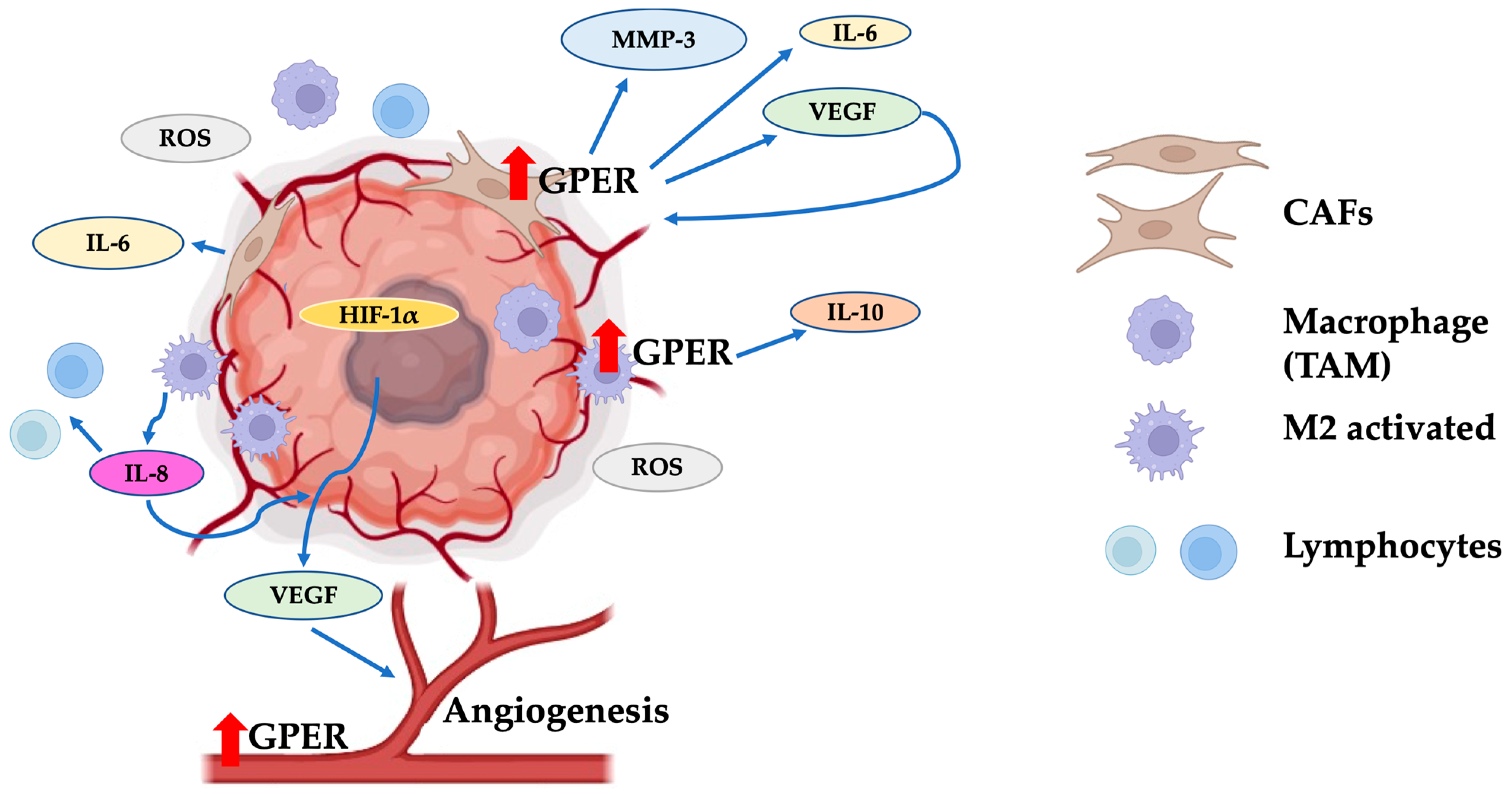
6. Role of GPER in Angiogenesis
7. Therapeutic Alternatives against Cancer Targeting GPER
8. Discussion
9. Conclusions
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Owman, C.; Blay, P.; Nilsson, C.; Lolait, S.J. Cloning of Human CDNA Encoding a Novel Heptahelix Receptor Expressed in Burkitt s Lymphoma and Widely Distributed in Brain and Peripheral Tissues Communication Both in CNS and in Peripheral Tissues. Biochem. Biophys. Res. Commun. 1996, 228, 285–292. [Google Scholar] [CrossRef]
- Carmeci, C.; Thompson, D.A.; Ring, H.Z.; Francke, U.; Weigel, R.J. Identification of a Gene (GPR30) With Homology to the G-protein-coupled Receptor Superfamily Associated with Estrogen Receptor Expression in Breast Cancer. Genomics 1997, 45, 607–617. [Google Scholar] [CrossRef]
- Kimura, M.; Mizukami, Y.; Miura, T.; Fujimoto, K.; Kobayashi, S.; Matsuzaki, M. Orphan G Protein-Coupled Receptor, GPR41, Induces Apoptosis via a P53/Bax Pathway during Ischemic Hypoxia and Reoxygenation. J. Biol. Chem. 2001, 276, 26453–26460. [Google Scholar] [CrossRef]
- Toran-Allerand, C.D.; Guan, X.; MacLusky, N.J.; Horvath, T.L.; Diano, S.; Singh, M.; Connolly, E.S.; Nethrapalli, I.S.; Tinnikov, A.A. ER-X: A Novel, Plasma Membrane-Associated, Putative Estrogen Receptor That Is Regulated during Development and after Ischemic Brain Injury. J. Neurosci. 2002, 22, 8391–8401. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.; Bland, K.I.; Frackelton, A.R.J. Estrogen-Induced Activation of Erk-1 and Erk-2 Requieres the G Protein-Couple Receptor Homolog, GPER, and Occurs via Trans-Activation of the Epidermal Growth Factor Receptor through Release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Chambliss, K.L.; Mineo, C.; Yuhanna, I.S.; Mendelsohn, M.E.; Mumby, S.M.; Shaul, P.W. Plasma Membrane Estrogen Receptors Are Coupled to Endothelial Nitric-Oxide Synthase through Gαi. J. Biol. Chem. 2001, 276, 27071–27076. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. 17β-Estradiol Stimulates the Growth of Human Keratinocytes by Inducing Cyclin D2 Expression. J. Investig. Dermatol. 2004, 123, 319–328. [Google Scholar] [CrossRef]
- Tutzauer, J.; Eilard, I.A.; De Valdivia, E.G.; Sw, K.; Broselid, S.; Kahn, R. Ligand-Independent G Protein—Coupled Estrogen Receptor/G Protein—Coupled Receptor 30 Activity: Lack of Receptor- Dependent Effects of G-1 and 17 b -Estradiol S. Mol. Pharmacol. 2021, 100, 271–282. [Google Scholar] [CrossRef]
- O’Dowd, B.F.; Nguyen, T.; Marchese, A.; Cheng, R.; Lynch, K.R.; Heng, H.H.; Kolakowski, L.F., Jr.; George, S.R. Discovery of Three Novel G-protein-coupled Receptor Genes. Genomics 1998, 47, 310–313. [Google Scholar] [CrossRef]
- Peña-Gutiérrez, K.M.; Hernández-Ortega, K.; Bello-Alvarez, C.; Camacho-Arroyo, I. Expression and Estrogen Regulation of G Protein-coupled Estrogen Receptor in Human Glioblastoma Cells. Oncol. Lett. 2022, 24, 397. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an Estrogen Membrane Receptor Coupled to a G Protein in Human Breast Cancer Cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the Novel Estrogen Receptor G Protein-Coupled Receptor 30 (GPER) at the Plasma Membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef]
