The Determination of Mitochondrial Mass Is a Prerequisite for Accurate Assessment of Peripheral Blood Mononuclear Cells’ Oxidative Metabolism
Abstract
:1. Introduction
2. Results
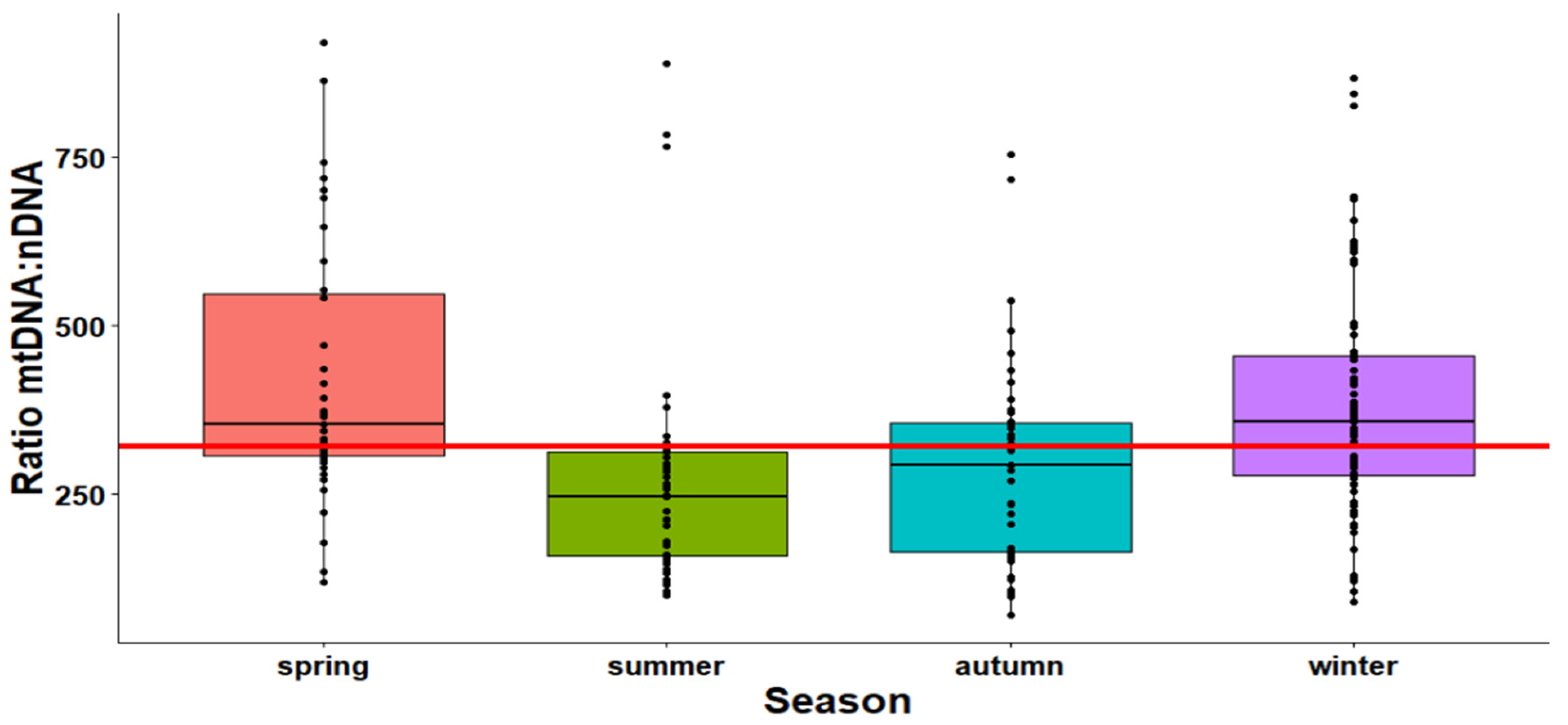
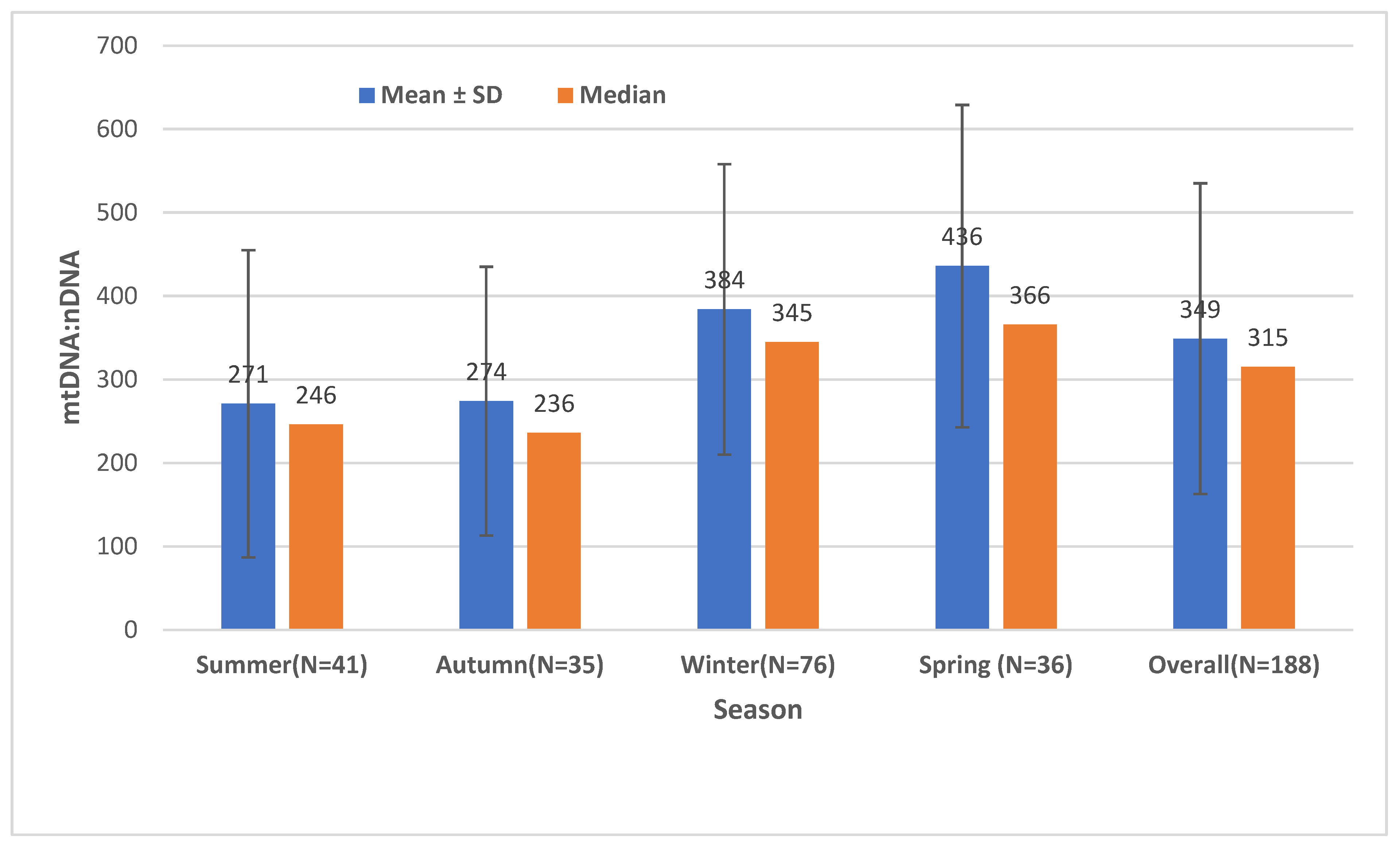
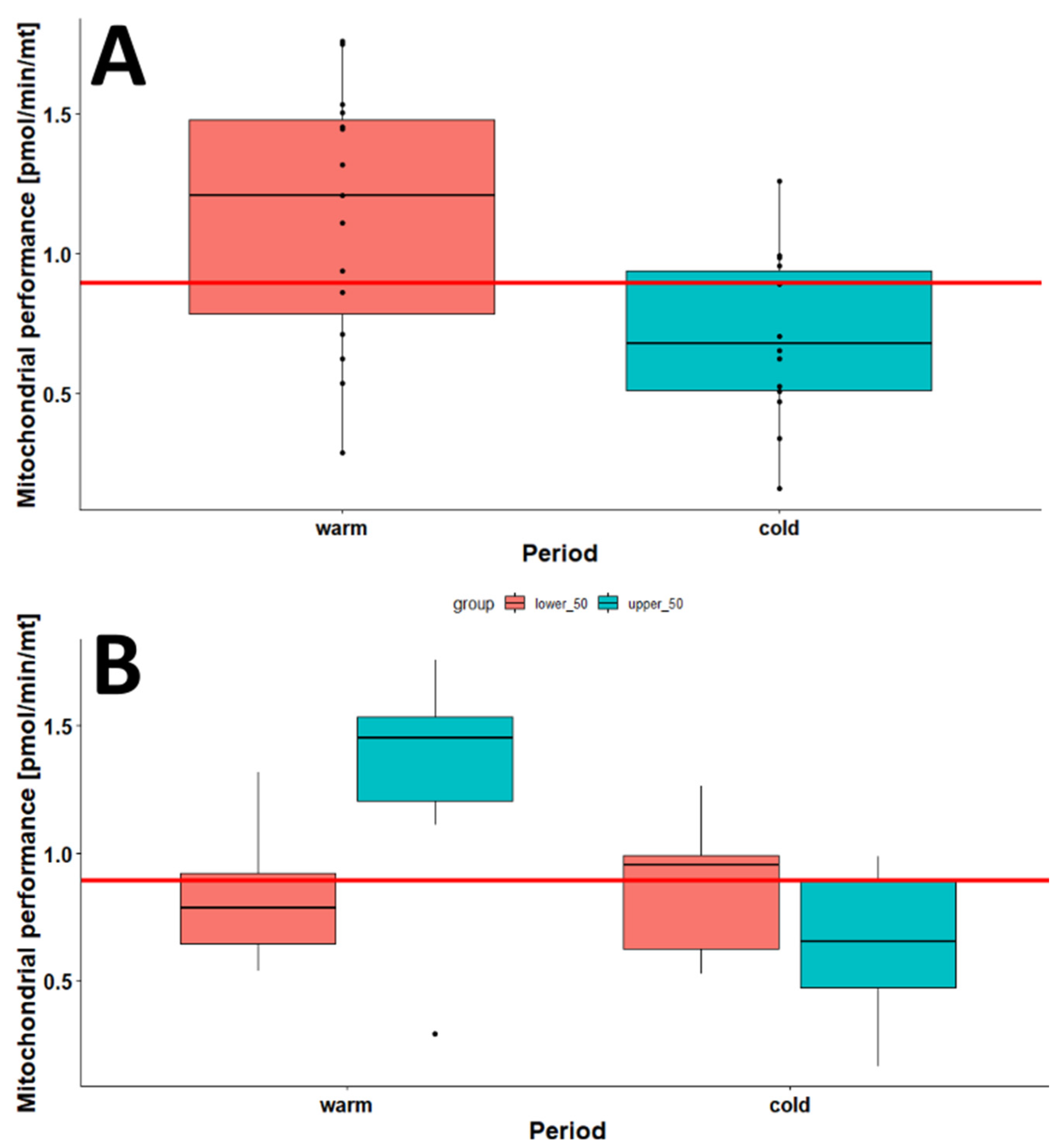
