1. Introduction
Menopause in women usually causes central obesity, leading to the development of obesity-related abnormalities, including dyslipidemia, hepatic steatosis, hyperglycemia, insulin resistance, and cardiovascular diseases [
1]. It is well known that menopause-associated hormonal changes are the predominant factors that cause central obesity and related metabolic abnormalities in women [
2]. Generally, loss of systemic estrogens has long been considered to be the primary mediator of central obesity and related metabolic abnormalities in menopausal women [
1]. However, recent studies using mouse models evidenced that the elevation of glucocorticoids (GCs; cortisol in humans and corticosterone in rodents) is the primary contributor to central obesity and related metabolic abnormalities in estrogen-depleted females [
3]. Because deprivation of GCs by adrenalectomy could completely prevent or reverse ovariectomy (OVX)-induced central obesity and related lipid metabolic abnormalities (e.g., hepatic steatosis) in female mice [
3]. Further, the sharp increase of follicle-stimulating hormone (FSH) has also been confirmed to be another key causative factor for the development of central obesity as well as related metabolic abnormalities in menopausal women and OVX mouse models [
3,
4,
5]. Therefore, the mechanisms underpinning menopause-induced central obesity and related metabolic abnormalities have not been fully explained so far. Even though it is clear that multiple altered endocrine hormones should act together to synergistically contribute to the development of central obesity and related metabolic abnormalities in menopausal women, thus, elucidation of the complex crosstalk between these altered endocrine hormones in the process of menopause-induced central obesity will provide key insights into such metabolic syndromes as well as better treatment.
Liver-derived fibroblast growth factor 21 (FGF21) is a recently recognized metabolic regulator for both carbohydrates and lipids, exerting great roles in maintaining glucose, lipid, and energy homeostasis [
6]. Amounting studies documented that, along with increased GCs, serum FGF21 levels were dramatically increased in both menopausal women [
7] and ovariectomized mice [
8] as well. Further, the parallel increase in circulating FGF21 and GCs levels was also observed in obese objects, including both rodents and humans [
9,
10,
11,
12], revealing that FGF21 may also play a key role in the development of obesity. Intriguingly, it has been evidenced that FGF21 and GCs regulate each other’s production in a feed-forward loop [
13]. Accordingly, we hypothesize that the increase of circulating FGF21 is an important causative factor for the development of central obesity in estrogen-depleted females via promoting GC production.
In this study, using OVX mice as a model, we first reported that liver-specific FGF21 knockout (FGF21 LKO) could completely reverse the elevation of circulating GCs and thus abrogate OVX-induced central obesity in mice. However, FGF21 LKO also caused the dissociation between decreased central obesity and the improvement of obesity-related metabolic syndromes, highlighting a complex role for hepatic FGF21 in the metabolic regulation of estrogen-depleted females. Our novel findings provide new insights into the etiology of central obesity in estrogen-depleted females and, meanwhile, are also crucial in developing effective prevention and management strategies for central obesity in menopausal women.
3. Discussion
The prevalence of central obesity and related metabolic abnormalities have become a major threat to the health and lifespan of menopausal women [
18]. Due to the complexity of its pathologies, so far, there is still no effective strategy to control the incidence and development of central obesity as well as related metabolic abnormalities in menopausal women [
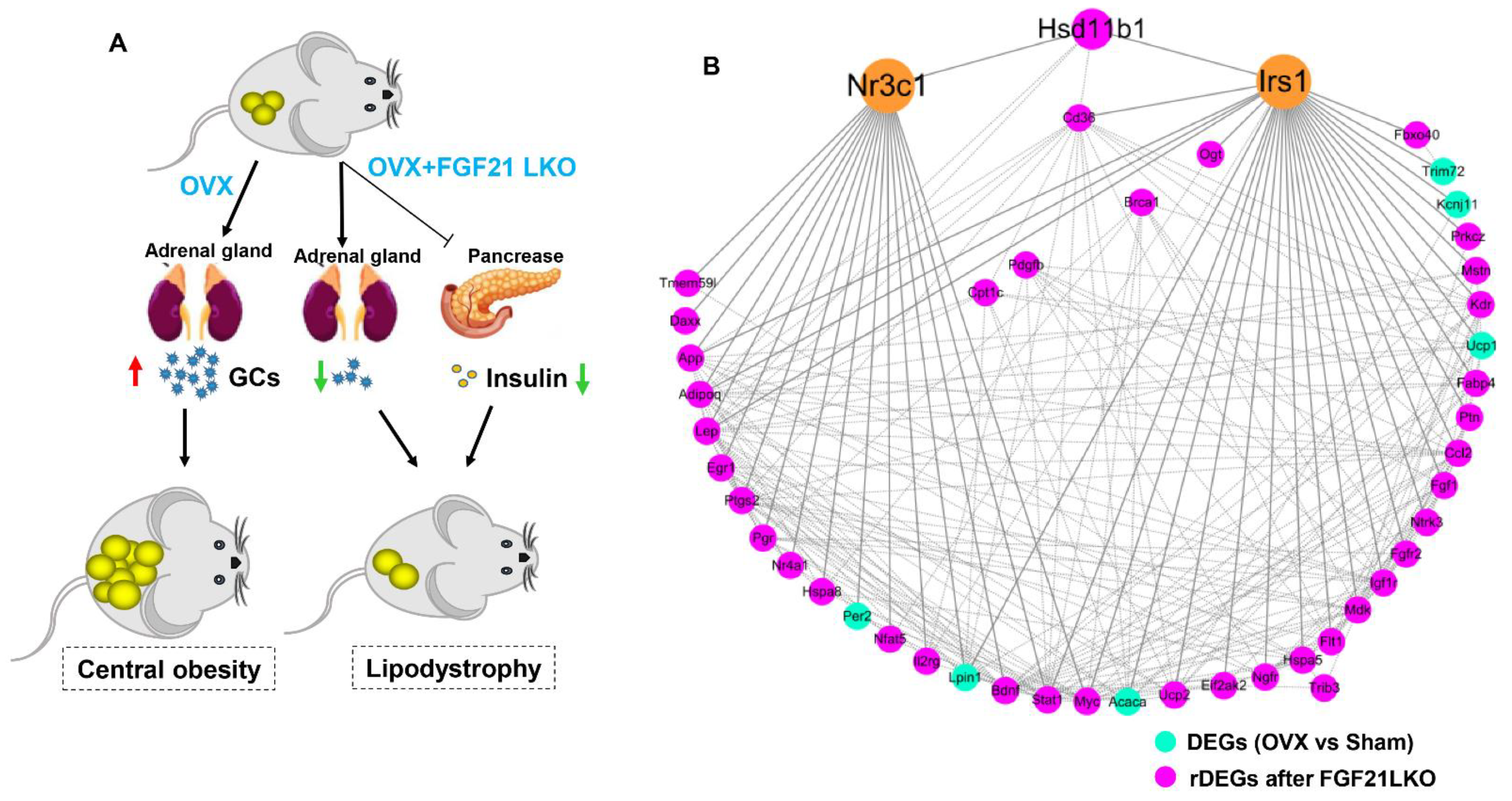
18]. In this study, we first found that liver-specific FGF21 knockout could completely abrogate OVX-induced central obesity by reversing high GC production in mice, highlighting an essential role of hepatic FGF21 in mediating the development of central obesity in estrogen-depleted females.
