The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer
Abstract
1. Introduction
2. The Role of PPARs in Endometrial Cancer
3. The Role of PPARα in Endometrial Cancer
4. The Role of PPARβ/δ in Endometrial Cancer
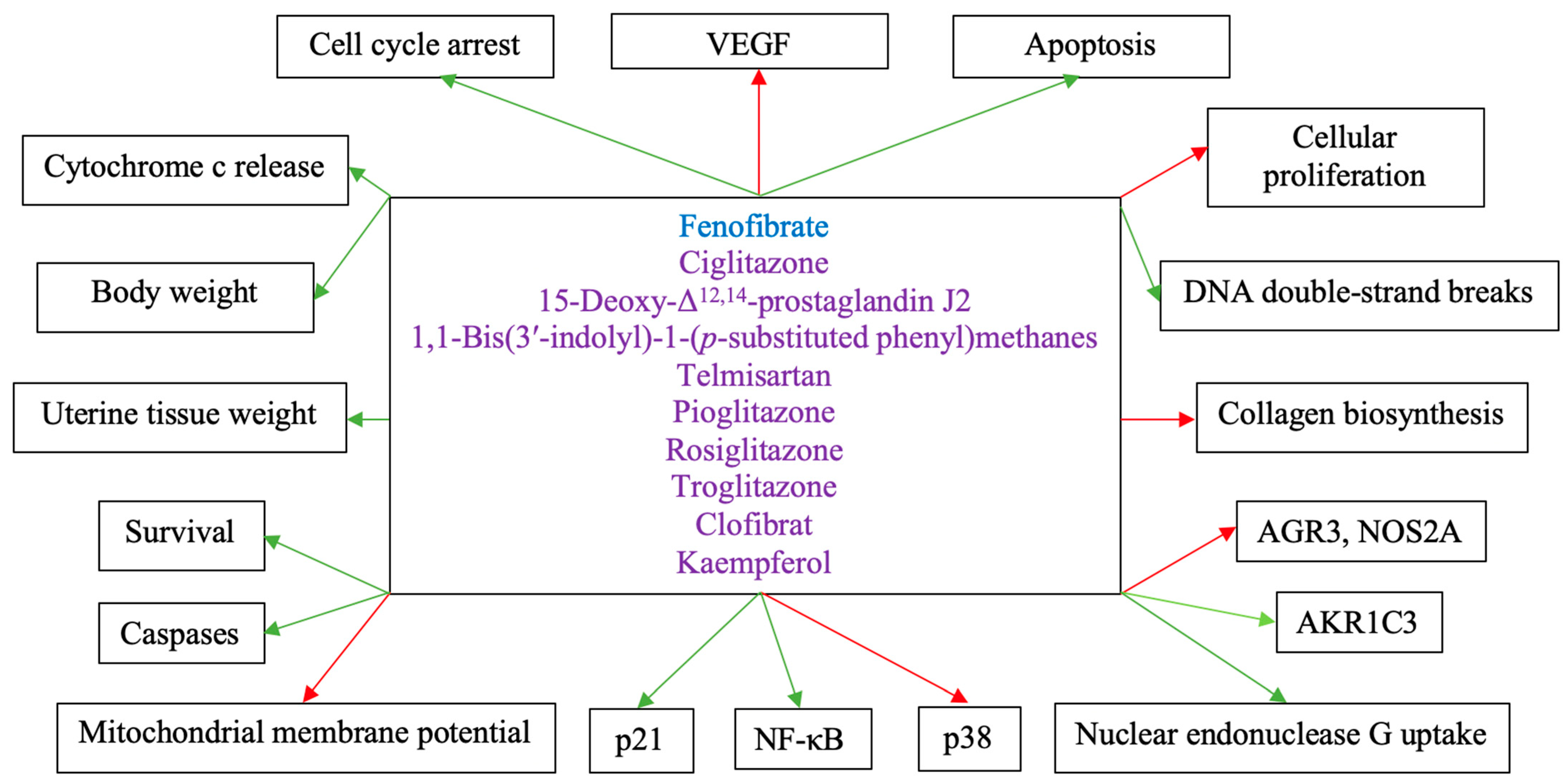
5. The Role of PPARγ in Endometrial Cancer
6. The Role of PPARs in Other Uterine Cancer Entities
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ACS. Key Statistics for Endometrial Cancer: American Cancer Society. 2023. Available online: https://www.cancer.org/cancer/types/endometrial-cancer/about/key-statistics.html (accessed on 16 April 2023).