2.1. The Effect of Season on the Mitochondrial Mass (mtDNA:nDNA Ratio)
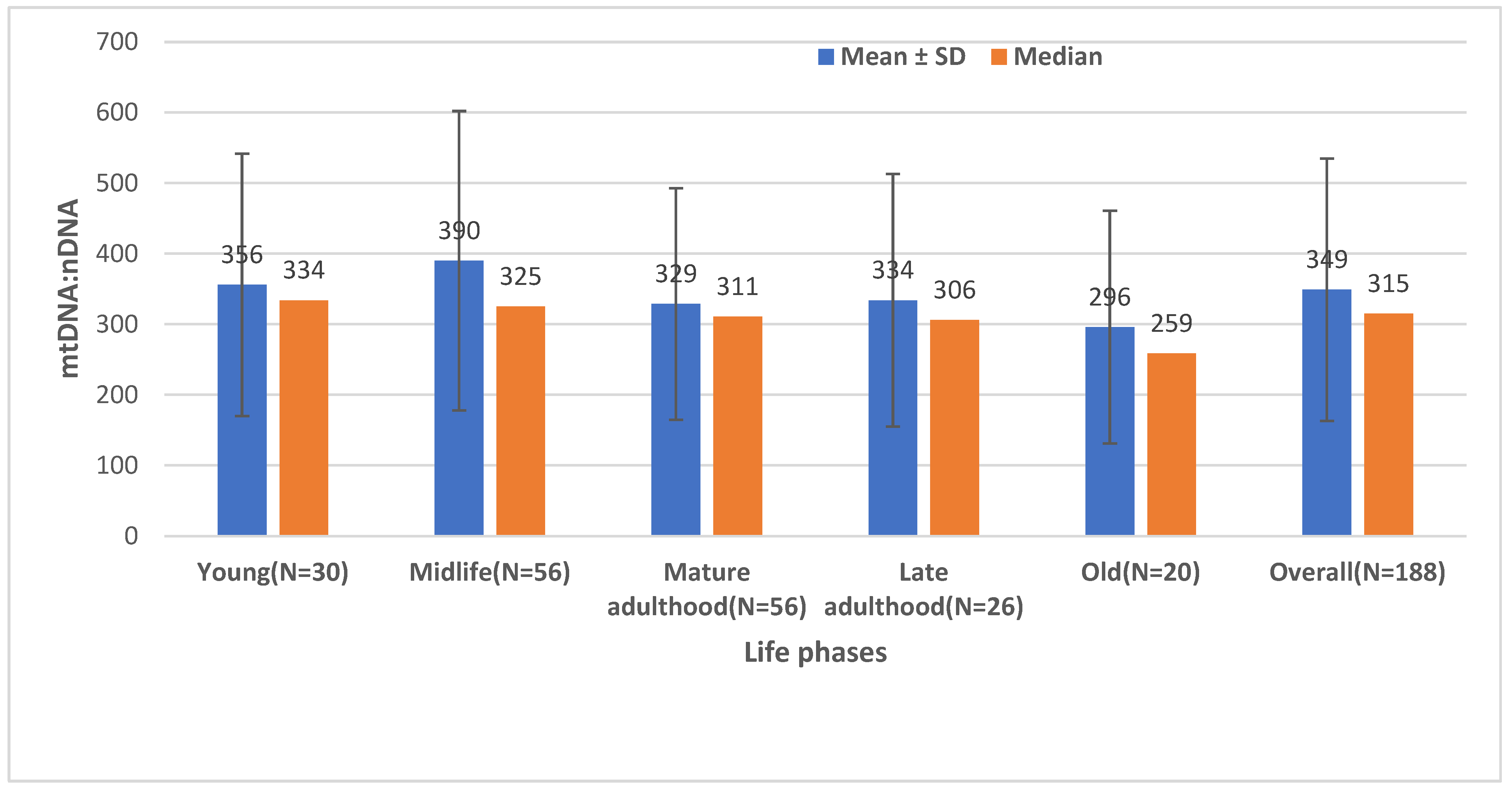
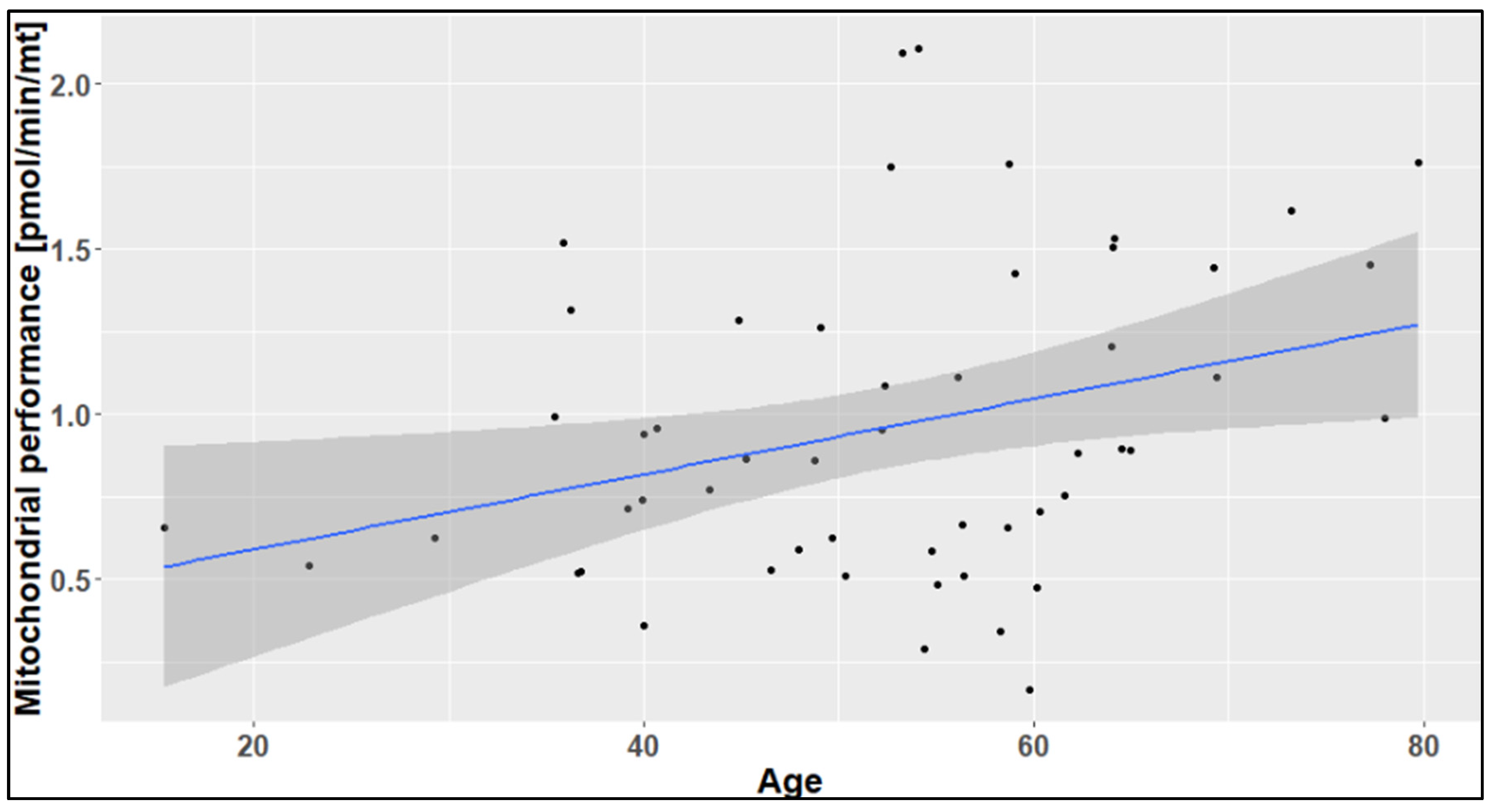
2.2. The Effect