Adrenal gland-secreted GCs act as a master factor for controlling nutrient substrate availability and systemic fuel partitioning, thereby essentially regulating adipose expansion, distribution, and whole-body energy homeostasis [
19]. In humans, excessive production of GCs causes Cushing’s syndrome, characterized by central obesity, while insufficient production of GCs causes Addison’s disease with minimal adipose deposition [
20]. Both menopausal women [
21] and OVX mice [
3] develop Cushing-like syndrome characterized by drastically increased circulating GCs levels and the development of central obesity. Intriguingly, abrogating high circulating GCs by adrenalectomy could completely prevent or reverse OVX-induced central obesity in mice [
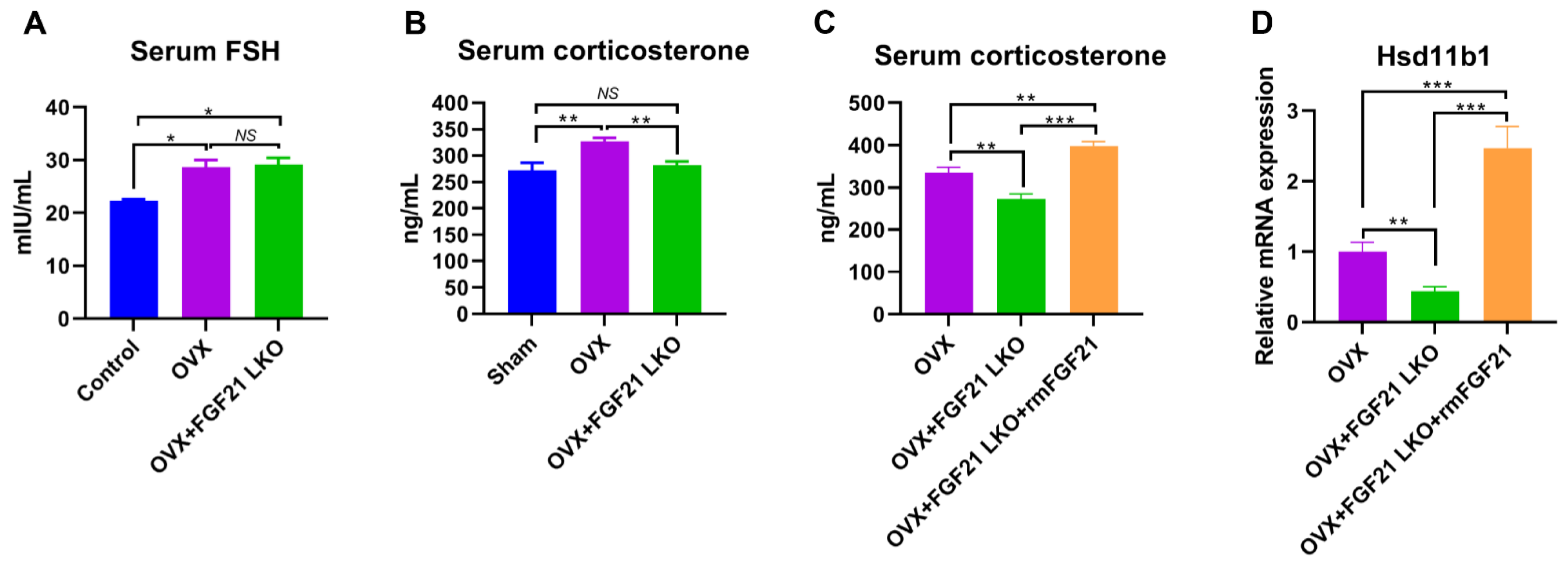
3], highlighting the essential role of GCs in mediating the development of central obesity in estrogen-depleted females. However, what factors cause increased GCs in estrogen-depleted females is still unclear so far. In this study, we found that circulating FGF21 and GCs were parallelly increased in female mice following OVX treatment and depletion of circulating FGF21 by FGF21 LKO completely reversed high circulating GCs, thus abrogating OVX-induced central obesity in female mice. Furthermore, transient replacement of recombinant FGF21 to OVX+FGF21 LKO mice could completely rescue OVX-induced high circulating GCs. All these results strongly demonstrate that liver-derived FGF21 plays an essential role in mediating OVX-induced central obesity by promoting high GC production.