- NIH. Cancer Stat Facts: Uterine Cancer: American Cancer Society. 2023. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 16 April 2023).
- Sherman, M.E.; Bur, M.E.; Kurman, R.J. p53 in endometrial cancer and its putative precursors: Evidence for diverse pathways of tumorigenesis. Hum. Pathol. 1995, 26, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Takai, N.; Narahara, H. Preclinical studies of chemotherapy using histone deacetylase inhibitors in endometrial cancer. Obstet. Gynecol. Int. 2010, 2010, 923824. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dowdy, S.C.; Meng, X.W.; Wang, Z.; Jones, M.B.; Podratz, K.C.; Jiang, S.W. Histone deacetylase inhibitors induce apoptosis in both Type I and Type II endometrial cancer cells. Gynecol. Oncol. 2007, 105, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Bestvina, C.M.; Fleming, G.F. Chemotherapy for Endometrial Cancer in Adjuvant and Advanced Disease Settings. Oncologist 2016, 21, 1250–1259. [Google Scholar] [CrossRef]
- Ueda, T.; Takai, N.; Nishida, M.; Nasu, K.; Narahara, H. Apicidin, a novel histone deacetylase inhibitor, has profound anti-growth activity in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 2007, 19, 301–308. [Google Scholar] [CrossRef]
- Psilopatis, I.; Pergaris, A.; Giaginis, C.; Theocharis, S. Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma. Dis. Markers 2021, 2021, 7850688. [Google Scholar] [CrossRef]
- ACS. Survival Rates for Endometrial Cancer: American Cancer Society. 2023. Available online: https://www.cancer.org/cancer/types/endometrial-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 16 April 2023).
- Auwerx, J.; Baulieu, E.; Beato, M.; Becker-Andre, M.; Burbach, P.H.; Camerino, G.; Chambon, P.; Cooney, A.; Dejean, A.; Dreyer, C.; et al. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999, 97, 161–163. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-beta/delta and PPAR-gamma. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Wagner, N. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 2010, 125, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Fleckenstein, F.N.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Preeclampsia. Cells 2023, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Vrettou, K.; Nousiopoulou, E.; Palamaris, K.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 2912. [Google Scholar] [CrossRef]
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Kumar, B.R.P. Peroxisome Proliferator-activated Receptor-gamma As A Novel and Promising Target For Treating Cancer Via Regulation of Inflammation: A Brief Review. Mini Rev. Med. Chem. 2022, 22, 3–14. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Hsu, J.Y.; Chowbina, S.R.; Lawrence, R.T.; Hoehn, K.L. Not all fat is equal: Differential gene expression and potential therapeutic targets in subcutaneous adipose, visceral adipose, and endometrium of obese women with and without endometrial cancer. Int. J. Gynecol. Cancer 2012, 22, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, J.; Fan, L. Comprehensive Analysis of Copy Number Variation, Nucleotide Mutation, and Transcription Level of PPAR Pathway-Related Genes in Endometrial Cancer. PPAR Res. 2022, 2022, 5572258. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Blachnio-Zabielska, A.; Jarzabek, K.; Wolczynski, S. Altered peroxisome-proliferator activated receptors expression in human endometrial cancer. PPAR Res. 2012, 2012, 471524. [Google Scholar] [CrossRef] [PubMed]