- Otto, C.; Rohde-Schulz, B.; Schwarz, G.; Fuchs, I.; Klewer, M.; Brittain, D.; Langer, G.; Bader, B.; Prelle, K.; Nubbemeyer, R.G. Protein-Coupled Receptor 30 Localizes to the Endoplasmic Reticulum and Is Not Activated by Estradiol. Endocrinology 2008, 149, 4846–4856. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Liu, M.; Yang, F.; Luo, H.; Li, Z.; Tu, G.; Yang, G. GPR30 as an Initiator of Tamoxifen Resistance in Hormone-Dependent Breast Cancer. Breast Cancer Res. 2013, 15, R114. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Yanai, A.; Shinoda, K.; Kawano, M.M.; Mizukami, Y. G Protein-Coupled Receptor 30 Is an Estrogen Receptor in the Plasma Membrane. Biochem. Biophys. Res. Commun. 2006, 346, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Unal, H.; Karnik, S. Domain Coupling in GPCRs: The Engine for Induced Conformational Changes. Trends Pharmacol. Sci. 2012, 33, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Christofides, K.; Menon, R.; Jones, C.E. Endocytosis of G Protein-Coupled Receptors and Their Ligands: Is There a Role in Metal Trafficking? Cell Biochem. Biophys. 2018, 76, 329–337. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Méndez-Luna, D.; Martínez-Archundia, M.; Maroun, R.C.; Ceballos-Reyes, G.; Fragoso-Vázquez, M.J.; González-Juárez, D.E.; Correa-Basurto, J. Deciphering the GPER/GPER-Agonist and Antagonists Interactions Using Molecular Modeling Studies, Molecular Dynamics, and Docking Simulations. J. Biomol. Struct. Dyn. 2015, 33, 2161–2172. [Google Scholar] [CrossRef]
- Pepermans, R.A.; Sharma, G.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor in Cancer and Stromal Cells: Functions and Novel Therapeutic Perspectives. Cells 2021, 10, 672. [Google Scholar] [CrossRef]
- Ding, Q.; Chorazyczewski, J.; Gros, R.; Motulsky, H.J.; Limbird, L.E.; Feldman, R.D. Correlation of Functional and Radioligand Binding Characteristics of GPER Ligands Confirming Aldosterone as a GPER Agonist. Pharmacol. Res. Perspect. 2022, 10, e00995. [Google Scholar] [CrossRef]
- Feng, B.; Wu, J.; Shen, B.; Jiang, F.; Feng, J. Cancer-Associated Fibroblasts and Resistance to Anticancer Therapies: Status, Mechanisms, and Countermeasures. Cancer Cell Int. 2022, 22, 166. [Google Scholar] [CrossRef]
- Lucas, T.F.G.; Royer, C.; Siu, E.R.; Lazari, M.F.M.; Porto, C.S. Expression and Signaling of G Protein-Coupled Estrogen Receptor 1 (GPER) in Rat Sertoli Cells. Biol. Reprod. 2010, 83, 307–317. [Google Scholar] [CrossRef]
- Gaudet, H.M.; Cheng, S.B.; Christensen, E.M.; Filardo, E.J. The G-Protein Coupled Estrogen Receptor, GPER: The inside and inside-out Story. Mol. Cell. Endocrinol. 2015, 418, 207–219. [Google Scholar] [CrossRef]
- Tran, Q.K. Reciprocality Between Estrogen Biology and Calcium Signaling in the Cardiovascular System. Front. Endocrinol. 2020, 11, 568203. [Google Scholar] [CrossRef]
- Tran, Q.K.; VerMeer, M.; Burgard, M.A.; Hassan, A.B.; Giles, J. Hetero-Oligomeric Complex between the G Protein-Coupled Estrogen Receptor 1 and the Plasma Membrane Ca2+-ATPase 4b. J. Biol. Chem. 2015, 290, 13293–13307. [Google Scholar] [CrossRef]
- Xu, S.; Yu, S.; Dong, D.; Lee, L.T.O. G Protein-Coupled Estrogen Receptor: A Potential Therapeutic Target in Cancer. Front. Endocrinol. 2019, 10, 725. [Google Scholar] [CrossRef]
- Pemberton, K.; Xu, F.; Rosato, M.; Dedert, C.; Deleon, C.; Arnatt, C. Differential Effects of the G-Protein-Coupled Estrogen Receptor (GPER) on Rat Embryonic (E18) Hippocampal and Cortical Neurons. eNeuro 2022, 9, 1–23. [Google Scholar] [CrossRef]