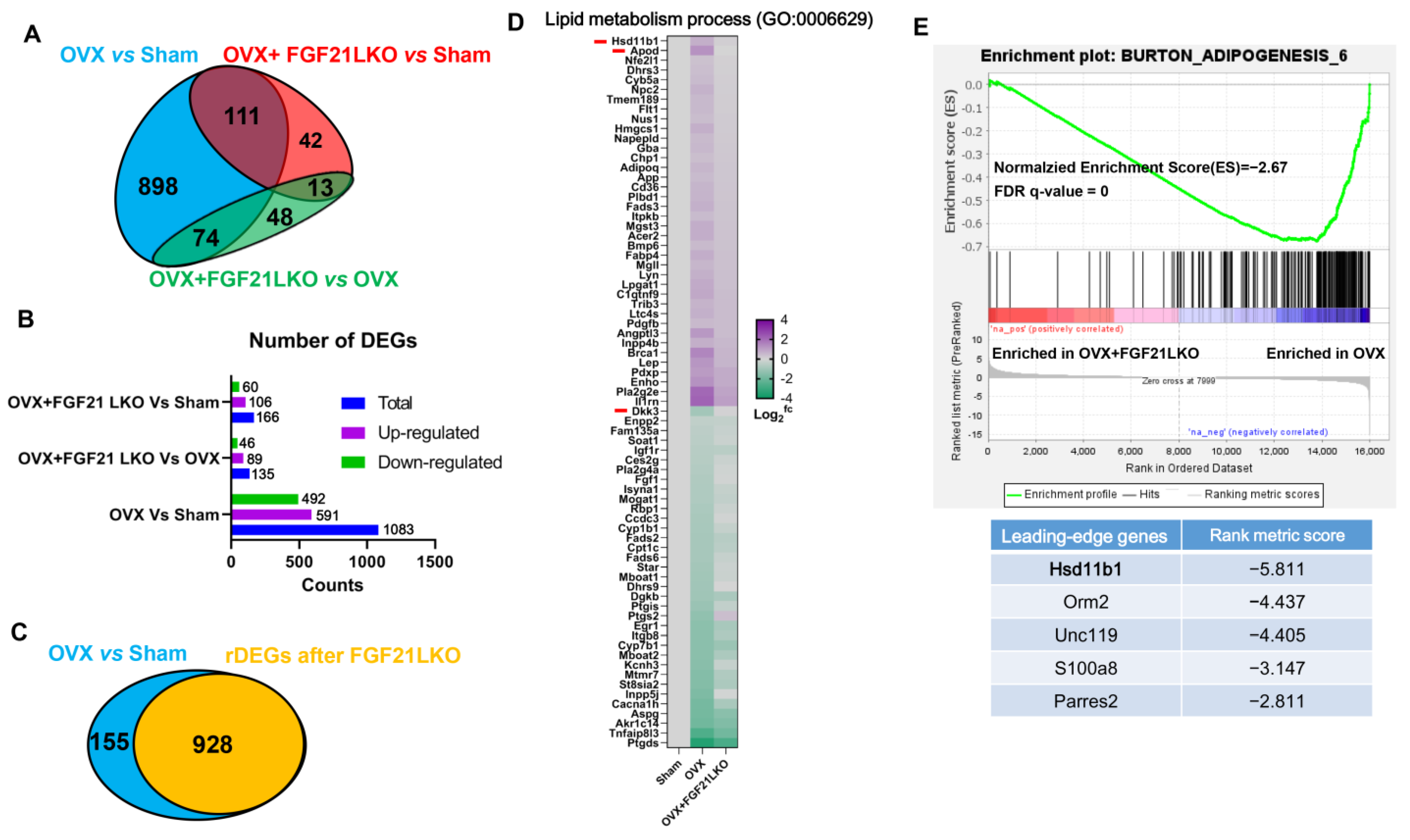
In addition to the regulated production of GCs by the hypothalamic-pituitary-adrenal (HPA) axis, the abundance of active GCs can be modulated by local tissue enzymes. 11Beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1) converts inactive cortisone (in humans) or 11-dehydrocorticosterone (in rodents) into its active form cortisol (in humans) or corticosterone (in rodents) [
22] thereby regulating the local availability of active GCs. Previous studies indicated that GCs excess acts as a feed-forward signal to increase tissue local GCs availability by stimulating 11β-HSD1 expression and activities, and global 11β-HSD1 knockout mice were protected from GCs excess-caused central obesity [
15], suggesting an essential role for 11β-HSD1 in mediating circulating GCs excess for causing visceral adipose accumulation and the incidence of central obesity. In the present study, we found FGF21 LKO completely abrogated OVX-induced
11β-HSD1 hyper-expression in visceral adipose tissues, and further replacement of recombinant FGF21 to the OVX+FGF21 LKO mice could significantly rescue their visceral adipose
11β-HSD1 hyper-expression. Accordingly, we suggested that the liver FGF21-adrenal GCs-visceral adipose
11β-HSD1 signaling cascade plays a central role in mediating OVX-induced central obesity. Thus, strategies targeting any element in such a signaling cascade may be applied to prevent and treat central obesity in estrogen-depleted females.