- Nickkho-Amiry, M.; McVey, R.; Holland, C. Peroxisome proliferator-activated receptors modulate proliferation and angiogenesis in human endometrial carcinoma. Mol. Cancer Res. 2012, 10, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.M.; Saidi, S.A.; Evans, A.L.; Sharkey, A.M.; Latimer, J.A.; Crawford, R.A.; Charnock-Jones, D.S.; Print, C.G.; Smith, S.K. Transcriptome analysis of endometrial cancer identifies peroxisome proliferator-activated receptors as potential therapeutic targets. Mol. Cancer Ther. 2004, 3, 993–1001. [Google Scholar] [CrossRef]
- Saidi, S.A.; Holland, C.M.; Charnock-Jones, D.S.; Smith, S.K. In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Mol. Cancer 2006, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.J.; Tan, J.; Tajeda, L.; Das, S.K.; Chapman, J.A.; DuBois, R.N.; Dey, S.K. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia 2000, 2, 483–490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.J.; Monsivais, D.; Dyson, M.T.; Coon, J.S.; Malpani, S.; Ono, M.; Zhao, H.; Xin, H.; Pavone, M.E.; Kim, J.J.; et al. Ligand-activated peroxisome proliferator-activated receptor beta/delta modulates human endometrial cancer cell survival. Horm. Cancer 2013, 4, 358–370. [Google Scholar] [CrossRef]
- Gu, C.; Yang, H.; Chang, K.; Zhang, B.; Xie, F.; Ye, J.; Chang, R.; Qiu, X.; Wang, Y.; Qu, Y.; et al. Melatonin alleviates progression of uterine endometrial cancer by suppressing estrogen/ubiquitin C/SDHB-mediated succinate accumulation. Cancer Lett. 2020, 476, 34–47. [Google Scholar] [CrossRef]
- Paynter, R.A.; Hankinson, S.E.; Colditz, G.A.; Hunter, D.J.; De Vivo, I. No evidence of a role for PPARgamma Pro12Ala polymorphism in endometrial cancer susceptibility. Pharmacogenetics 2004, 14, 851–856. [Google Scholar] [CrossRef]
- Smith, W.M.; Zhou, X.P.; Kurose, K.; Gao, X.; Latif, F.; Kroll, T.; Sugano, K.; Cannistra, S.A.; Clinton, S.K.; Maher, E.R.; et al. Opposite association of two PPARG variants with cancer: Overrepresentation of H449H in endometrial carcinoma cases and underrepresentation of P12A in renal cell carcinoma cases. Hum. Genet. 2001, 109, 146–151. [Google Scholar] [CrossRef]
- Huang, M.; Chen, L.; Mao, X.; Liu, G.; Gao, Y.; You, X.; Gao, M.; Sehouli, J.; Sun, P. ERRalpha inhibitor acts as a potential agonist of PPARgamma to induce cell apoptosis and inhibit cell proliferation in endometrial cancer. Aging 2020, 12, 23029–23046. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, X.; Gao, S. Stimulation of peroxisome proliferator-activated receptor gamma inhibits estrogen receptor alpha transcriptional activity in endometrial carcinoma cells. Oncol. Rep. 2015, 33, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Cormio, A.; Guerra, F.; Cormio, G.; Pesce, V.; Fracasso, F.; Loizzi, V.; Cantatore, P.; Selvaggi, L.; Gadaleta, M.N. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem. Biophys. Res. Commun. 2009, 390, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yang, H.; Wang, C.; Ma, X. The effects of PGC-1alpha on the proliferation and energy metabolism of malignant endometrial cancer cells. Onco Targets Ther. 2015, 8, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Wersall, O.C.; Lofstedt, L.; Govorov, I.; Mints, M.; Gabrielson, M.; Shoshan, M. PGC1alpha and VDAC1 expression in endometrial cancer. Mol. Clin. Oncol. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, R.; Liu, H.; Ren, Z.; Kong, F.; Li, D.; Ma, X. Synergism between PGC-1alpha and estrogen in the survival of endometrial cancer cells via the mitochondrial pathway. Onco Targets Ther. 2016, 9, 3963–3973. [Google Scholar] [CrossRef]
- Yang, H.; Yang, R.; Liu, H.; Ren, Z.; Wang, C.; Li, D.; Ma, X. Knockdown of peroxisome proliferator-activated receptor gamma coactivator-1 alpha increased apoptosis of human endometrial cancer HEC-1A cells. Onco Targets Ther. 2016, 9, 5329–5338. [Google Scholar] [CrossRef]