- Fredette, N.C.; Meyer, M.R.; Prossnitz, E.R. Role of GPER in Estrogen-Dependent Nitric Oxide Formation and Vasodilation. J. Steroid Biochem. Mol. Biol. 2018, 176, 65–72. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Maggiolini, M. Mechanisms of Estrogen Signaling and Gene Expression Via GPR30. Mol. Cell. Endocrinol. 2009, 308, 32–38. [Google Scholar] [CrossRef]
- Deng, Y.; Miki, Y.; Nakanishi, A. Estradiol/GPER Affects the Integrity of Mammary Duct-like Structures in Vitro. Sci. Rep. 2020, 10, 1386. [Google Scholar] [CrossRef]
- Scaling, A.L.; Prossnitz, E.R.; Hathaway, H.J. GPER Mediates Estrogen-Induced Signaling and Proliferation in Human Breast Epithelial Cells and Normal and Malignant Breast. Horm. Cancer 2014, 5, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Liu, M.Y.; Liu, Z.; Zhao, J.K.; Zhao, Y.G.; He, L.; Li, W.; Zhang, J.Q. GPER-Mediated Estrogenic Regulation of Actin Polymerization and Spatial Memory Involves SRC-1 and PI3K-MTORC2 in the Hippocampus of Female Mice. CNS Neurosci. Ther. 2019, 25, 714–733. [Google Scholar] [CrossRef] [PubMed]
- Roque, C.; Mendes-Oliveira, J.; Duarte-Chendo, C.; Baltazar, G. The Role of G Protein-Coupled Estrogen Receptor 1 on Neurological Disorders. Front. Neuroendocrinol. 2019, 55, 100786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Q.; Yu, C.Y.; Wang, F.; Shao, Y.; Sun, K.S.; Sun, T.; Liu, J. G Protein-Coupled Estrogen Receptor 1 Knockout Deteriorates MK-801-Induced Learning and Memory Impairment in Mice. Front. Behav. Neurosci. 2020, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Prossnitz, E.R. Targeting the G Protein-Coupled Estrogen Receptor (GPER) in Obesity and Diabetes. Endocr. Metab. Sci. 2021, 2, 100080. [Google Scholar] [CrossRef]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Estrogen Receptor in Vivo? Front. Endocrinol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Vo, D.K.H.; Hartig, R.; Weinert, S.; Haybaeck, J.; Nass, N. G-Protein-Coupled Estrogen Receptor (GPER)-Specific Agonist G1 Induces ER Stress Leading to Cell Death in MCF-7 Cells. Biomolecules 2019, 9, 503. [Google Scholar] [CrossRef]
- Jung, J. Role of G Protein-Coupled Estrogen Receptor in Cancer Progression. Toxicol. Res. 2019, 35, 209–214. [Google Scholar] [CrossRef]
- Notas, G.; Kampa, M.; Castanas, E. G Protein-Coupled Estrogen Receptor in Immune Cells and Its Role in Immune-Related Diseases. Front. Endocrinol. 2020, 11, 579420. [Google Scholar] [CrossRef]
- Tian, S.; Zhan, N.; Li, R.; Dong, W. Downregulation of G Protein-Coupled Estrogen Receptor (GPER) Is Associated with Reduced Prognosis in Patients with Gastric Cancer. Med. Sci. Monit. 2019, 25, 3115–3126. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Heublein, S.; Jeschke, U.; Kuhn, C.; Hester, A.; Czogalla, B.; Mahner, S.; Rottmann, M.; Mayr, D.; Schmoeckel, E. The G-Protein-Couple Estrogen Receptor (GPER) Regulates Trimethylation of Histone H3 at Lysine 4 and Represses Migration and Proliferation of Ovarian Cancer Cells in Vitro. Cells 2021, 10, 619. [Google Scholar] [CrossRef]