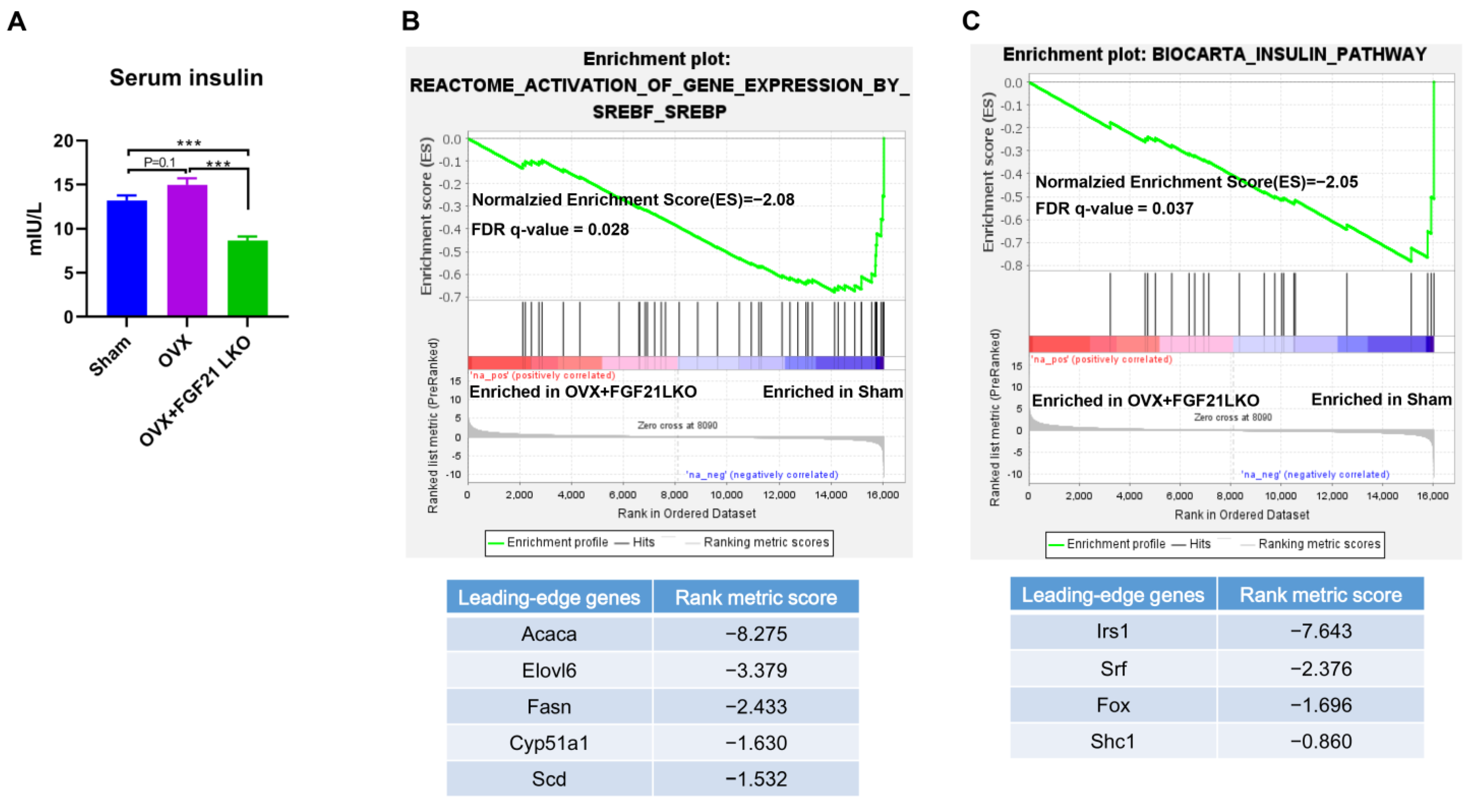
It is well known that insulin plays a crucial role in both adipogenesis and lipogenesis, and the deletion of the constitutive insulin receptor (IR) in adipocytes causes lipodystrophy [
23]. Intriguingly, recent studies newly evidenced that GCs excess causes hyperinsulinemia, which is a primary factor inducing central obesity [
24]. In the present study, OVX increased circulating GCs and caused hyperinsulinemia, while FGF21 LKO completely reversed OVX-induced high GCs and caused hypoinsulinemia in mice. Therefore, the drop in GCs likely decreased insulin levels, which may be another important mechanism that mediates FGF21 LKO to abrogate OVX-induced central obesity in females. Further, FGF21 itself also plays a crucial role in preserving β-cell function and survival and in the maintenance of normal islet cell growth, islet function, and insulin synthesis [
25]. Thus, in addition to decreasing GCs’s production, deficiency of FGF21 per se also should have direct effects to reduce insulin production/secretion and, in turn, visceral adipose deposition in OVX+FGF21 LKO mice. This was possibly why the serum insulin levels and visceral fat weight of OVX+FGF21 LKO mice were even significantly lower or tended to be lower than those of the sham controls.
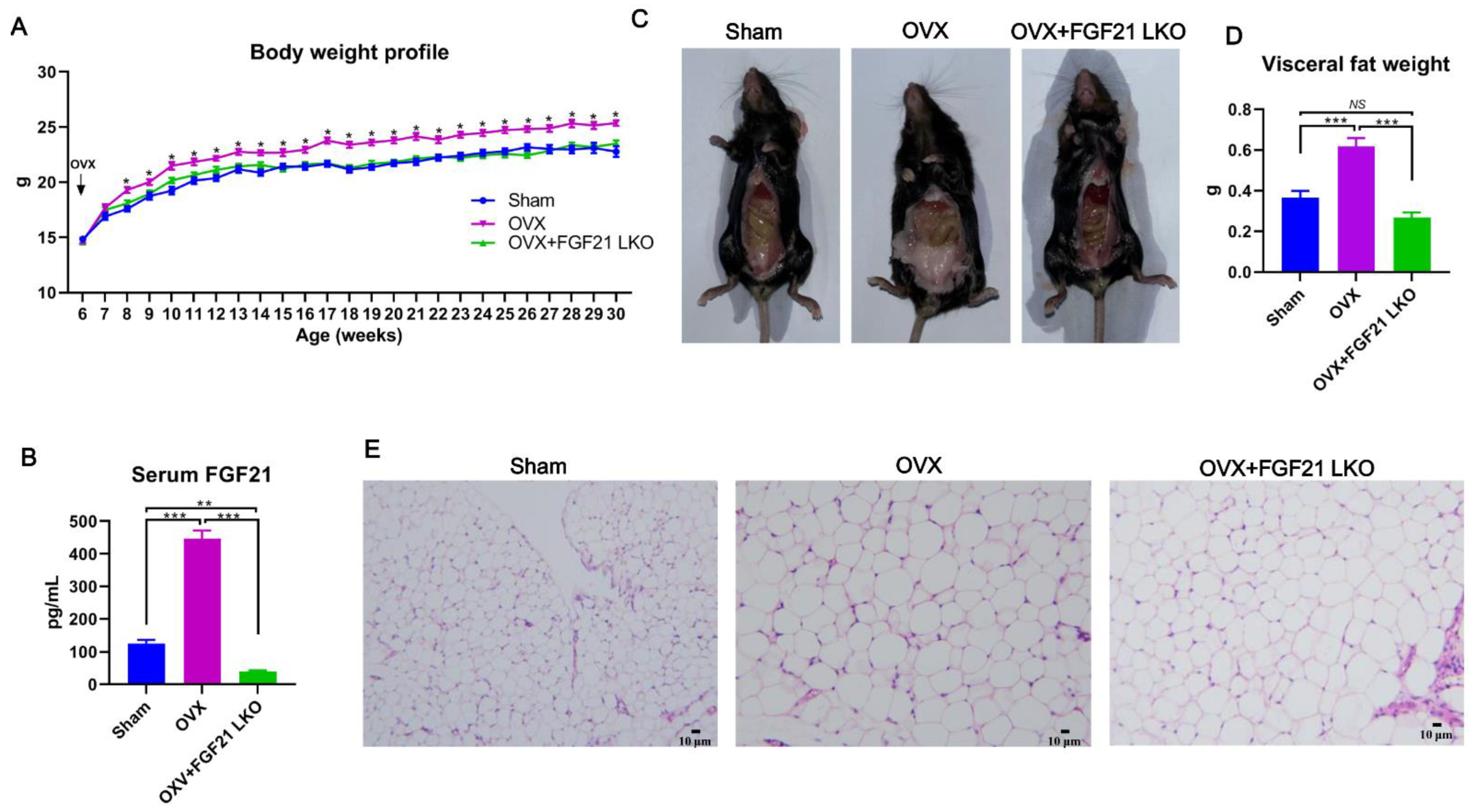
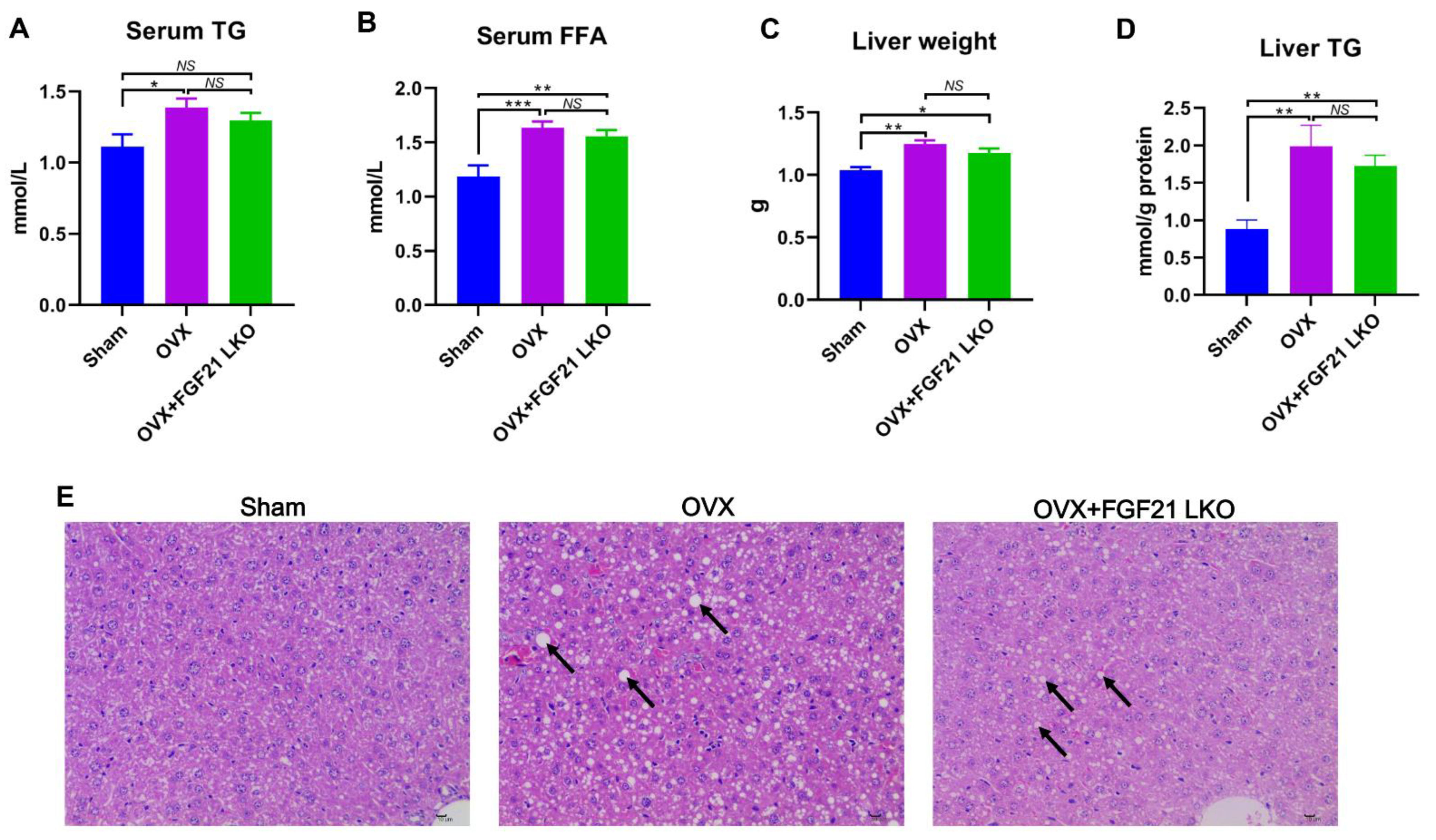
The incidence of central obesity usually causes metabolic syndromes, such as dyslipidemia, hepatic steatosis, and insulin resistance [
26]. Consistently, all these metabolic syndromes occurred in female mice following OVX in the present study. Unexpectedly, FGF21 LKO completely abrogated OVX-induced central obesity but not these obesity-related metabolic disorders. Thus, it appears that the increased visceral adipose accumulation is not the major or determinant factor for causing these metabolic syndromes, or in other words, there is a dissociation between pure increased visceral adipose deposition and the incidence of metabolic disorders in estrogen-depleted females. Indeed, in both menopausal women and OVX mice, multiple factors or mechanisms have been verified to be involved in their metabolic deterioration [
1,
3]. For example, the drastic elevation of FSH has been well established to exert great roles in causing dyslipidemia, hepatic steatosis, and insulin resistance in both menopausal women and OVX mice [
3,
5,
27]. However, serum FSH levels were not reversed by FGF21 LKO in OVX mice. Collectively, our results suggest that the altered endocrine hormones like increased FSH rather than mere increased visceral adipose deposition may play a more important role in promoting the development of metabolic abnormalities, including dyslipidemia, hepatic steatosis, and insulin resistance in estrogen-depleted females.