- Yoriki, K.; Mori, T.; Kokabu, T.; Matsushima, H.; Umemura, S.; Tarumi, Y.; Kitawaki, J. Estrogen-related receptor alpha induces epithelial-mesenchymal transition through cancer-stromal interactions in endometrial cancer. Sci. Rep. 2019, 9, 6697. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Ito, K.; Suzuki, T.; Saito, S.; Tamura, M.; Hayashi, S.; Okamura, K.; Sasano, H.; Yaegashi, N. Peroxisome proliferator-activated receptor gamma and growth inhibition by its ligands in uterine endometrial carcinoma. Clin. Cancer Res. 2006, 12, 4200–4208. [Google Scholar] [CrossRef]
- Li, H.; Narahara, H. 15-Deoxy-Delta(12,14)-prostaglandin J(2) induces growth inhibition, cell cycle arrest and apoptosis in human endometrial cancer cell lines. Int. J. Mol. Med. 2013, 31, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Samudio, I.; Chintharlapalli, S.; Safe, S. 1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methanes decrease mitochondrial membrane potential and induce apoptosis in endometrial and other cancer cell lines. Mol. Carcinog. 2008, 47, 492–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koyama, N.; Nishida, Y.; Ishii, T.; Yoshida, T.; Furukawa, Y.; Narahara, H. Telmisartan induces growth inhibition, DNA double-strand breaks and apoptosis in human endometrial cancer cells. PLoS ONE 2014, 9, e93050. [Google Scholar] [CrossRef]
- Kumari, G.K.; Kiran, A.; Krishnamurthy, P.T. Preliminary evaluation on the beneficial effects of pioglitazone in the treatment of endometrial cancer. Med. Oncol. 2021, 38, 71. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Celestino, J.; Milam, M.R.; Schmeler, K.M.; Broaddus, R.R.; Ellenson, L.H.; Lu, K.H. Primary chemoprevention of endometrial hyperplasia with the peroxisome proliferator-activated receptor gamma agonist rosiglitazone in the PTEN heterozygote murine model. Int. J. Gynecol. Cancer 2008, 18, 329–338. [Google Scholar] [CrossRef]
- Surazynski, A.; Jarzabek, K.; Miltyk, W.; Wolczynski, S.; Palka, J. Estrogen-dependent regulation of PPAR-gamma signaling on collagen biosynthesis in adenocarcinoma endometrial cells. Neoplasma 2009, 56, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.Y.; Ye, L.X.; Lin, J.S.; Lin, H.Y.; Yu, L.R.; Wang, C.Y.; Mao, X.D.; Zhang, S.H.; Sun, P.M. An integrated approach of network pharmacology, molecular docking, and experimental verification uncovers kaempferol as the effective modulator of HSD17B1 for treatment of endometrial cancer. J. Transl. Med. 2023, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- ACS. What Is Cervical Cancer?: Cancer: American Cancer Society. 2020. Available online: https://www.cancer.org/cancer/types/cervical-cancer/about/what-is-cervical-cancer.html (accessed on 16 April 2023).
- ACS. Key Statistics for Cervical Cancer: American Cancer Society. 2023. Available online: https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html (accessed on 16 April 2023).
- ACS. Risk Factors for Cervical Cancer: American Cancer Society. 2020. Available online: https://www.cancer.org/cancer/types/cervical-cancer/causes-risks-prevention/risk-factors.html (accessed on 16 April 2023).
- ACS. Signs and Symptoms of Cervical Cancer: American Cancer Society. 2020. Available online: https://www.cancer.org/cancer/types/cervical-cancer/detection-diagnosis-staging/signs-symptoms.html (accessed on 16 April 2023).
- ACS. Treatment Options for Cervical Cancer, by Stage: American Cancer Society. 2021. Available online: https://www.cancer.org/cancer/types/cervical-cancer/treating/by-stage.html (accessed on 16 April 2023).
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- ACS. Survival Rates for Cervical Cancer: American Cancer Society. 2023. Available online: https://www.cancer.org/cancer/types/cervical-cancer/detection-diagnosis-staging/survival.html (accessed on 16 April 2023).