- Qiu, Y.A.; Xiong, J.; Fu, Q.; Dong, Y.; Liu, M.; Peng, M.; Jin, W.; Zhou, L.; Xu, X.; Huang, X. GPER-Induced ERK Signaling Decreases Cell Viability of Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 638171. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Jiang, G.; Zhou, Y.; Yang, X.; Huang, H.; Liu, H.; Du, J.; Wang, H. Epigenetic down Regulation of G Protein-Coupled Estrogen Receptor (GPER) Functions as a Tumor Suppressor in Colorectal Cancer. Mol. Cancer 2017, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.K.Y.; Lam, H.; Ng, C.; Lee, A.Y.Y.; Chan, E.S.Y.; Ng, K.; Ho, S.; Lau, K. Activation of GPER Inhibits Growth of Prostate Cancer Cells via Sustained Activation of Erk1/2, c-Jun/c-Fos-Dependent Upregulation of P21, and Induction of G2 Cell-Cycle Arrest. Cell Death Differ. 2010, 17, 1511–1523. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Skrzypczak, M.; Ignatov, T.; Ignatov, A.; Ortmann, O.; Treeck, O.G. Protein-Coupled Estrogen Receptor 1 (GPER-1) and Agonist G-1 Inhibit Growth of Ovarian Cancer Cells by Activation of Anti-Tumoral Transcriptome Responses: Impact of GPER-1 MRNA on Survival. J. Cancer Res. Clin. Oncol. 2020, 146, 3175–3188. [Google Scholar] [CrossRef] [PubMed]
- Cortes, E.; Sarper, M.; Robinson, B.; Lachowski, D.; Chronopoulos, A.; Thorpe, S.D.; Lee, D.A.; Río-Hernández, A.E. GPER Is a Mechanoregulator of Pancreatic Stellate Cells and the Tumor Microenvironment. EMBO Rep. 2019, 20, e46556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.X.; Xiong, W.; Wang, M.L.; Yang, J.; Shi, H.J.; Chen, H.Q.; Niu, G. Nuclear G Protein-Coupled Oestrogen Receptor (GPER) Predicts Poor Survival in Patients with Ovarian Cancer. J. Int. Med. Res. 2018, 46, 723–731. [Google Scholar] [CrossRef]
- Jala, V.R.; Radde, B.N.; Haribabu, B.; Klinge, C.M. Enhanced Expression of G-Protein Coupled Estrogen Receptor (GPER/GPER) in Lung Cancer. BMC Cancer 2012, 12, 624. [Google Scholar] [CrossRef]
- Akimoto, T.; Takasawa, A.; Takasawa, K.; Aoyama, T.; Murata, M.; Osanai, M.; Saito, T.; Sawada, N. Estrogen/GPER Signaling Contributes to the Malignant Potentials of ER-Negative Cervical Adenocarcinoma via Regulation of Claudin-1 Expression. Neoplasia 2018, 20, 1083–1093. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, J.; Hu, S.; Cui, Y.; Xiao, J.; Yu, W.; Peng, J.; Han, W.; He, Q.; Fan, Y. Estrogen and G Protein-Coupled Estrogen Receptor Accelerate the Progression of Benign Prostatic Hyperplasia by Inducing Prostatic Fibrosis. Cell Death Dis. 2022, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ma, D.; Chen, S.; Tang, R.; Yang, J.; Meng, C.; Feng, Y.; Liu, L.; Wang, J.; Luo, H. High GPER Expression in Triple-Negative Breast Cancer Is Linked to pro-Metastatic Pathways and Predicts Poor Patient Outcomes. NPJ Breast Cancer 2022, 8, 100. [Google Scholar] [CrossRef]
- Gutierrez-Almeida, C.E.; Santerre, A.; Leon-Moreno, L.C.; Aguilar-Garcia, I.G.; Castaneda-Arellano, R.; Duenas-Jimenez, S.H.; Duenas-Jimenez, J.M. Proliferation and Apoptosis Regulation by G Protein-Coupled Estrogen Receptor in Glioblastoma C6 Cells. Oncol. Lett. 2022, 24, 217. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.L.C.; Pandey, V.; Zhu, T.; Ma, L.; Basappa; Lobie, P.E. Bad Phosphorylation as a Target of Inhibition in Oncology. Cancer Lett. 2018, 415, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.T.; Lai, A.C.Y.; Lin, R.J.; Wang, Y.H.; Wang, Y.T.; Chang, W.W.; Wu, H.Y.; Lin, Y.J.; Chang, W.Y.; Wu, J.C. GPER-Induced Signaling Is Essential for the Survival of Breast Cancer Stem Cells. Int. J. Cancer 2020, 147, 1674–1685. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells. 2021, 10, 2999. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Wang, Z.; Feng, X.; Liu, P.; Lv, X.B.; Li, F.; Yu, F.X.; Sun, Y.; Yuan, H. Estrogen Regulates Hippo Signaling via GPER in Breast Cancer. J. Clin. Investig. 2015, 125, 2123–2135. [Google Scholar] [CrossRef]