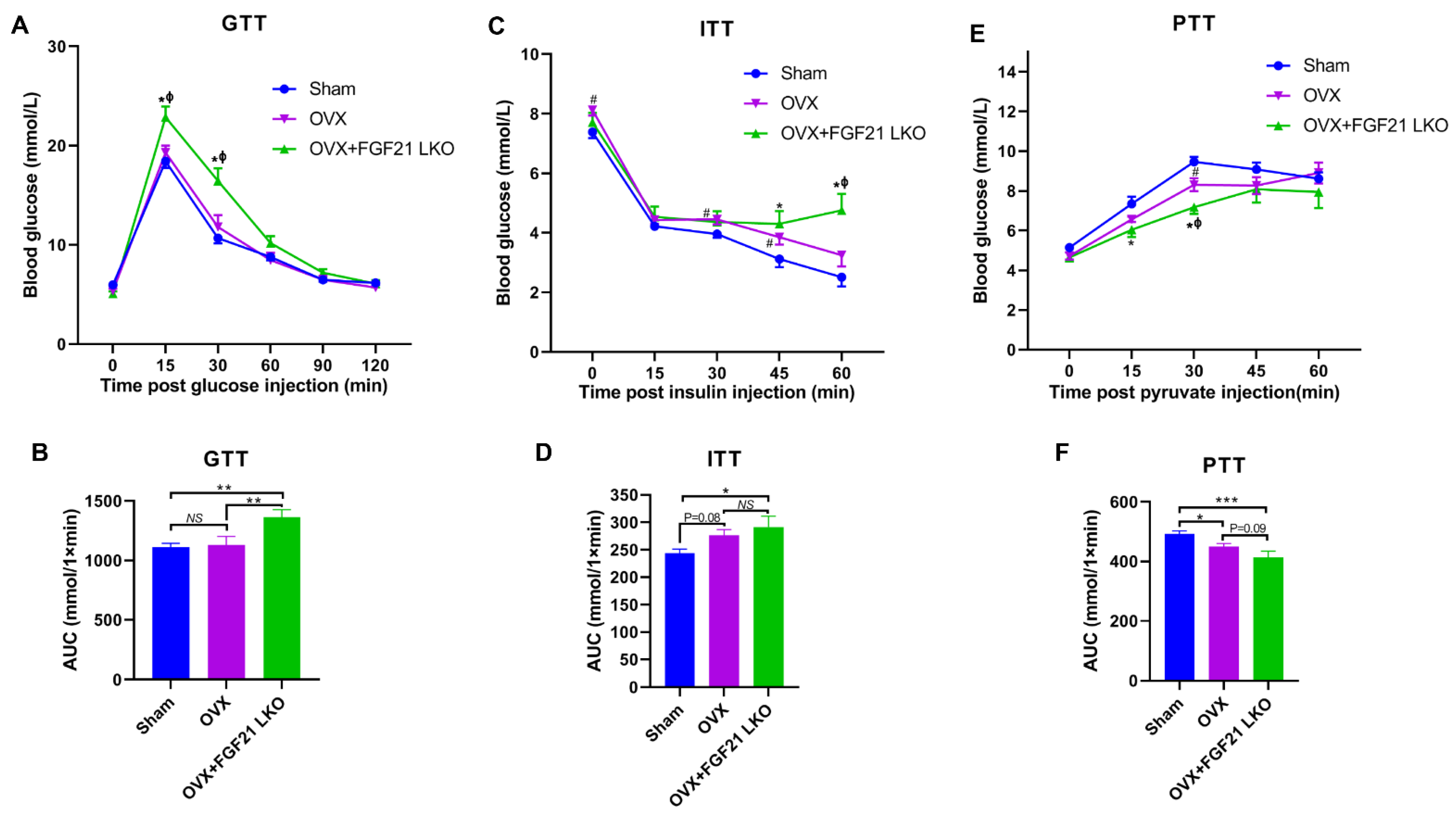
Surprisingly, FGF21 LKO did not alleviate but even exacerbated whole body glucose metabolic abnormalities in OVX mice, as evidenced by more impaired glucose and pyruvate tolerance, and worsened insulin resistance in OVX+FGF21 LKO mice compared to OVX mice. Integrated data analysis also indicated that FGF21 LKO impaired insulin signaling in OVX mice. Insulin plays a central role in regulating whole-body glucose homeostasis [
17]. In the present study, FGF21 LKO drastically decreased the circulating insulin levels in OVX mice, which could well explain why the metabolic abnormality in OVX mice was further exacerbated by FGF21 KO. Furthermore, in addition to acting as a master factor for modulating glucose homeostasis, insulin is also essential for the formation, maintenance, and function of white adipose tissue [
17]. Deficiency of insulin signaling permits unregulated adipose tissue lipolysis, leading to dyslipidemia and the ectopic deposition of lipids in non-adipose tissues such as the liver, further causing hepatic steatosis and exacerbating systemic insulin resistance [
28]. Thus, reduced insulin production may be the central factor responsible for the dissociation between decreased visceral adipose deposition and the improvement of metabolic abnormalities in OVX+FGF21 LKO mice. Accordingly, mild suppression of hyper-FGF21 without unfavorable insulin-associated metabolic changes should be an interesting strategy to prevent or treat OVX/menopause-caused central obesity.