- Chang, H.K.; Kim, D.S.; Chae, J.J.; Kim, M.; Myong, J.P.; Lee, K.H.; Lee, M.W.; Park, T.C. Inhibition of ERK activity enhances the cytotoxic effect of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists in HeLa cells. Biochem. Biophys. Res. Commun. 2017, 482, 843–848. [Google Scholar] [CrossRef]
- Wuertz, B.R.; Darrah, L.; Wudel, J.; Ondrey, F.G. Thiazolidinediones abrogate cervical cancer growth. Exp. Cell Res. 2017, 353, 63–71. [Google Scholar] [CrossRef]
- Plissonnier, M.L.; Fauconnet, S.; Bittard, H.; Mougin, C.; Rommelaere, J.; Lascombe, I. Cell death and restoration of TRAIL-sensitivity by ciglitazone in resistant cervical cancer cells. Oncotarget 2017, 8, 107744–107762. [Google Scholar] [CrossRef]
- Tian, R.; Li, X.; Gao, Y.; Li, Y.; Yang, P.; Wang, K. Identification and validation of the role of matrix metalloproteinase-1 in cervical cancer. Int. J. Oncol. 2018, 52, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Resendiz, I.; Gallardo-Perez, J.C.; Lopez-Macay, A.; Robledo-Cadena, D.X.; Garcia-Villa, E.; Gariglio, P.; Saavedra, E.; Moreno-Sanchez, R.; Rodriguez-Enriquez, S. Mutant p53(R248Q) downregulates oxidative phosphorylation and upregulates glycolysis under normoxia and hypoxia in human cervix cancer cells. J. Cell Physiol. 2019, 234, 5524–5536. [Google Scholar] [CrossRef] [PubMed]
- Lutzen, U.; Zhao, Y.; Lucht, K.; Zuhayra, M.; Marx, M.; Cascorbi, I.; Culman, J. Pioglitazone induces cell growth arrest and activates mitochondrial apoptosis in human uterine leiomyosarcoma cells by a peroxisome proliferator-activated receptor gamma-independent mechanism. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 37–48. [Google Scholar] [CrossRef]
- Lützen, U.; Zhao, Y.; Lucht, K.; Zuhayra, M.; Hedderich, J.; Cascorbi, I.; Culman, J. Activation of the cell membrane angiotensin AT2 receptors in human leiomyosarcoma cells induces differentiation and apoptosis by a PPARgamma—Dependent mechanism. Neoplasma 2017, 64, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Mineda, A.; Nishimura, M.; Kagawa, T.; Takiguchi, E.; Kawakita, T.; Abe, A.; Irahara, M. Resveratrol suppresses proliferation and induces apoptosis of uterine sarcoma cells by inhibiting the Wnt signaling pathway. Exp. Ther. Med. 2019, 17, 2242–2246. [Google Scholar] [CrossRef]
- Bogacka, I.; Kurzynska, A.; Bogacki, M.; Chojnowska, K. Peroxisome proliferator-activated receptors in the regulation of female reproductive functions. Folia Histochem. Cytobiol. 2015, 53, 189–200. [Google Scholar] [CrossRef]