- Chuang, S.C.; Chen, C.H.; Chou, Y.S.; Ho, M.L.; Chang, J.K. G Protein-Coupled Estrogen Receptor Mediates Cell Proliferation through the Camp/Pka/Creb Pathway in Murine Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 6490. [Google Scholar] [CrossRef]
- Talia, M.; De Francesco, E.M.; Rigiracciolo, D.C.; Muoio, M.G.; Muglia, L.; Belfiore, A.; Maggiolini, M.; Sims, A.H.; Lappano, R. The G Protein-Coupled Estrogen Receptor (GPER) Expression Correlates with Pro-Metastatic Pathways in ER-Negative Breast Cancer: A Bioinformatics Analysis. Cells 2020, 9, 622. [Google Scholar] [CrossRef]
- Weisz, A.; Rosales, R. Identification of an Estrogen Response Element Upstream of the Human C-Fos Gene That Binds the Estrogen Receptor and the AP-1 Transcription Factor. Nucleic Acids Res. 1990, 18, 5097–5106. [Google Scholar] [CrossRef]
- Welboren, W.J.; Van Driel, M.A.; Janssen-Megens, E.M.; Van Heeringen, S.J.; Sweep, F.C.; Span, P.N.; Stunnenberg, H.G. ChIP-Seq of ERα and RNA Polymerase II Defines Genes Differentially Responding to Ligands. EMBO J. 2009, 28, 1418–1428. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Luca, A.; Nocito, M.C.; Avena, P.; La Padula, D.; Zavaglia, L.; Pezzi, V. Role of GPER-Mediated Signaling in Testicular Functions and Tumorigenesis. Cells 2020, 9, 2115. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Krajewska, W.M. Significance of G Protein-Coupled Estrogen Receptor in the Pathophysiology of Irritable Bowel Syndrome, Inflammatory Bowel Diseases and Colorectal Cancer. Front. Endocrinol. 2020, 11, 390. [Google Scholar] [CrossRef]
- Jacenik, D.; Beswick, E.J.; Krajewska, W.M.; Prossnitz, E.R. G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis. World J. Gastroenterol. 2019, 25, 4092–4104. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, A.; Sebastiani, A.; Miglietta, A.M.; Rigiracciolo, D.C.; Cirillo, F.; Galli, G.R.; Talia, M.; Santolla, M.F.; Lappano, R.; Giordano, F. MiR-338-3p Is Regulated by Estrogens through GPER in Breast Cancer Cells and Cancer-Associated Fibroblasts (CAFs). Cells 2018, 7, 203. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liao, H.; Wang, Q. Activation of GPER by E2 Promotes Proliferation, Invasion and Migration of Breast Cancer Cells by Regulating the MiR-124/CD151 Pathway. Oncol. Lett. 2021, 21, 432. [Google Scholar] [CrossRef]
- He, S.Z.; Li, J.; Bao, H.C.; Wang, M.M.; Wang, X.R.; Huang, X.; Li, F.H.; Zhang, W.; Xu, A.L.; Fang, H.C. G Protein-Coupled Estrogen Receptor/MiR-148a/Human Leukocyte Antigen-G Signaling Pathway Mediates Cell Apoptosis of Ovarian Endometriosis. Mol. Med. Rep. 2018, 18, 1141–1148. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, Z.; Wang, W. MicroRNA-424 Suppresses Estradiol-Induced Cell Proliferation via Targeting GPER in Endometrial Cancer Cells. Cell. Mol. Biol. 2015, 61, 96–101. [Google Scholar] [CrossRef]
- Deng, J.; Wang, W.; Yu, G.; Ma, X. MicroRNA-195 Inhibits Epithelial-mesenchymal Transition by Targeting G Protein-coupled Estrogen Receptor 1 in Endometrial Carcinoma. Mol. Med. Rep. 2019, 20, 4023–4032. [Google Scholar] [CrossRef]