In summary, liver-derived FGF21 plays an essential role in mediating the development of central obesity in OVX mice by promoting GC production. However, FGF21 LKO also reduced circulating insulin levels, leading to the dissociation between decreased visceral adipose deposition and the improvement of metabolic abnormalities, highlighting the detrimental role of hepatic FGF21 signaling in the development of central obesity but a beneficial role in protecting metabolic abnormality from further exacerbation in estrogen-depleted females. Our novel findings provide new insights into the pathology of central obesity and related metabolic disorders in estrogen-depleted females and will facilitate the development of new strategies for preventing or treating central obesity and related metabolic abnormalities in menopausal women.
5. Materials and Methods
5.1. Animals and Treatments
The liver-specific FGF21 knockout mice were generated as in our previous descriptions [
29]. Namely, the FGF21
loxp/loxp mice (Jackson Laboratory; Stock # 022361; Bar Harbor, ME, USA) were crossed to Alb
Cre/Cre mice (Jackson Laboratory; Stock # 003574; Bar Harbor, ME, USA) to generate Alb
Cre/; FGF21
loxp/− mice. Then, the Alb
Cre/; FGF21
loxp/− mice were intercrossed to generate Alb
Cre+; FGF21
loxp/loxp mice (FGF21 LKO) and Alb
Cre−; FGF21
loxp/loxp mice (WT) for this study. For studying the effects of liver-derived FGF21 on OVX-induced central obesity in female mice, 12 FGF21 LKO and 24 WT female littermates were selected. At 6 weeks of age, 12 FGF21 LKO (OVX+FGF21 LKO) and 12 randomly selected WT (OVX) were ovariectomized under anesthesia. To clarify the effects of OVX and OVX+FGF21 LKO on adipose deposition and related metabolic abnormalities, the remaining 12 WT littermates were undergone sham surgery and used as the control. After surgery, all mice were maintained in a controlled microenvironment with a temperature of 21 ± 2 °C, a relative humidity of 50–60%, and a 12 h light/12 h dark cycle (lights on at 06:00 a.m.) as measured using the facility monitoring system (Phoenix Controls, Newtown Square, PA, and Comdale Systems, Toronto, Ontario, Canada). All mice had free access to water and a standard chow. Additionally, lighting conditions were controlled using a programmable electronic timer (ChronTrol XT, ChronTrol Corp., San Diego, CA, USA). Mice were weighted weekly until sacrifice at age of 30 weeks. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Sichuan Agricultural University (No: 20220612). All animal experiments were conducted in accordance with the institutional guidelines for laboratory animals established by the animal care and use committee of Sichuan Agricultural University, and all methods strictly obeyed the Guide for the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines 2.0 [
30].
5.2. Glucose, Insulin and Pyruvate Tolerance Tests
One to two week(s) before decapitation, all mice have undergone glucose, insulin, and pyruvate tolerance tests according to previous studies with minor modifications [
31]. Namely, for the glucose tolerance test (GTT), all mice were fasting overnight for 18 h, then intraperitoneally (i.p.) injected with D-glucose (Sigma-Aldrich, St. Louis, MI, USA) at a dose of 1 g/kg body weight. Blood glucose concentrations were measured at 0, 15, 30, 60, 90, and 120 min with a handheld glucometer (Roche Diagnostics, Mannheim, Germany). For the insulin tolerance test (ITT) and pyruvate tolerance test (PTT), the mice were i.p. injected with the regular 30/70 recombinant human insulin (Eli Lily, Indianapolis, IN, USA) at a dose of 0.75 U/kg body weight after a 4 h fasting, or pyruvate (Sigma-Aldrich) at a dose of 1.5 mg/kg body weight after a 18 h fasting. Then, blood glucose concentrations were measured at 0, 15, 30, 45, and 60 min for both tests with a handheld glucometer (Roche Diagnostics). Areas under the curve (AUCs) were calculated for glucose, insulin and pyruvate tolerance tests using the blood glucose data.
5.3. Sample Collections and Parameter Measurements at Decapitation
At 30 weeks of age, all mice were anesthetized with isoflurane (Fluriso; VetOne, Boise, ID, USA), weighed and euthanized. For decreasing the influence of estrus cyclicity on experimental results, all mice from the sham group were sacrificed at the dioestrus phase. The trunk blood was collected and centrifuged at 2000× g for 15 min at 4 °C, and the sera were stored at −20 °C, pending analyses. After sacrifice, the abdominal adipose tissues were collected and weighed, one portion of the abdominal adipose tissues were immediately frozen in liquid nitrogen and stored at −80 °C for RNA-sequencing analysis, and the remaining abdominal adipose tissues were fixed in 10% buffered formalin and used for histology analysis.
5.4. Serum Hormone and Lipid Determination
Serum FGF21 (MF2100; R&D Systems, Minneapolis, MN, USA), FSH (CSB-E06871m; CUSABIO, Houston, TX, USA), corticosterone (KGE009; R&D Systems, Minneapolis, MN, USA) and insulin (80-INSHU-CH01; ALPCO, Salem, NH, USA) concentrations were determined with commercial ELISA kits, according to the manufacturer’s instruction. Serum-free fat acid (FFA) and triglyceride (TG) levels were measured via a nonesterified free fatty acids assay kit (A042-2-1) and triglyceride assay Kit (A110-1-1) from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), as our previous descriptions [
32]. The liver tissue of mice was added with nine times the amount of saline to prepare 10% tissue homogenization in the ice water bath, and TG levels in the liver tissue of mice were measured using the enzymic method according to the kit instructions.