- MacLaren, L.A.; Guzeloglu, A.; Michel, F.; Thatcher, W.W. Peroxisome proliferator-activated receptor (PPAR) expression in cultured bovine endometrial cells and response to omega-3 fatty acid, growth hormone and agonist stimulation in relation to series 2 prostaglandin production. Domest. Anim. Endocrinol. 2006, 30, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Cammas, L.; Reinaud, P.; Bordas, N.; Dubois, O.; Germain, G.; Charpigny, G. Developmental regulation of prostacyclin synthase and prostacyclin receptors in the ovine uterus and conceptus during the peri-implantation period. Reproduction 2006, 131, 917–927. [Google Scholar] [CrossRef]
- Nishimura, K.; Yamauchi, N.; Chowdhury, V.S.; Torii, M.; Hattori, M.A.; Kaneto, M. Expression of peroxisome proliferator-activated receptor isoforms in the rat uterus during early pregnancy. Cell Tissue Res. 2011, 345, 275–284. [Google Scholar] [CrossRef]
- Socha, B.M.; Socha, P.; Szostek-Mioduchowska, A.Z.; Nowak, T.; Skarzynski, D.J. Peroxisome proliferator-activated receptor expression in the canine endometrium with cystic endometrial hyperplasia-pyometra complex. Reprod. Domest. Anim. 2022, 57, 771–783. [Google Scholar] [CrossRef]
- Gunin, A.G.; Bitter, A.D.; Demakov, A.B.; Vasilieva, E.N.; Suslonova, N.V. Effects of peroxisome proliferator activated receptors-alpha and -gamma agonists on estradiol-induced proliferation and hyperplasia formation in the mouse uterus. J. Endocrinol. 2004, 182, 229–239. [Google Scholar] [CrossRef] [PubMed]
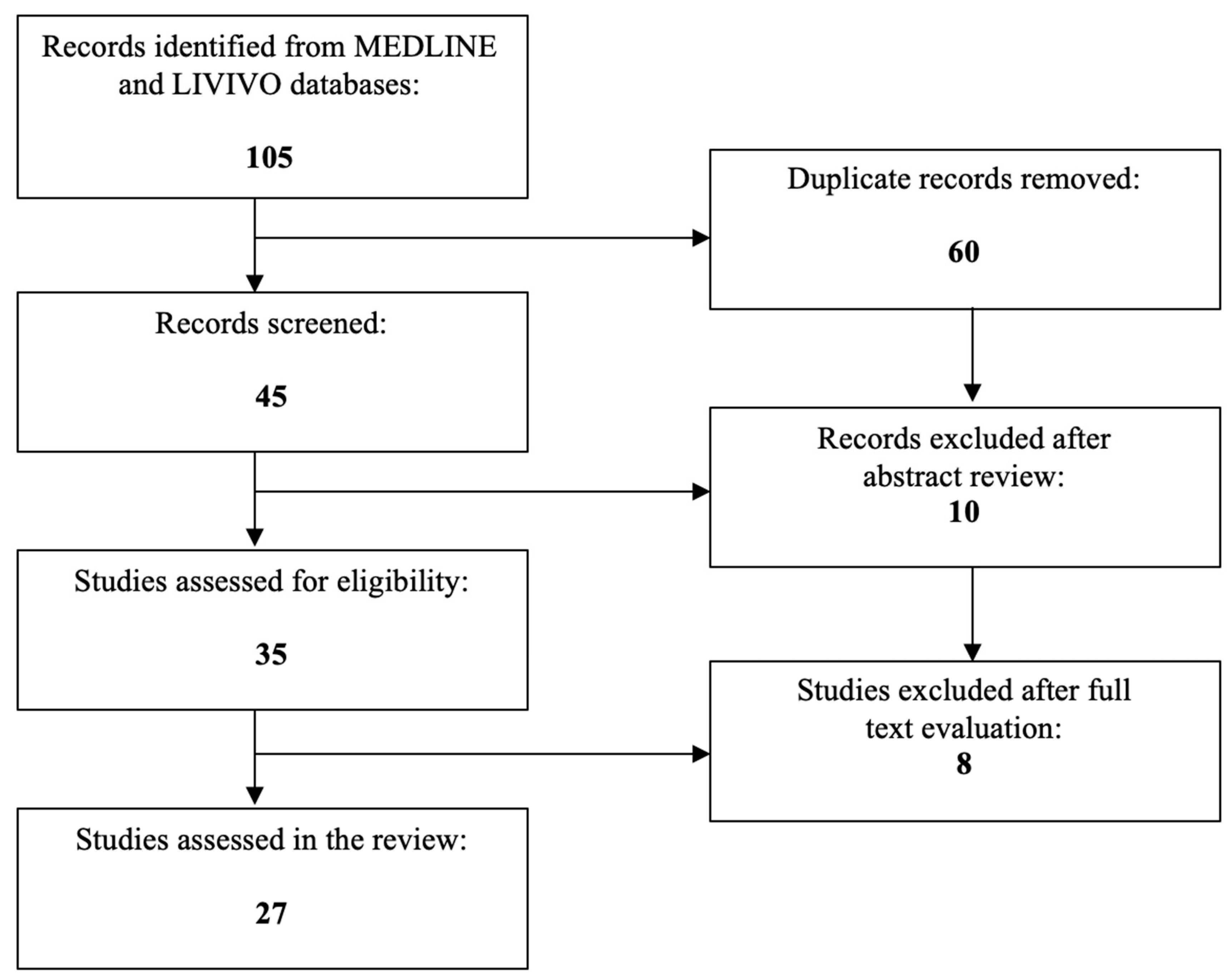
| Study | PPAR Isoform | Effect in Endometrial Cancer |
|---|---|---|
| Modesitt et al. [23] | Not specified | Upregulation in both endometrial and visceral adipose tissue Downregulation in subcutaneous adipose tissue |
| Tang et al. [24] | Not specified | Copy number variations and nucleotide mutations in the exon regions differentially influence disease prognosis and overall survival DBI, CPT1A, CYP27A1, and ME1, constitute the most significant PPAR-related genes linked to patient prognosis |
| Knapp et al. [25] | PPARα PPARβ/δ PPARγ | Upregulation of PPARα and PPARβ/δ Downregulation of PPARγ |
| Nickkho-Amiry et al. [26] | PPARα PPARβ/δ PPARγ | Upregulation of PPARα and PPARβ/δ Downregulation of PPARγ |
| Holland et al. [27] | PPARα PPARγ | Elevated PPARα and PPARγ levels |
| Tong et al. [29] | PPARβ/δ | High PPARβ/δ expression levels |
| Ma et al. [30] | PPARβ/δ | Significant growth inhibitory and apoptotic effects Increased Protein Kinase B and GSK3β dephosphorylation Enhanced β-catenin phosphorylation |
| Paynter et al. [32] | PPARγ | Pro12Ala polymorphism does not mediate circulating estrogen levels or endometrial cancer susceptibility |