- Huang, R.; Li, J.; Pan, F.; Zhang, B.; Yao, Y. The Activation of GPER Inhibits Cells Proliferation, Invasion and EMT of Triple-Negative Breast Cancer via CD151/MiR-199a-3p Bio-Axis. Am. J. Transl. Res. 2020, 12, 32–44. [Google Scholar] [PubMed]
- Maitra, R.; Malik, P.; Mukherjee, T.K. Targeting Estrogens and Various Estrogen-Related Receptors against Non-Small Cell Lung Cancers: A Perspective. Cancers 2022, 14, 80. [Google Scholar] [CrossRef]
- Guan, B.Z.; Yan, R.L.; Huang, J.W.; Li, F.L.; Zhong, Y.X.; Chen, Y.; Liu, F.N.; Hu, B.; Huang, S.B.; Yin, L.H. Activation of G Protein Coupled Estrogen Receptor (GPER) Promotes the Migration of Renal Cell Carcinoma via the PI3K/AKT/MMP-9 Signals. Cell Adhes. Migr. 2018, 12, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Rigiracciolo, D.C.; Santolla, M.F.; Lappano, R.; Vivacqua, A.; Cirillo, F.; Galli, G.R.; Talia, M.; Muglia, L.; Pellegrino, M.; Nohata, N. Focal Adhesion Kinase (FAK) Activation by Estrogens Involves GPER in Triple-Negative Breast Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 58. [Google Scholar] [CrossRef] [PubMed]
- Avena, P.; Casaburi, I.; Zavaglia, L.; Nocito, M.C.; La Padula, D.; Rago, V.; Dong, J.; Thomas, P.; Mineo, C.; Sirianni, R. 27-Hydroxycholesterol Binds GPER and Induces Progression of Estrogen Receptor-Negative Breast Cancer. Cancers 2022, 14, 1521. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, F.Y.; Chen, W.; Fu, P.F.; Yao, M.Y.; Zheng, S.S. G15 Sensitizes Epithelial Breast Cancer Cells to Doxorubicin by Preventing Epithelial-Mesenchymal Transition through Inhibition of GPER. Am. J. Transl. Res. 2015, 7, 967–975. [Google Scholar]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging Role of Tumor Cell Plasticity in Modifying Therapeutic Response. Signal Transduct. Target 2020, 5, 228. [Google Scholar]
- De Francesco, E.M.; Maggiolini, M.; Musti, A.M. Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 2018, 19, 2011. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wei, W.; Jiang, G.M.; Liu, H.; Wei, W.D.; Yang, X.; Wu, Y.M.; Liu, H.; Wong, C.K.C.; Du, J. Activation of GPER Suppresses Epithelial Mesenchymal Transition of Triple Negative Breast Cancer Cells via NF-ΚB Signals. Mol. Oncol. 2016, 10, 775–788. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-Associated Fibroblasts: Overview, Progress, Challenges, and Directions. Cancer Gene Ther. 2021, 28, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. Tumor Microencironment. Physiol. Behav. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Cortés, M.; Sanchez-Moral, L.; de Barrios, O.; Fernández-Aceñero, M.J.; Martínez-Campanario, M.; Esteve-Codina, A.; Darling, D.S.; Győrffy, B.; Lawrence, T.; Dean, D.C. Tumor-associated Macrophages (TAMs) Depend on ZEB1 for Their Cancer-promoting Roles. EMBO J. 2017, 36, 3336–3355. [Google Scholar] [CrossRef]
- Wu, H.T.; Zhong, H.T.; Li, G.W.; Shen, J.X.; Ye, Q.Q.; Zhang, M.L.; Liu, J. Oncogenic Functions of the EMT-Related Transcription Factor ZEB1 in Breast Cancer. J. Transl. Med. 2020, 18, 51. [Google Scholar] [CrossRef]
- Sun, X.; Mao, Y.; Wang, J.; Zu, L.; Hao, M.; Cheng, G.; Qu, Q.; Cui, D.; Keller, E.T.; Chen, X. IL-6 Secreted by Cancer-Associated Fibroblasts Induces Tamoxifen Resistance in Luminal Breast Cancer. Oncogene 2014, 33, 4450. [Google Scholar] [CrossRef]