5.5. RNA-Sequencing
Total RNA was extracted from the crushed visceral adipose tissue samples using TRIzol™ Reagent (Invitrogen, Carlsbad, CA, USA). RNA quantity and quality were determined by using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA). 1000 ng of total RNA per sample with RNA integrity numbers (RINs) greater than or equal to 8.0 was used as input material to prepare the RNA sample. Briefly, mRNA was purified from total RNA by using oligo (dT) magnetic capture beads. Libraries were synthesized by using the NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB, Ipswich, MA, USA) according to the manufacturer’s instructions. Then, the cDNA library was sequenced on an illu-mina Novaseq platform and generated 150 bp paired-end reads. Fastp (Version 0.19.7) was used to filter low-quality reads (defined as reads with more than 50% beads scoring Qphred ≤ 20) and to remove adapter sequences to generate clean reads. Mouse reference genome (
http://ftp.ensembl.org/pub/release-105/fasta/mus_musculus/dna/, accessed on 16 May 2022) and gene annotation files (
http://ftp.ensembl.org/pub/release-105/gtf/mus_musculus/, accessed on 16 May 2022) were down-loaded from Ensembl directly to build the index of the reference genome; then clean reads were then aligned to the reference genome using Hisat2 (v2.0.5). For gene expression level quantification, featureCounts (v1.5.0-p3) was used to count the reads numbers mapped to each gene. The R package DESeq2 (1.20.0) was used to perform pairwise comparisons among groups; the resulting
p-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate (FDR), the gene met the criteria of FDR < 0.05 were considered as a differentially expressed gene (DEG).
5.6. Functional Enrichment Analysis
Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics database (v2023q2) was used to perform gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. For all GO terms and KEGG pathways, a threshold of p value < 0.05 was set for significance.
5.7. Protein–Protein Interaction Network Analysis
Protein–protein interaction (PPI) network was built up using the STRING APP of Cytoscape (3.9.1); the minimum required interaction score was set as 0.4. Cytoscape (3.9.1) was used for network visualization.
5.8. FGF21 Administration In Vivo
To evaluate whether FGF21 replacement could rescue OVX-induced high circulating corticosterone levels in OVX+FGF21 LKO mice, eight wild-types (Alb
Cre-; FGF21
loxp/loxp) and 16 FGF21 LKO (Alb
Cre+; FGF21
loxp/loxp) female mice from the breeding cages were selected, and all mice were bilaterally ovariectomized at 6 wk of age, and randomly allocated into two subgroups (n = 8). Four weeks after surgery procedures (i.e., at 10 week of age), a subgroup of OVX+FGF21 LKO mice were injected via tail vein with recombinant mouse FGF21 (RD272108100, BioVendor, Prague, Czech Republic) at a dose of 1 mg/kg body weight at 0800 AM, according to our previous descriptions [
29]. Additionally, the other subgroup of OVX+FGF21 LKO and OVX mice were injected with vehicle (0.9% saline). Two hours after injection, all mice were decapitated, the trunk blood was collected, and visceral adipose was obtained and stored at −80 °C.
5.9. Quantitative Reverse Transcription PCR (qRT-PCR)
To verify the effects of FGF21 replacement on visceral adipose Hsd11b1 expression, the visceral adipose tissues from animals were collected for RT-qPCR analysis. Briefly, total RNA was isolated from visceral adipose tissues using TRIZOL(Invitrogen Co., Carlsbad, CA, USA), according to the manufacturer’s instructions. Quantitative and qualitative analyses of isolated RNA were assessed from the ratio of absorbance at 260 and 280 nm and agarose gel electrophoresis. First-strand complementary DNA (cDNA) was reverse-transcribed (500 ng total RNA) using a PrimeScript® RT reagent kit with gDNA Eraser (TaKaRa Bio, Co., Ltd., Dalian, China) and used as the template for subsequent real-time quantitative PCR reaction (RT-qPCR), done in triplicate on a CFX96 Real-Time PCR detection system (Bio-Rad, Hercules, CA, USA) with SYBR® greenII. RT-qPCR was conducted for 43 cycles with 5 s at 94 °C for denaturing, 25 s at 60 °C for annealing and primer extension, and a final melting curve analysis to monitor the purity of the PCR product. Each PCR reaction (10 μL) contained the same amount of cDNA (1 μL template), 500 nmol/L each of forward and reversed primers, and 2XSYBR® premix TaqTM (TaKaRa Bio Co., Ltd., Beijing, China). The cycle threshold value was analyzed (CFX96 detection system) and transformed to a relative quantity using a standard curve. Relative gene expression levels were normalized to GAPDH. Outcomes were expressed as fold changes relative to average mRNA levels of genes in sham controls. The sequences of primers used for qRT-PCR are as follows: Hsd11b1 forward: 5′- CAGAAATGCTCCAGGGAAAGAA-3′, reverse: 5′- GCAGTCAATACCACATGGGC-3′; GAPDH forward: 5′- AGGTCGGTGTGAACGGATTTG-3′, reverse: 5′- TGTAGACCATGTAGTTGAGGTCA-3′.
5.10. Statistical Analyses
All statistical analyses except RNA-sequencing data were performed with GraphPad Prism 9.2 software (La Jolla, CA, USA). Comparisons among groups were carried out via one-way ANOVA followed by Turkey’s test. For analysis of the effect of treatment in repeated measures (i.e., body weight), two-way ANOVA followed by Sidak’s multiple comparisons test was used. All values were presented as mean ± SEM. Significance is given as * p < 0.05, ** p < 0.01 and *** p < 0.001.