| Smith et al. [33] | PPARγ | Overrepresentation of the H449H variant |
| Huang et al. [34] | PPARγ | Negative association between PPARγ and estrogen-related receptor-α expression Cancer cell proliferation and apoptosis regulation PPARγ/estrogen-related receptor-α ratio ≤ 1.86 represents an independent risk factor for endometrial carcinogenesis PPARγ(−)/estrogen-related receptor-α(+) status correlates with the worst overall survival and disease-free survival rates |
| Zhang et al. [35] | PPARγ | Significant correlation with estrogen receptor α expression levels, pathological grade and clinical stage |
| Cormio et al. [36] | PPARγ (PGC-1α) | Direct association with the enhanced mitochondrial biogenesis in type I endometrial carcinoma |
| Ren et al. [37] | PPARγ (PGC-1α) | More profound mRNA expression in high-grade malignant tissues, especially those of endometrial cancer patients with type 2 diabetes Positive association with the concentrations of the estrogen-related receptor gamma, the pyruvate kinase, and the isocitrate dehydrogenase |
| Wersäll et al. [38] | PPARγ (PGC-1α) | Significant downregulation No correlation with patient survival, grade, stage, p53 status, Ki-67, or clinical resistance |
| Yang et al. [39,40] | PPARγ (PGC-1α) | Synergy via the mitochondrial pathway with estrogen to ensure endometrial cancer cell survival |
| Yoriki et al. [41] | PPARγ (PGC-1α) | Decreased E-cadherin expression Upregulated vimentin, Snail, and ZEB1 expression after TGF-β exposure |
| Ota et al. [42] | PPARγ | Significantly lower expression Positive correlation with the p21 expression Negative correlation with the patients’ body mass index |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopatis, I.; Vrettou, K.; Troungos, C.; Theocharis, S. The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 9190. https://doi.org/10.3390/ijms24119190
Psilopatis I, Vrettou K, Troungos C, Theocharis S. The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer. International Journal of Molecular Sciences. 2023; 24(11):9190. https://doi.org/10.3390/ijms24119190
Chicago/Turabian StylePsilopatis, Iason, Kleio Vrettou, Constantinos Troungos, and Stamatios Theocharis. 2023. "The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer" International Journal of Molecular Sciences 24, no. 11: 9190. https://doi.org/10.3390/ijms24119190
APA StylePsilopatis, I., Vrettou, K., Troungos, C., & Theocharis, S. (2023). The Role of Peroxisome Proliferator-Activated Receptors in Endometrial Cancer. International Journal of Molecular Sciences, 24(11), 9190. https://doi.org/10.3390/ijms24119190