- Dittmer, A.; Lange, T.; Leyh, B.; Dittmer, J. Protein- And Growth-Modulatory Effects of Carcinoma-Associated Fibroblasts on Breast Cancer Cells: Role of Interleukin-6. Int. J. Oncol. 2020, 56, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling Pathways in Cancer-Associated Fibroblasts and Targeted Therapy for Cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The Protective Role of Estrogen and Estrogen Receptors in Cardiovascular Disease and the Controversial Use of Estrogen Therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 1, 121–126. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Maddalon, A.; Iulini, M.; Linciano, P.; Galbiati, V.; Marinovich, M.; Racchi, M.; Corsini, E.; Buoso, E. Effects of endocrine disrupting chemicals on the expression of RACK1 and LPS-induced THP-1 cell activation. Toxicology 2022, 480, 153321. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Galbiati, V.; Maddalon, A.; Iulini, M.; Kenda, M.; Sollner, D.M.; Marinovich, M.; Racchi, M.; Corsini, E. Effect of estrogen-active compounds on the expression of RACK1 and immunological implications. Arch. Toxicol. 2020, 94, 2081–2095. [Google Scholar] [CrossRef]
- Maddalon, A.; Masi, M.; Iulini, M.; Linciano, P.; Galbiati, V.; Marinovich, M.; Racchi, M.; Buoso, E.; Corsini, E. Effects of endocrine active contaminating pesticides on RACK1 expression and immunological consequences in THP-1 cells. Environ. Toxicol. Pharmacol. 2022, 95, 103971. [Google Scholar] [CrossRef]
- Maddalon, A.; Cari, L.; Iulini, M. Impact of endocrine disruptors on peripheral blood mononuclear cells in vitro: Role of gender. Arch. Toxicol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Holm, A.; Baldetorp, B.; Olde, B.; Leeb-Lundberg, L.M.F.; Nilsson, B.O. The GPER1 Agonist G-1 Attenuates Endothelial Cell Proliferation by Inhibiting DNA Synthesis and Accumulating Cells in the S and G2 Phases of the Cell Cycle. J. Vasc. Res. 2011, 48, 327–335. [Google Scholar] [CrossRef]
- Chen, Z.; Yuhanna, I.S.; Galcheva-Gargova, Z.; Karas, R.H.; Mendelsohn, M.E.; Shaul, P.W. Estrogen Receptor α Mediates the Nongenomic Activation of Endothelial Nitric Oxide Synthase by Estrogen. J. Clin. Investig. 1999, 103, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Lindner, V.; Kim, S.K.; Karas, R.H.; Kuiper, G.G.J.M.; Mendelsohn, M.E. Increased Expression of Estrogen Receptor-b MRNA in Male Blood Vessels After Vascular Injury. Circ. Res. 1998, 83, 224–229. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Mao, X.; Qi, H.; Baker, P.N.; Hua, Z. G-Protein-Coupled Receptor 30 Mediates the Effects of Estrogen on Endothelial Cell Tube Formation in Vitro. Int. J. Mol. Med. 2017, 39, 1461–1467. [Google Scholar] [CrossRef]
- Lappano, R.; Rigiracciolo, D.; De Marco, P.; Avino, S.; Cappello, A.R.; Rosano, C.; Maggiolini, M.; De Francesco, E.M. Recent Advances on the Role of G Protein-Coupled Receptors in Hypoxia-Mediated Signaling. AAPS J. 2016, 18, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Grände, P.O.; Ludueña, R.F.; Olde, B.; Prasad, V.; Leeb-Lundberg, L.M.F.; Nilsson, B.O. The G Protein-Coupled Oestrogen Receptor 1 Agonist G-1 Disrupts Endothelial Cell Microtubule Structure in a Receptor-Independent Manner. Mol. Cell. Biochem. 2012, 366, 239–249. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Pellegrino, M.; Santolla, M.F.; Lappano, R.; Ricchio, E.; Abonante, S.; Maggiolini, M. GPER Mediates Activation of HIF1α/VEGF Signaling by Estrogens. Cancer Res. 2014, 74, 4053–4064. [Google Scholar] [CrossRef]
- Maggiolini, M.; Santolla, M.F.; Avino, S.; Aiello, F.; Rosano, C.; Garofalo, A.; Grande, F. Identification of Two Benzopyrroloxazines Acting as Selective GPER Antagonists in Breast Cancer Cells and Cancer-Associated Fibroblasts. Futur. Med. Chem. 2015, 7, 437–448. [Google Scholar] [CrossRef]
- Rouhimoghadam, M.; Lu, A.S.; Salem, A.K.; Filardo, E.J. Therapeutic Perspectives on the Modulation of G-Protein Coupled Estrogen Receptor, GPER, Function. Front. Endocrinol. 2020, 11, 591217. [Google Scholar] [CrossRef]
- Arterburn, J.B.; Prossnitz, E.R. G Protein-Coupled Estrogen Receptor GPER: Molecular Pharmacology and Therapeutic Applications. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 295–320. [Google Scholar] [CrossRef]
- Geng, X.; Chen, H.; Zhao, L.; Hu, J.; Yang, W.; Li, G.; Cheng, C.; Zhao, Z.; Zhang, T.; Sun, B. Cancer-Associated Fibroblast (CAF) Heterogeneity and Targeting Therapy of CAFs in Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 655152. [Google Scholar] [CrossRef]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N.; Mei, Y.; Zhang, J. Cancer Metastasis: Issues and Challenges. Chin. J. Cancer 2017, 36, 36–39. [Google Scholar] [CrossRef]
- Jin, L.; Han, B.; Siegel, E.; Cui, Y.; Giuliano, A.; Cui, X. Breast Cancer Lung Metastasis: Molecular Biology and Therapeutic Implications. Cancer Biol. Ther. 2018, 19, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Muders, M.; Zhang, H.; Tindall, D.J. Mechanism of Lymph Node Metastasis in Prostate Cancer. Futur. Oncol. 2010, 6, 823–836. [Google Scholar] [CrossRef] [PubMed]


| miRNA | Target | GPER Regulation | Effect |
|---|---|---|---|
| miR338-3p | c-fos | Negative | Proliferation decreases in hepatocarcinoma [66] |
| miR-124 | CD151 | Negative | Proliferation decreases in luminal breast cancer [67] |
| miR148-a | Human leukocyte antigen-G | Negative | Cell death in ovarian endometriosis [68] |
| miR-424 | GPER | Negative | E2-induced proliferation in endometrial carcinoma [69] |
| miR-195 | GPER | Negative | PI3K/AKT-induced MMP-2/9 expression in endometrial carcinoma [70] |
| miR-199a-3p | CD151 | Positive | Promotes EMT in triple-negative breast cancer [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirado-Garibay, A.C.; Falcón-Ruiz, E.A.; Ochoa-Zarzosa, A.; López-Meza, J.E. GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments. Int. J. Mol. Sci. 2023, 24, 14993. https://doi.org/10.3390/ijms241914993
Tirado-Garibay AC, Falcón-Ruiz EA, Ochoa-Zarzosa A, López-Meza JE. GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments. International Journal of Molecular Sciences. 2023; 24(19):14993. https://doi.org/10.3390/ijms241914993
Chicago/Turabian StyleTirado-Garibay, Ana Carolina, Elba Andrea Falcón-Ruiz, Alejandra Ochoa-Zarzosa, and Joel E. López-Meza. 2023. "GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments" International Journal of Molecular Sciences 24, no. 19: 14993. https://doi.org/10.3390/ijms241914993
APA StyleTirado-Garibay, A. C., Falcón-Ruiz, E. A., Ochoa-Zarzosa, A., & López-Meza, J. E. (2023). GPER: An Estrogen Receptor Key in Metastasis and Tumoral Microenvironments. International Journal of Molecular Sciences, 24(19), 14993. https://doi.org/10.3390/ijms241914993