of Age on the Mitochondrial Mass (mtDNA:nDNA Ratio)
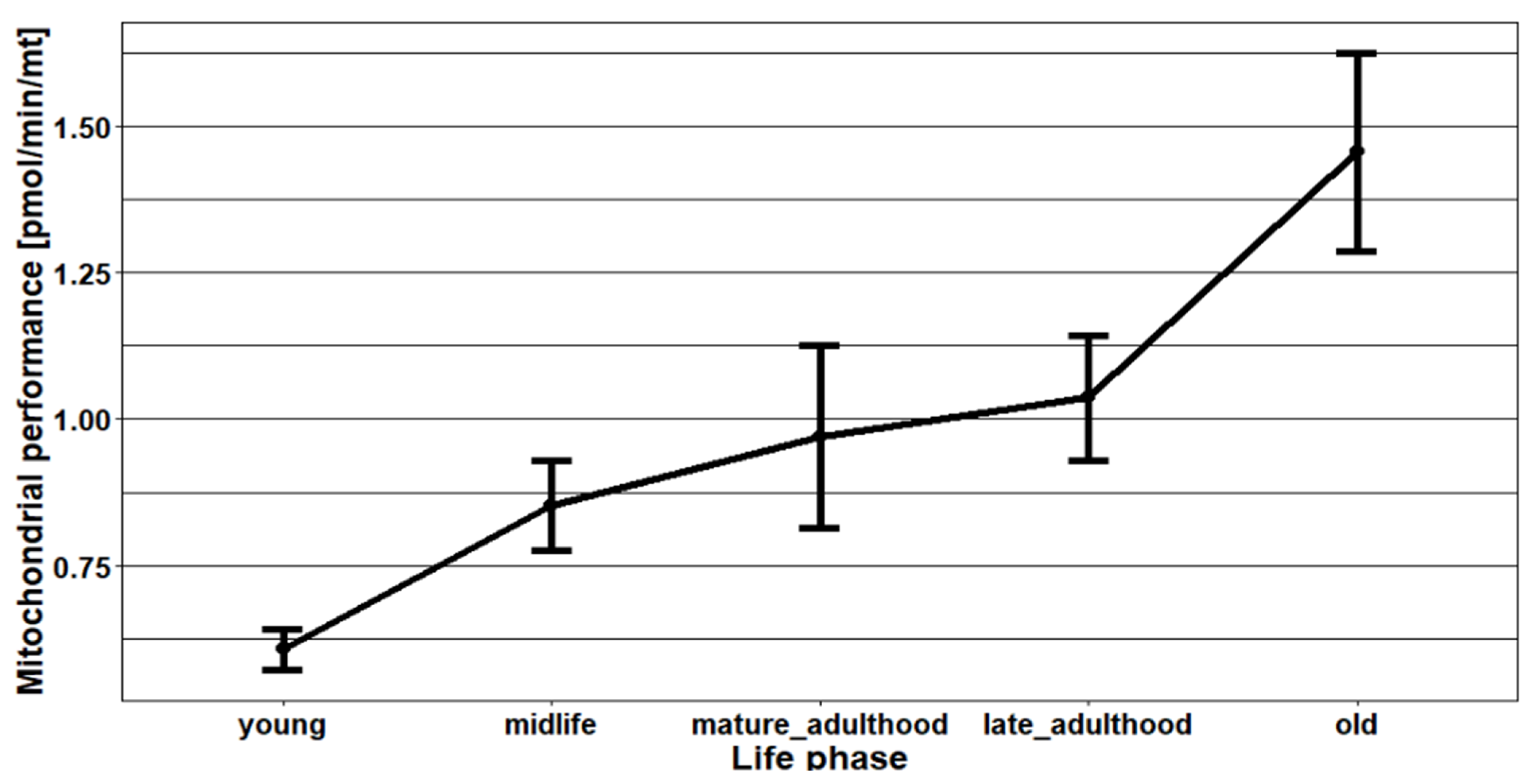
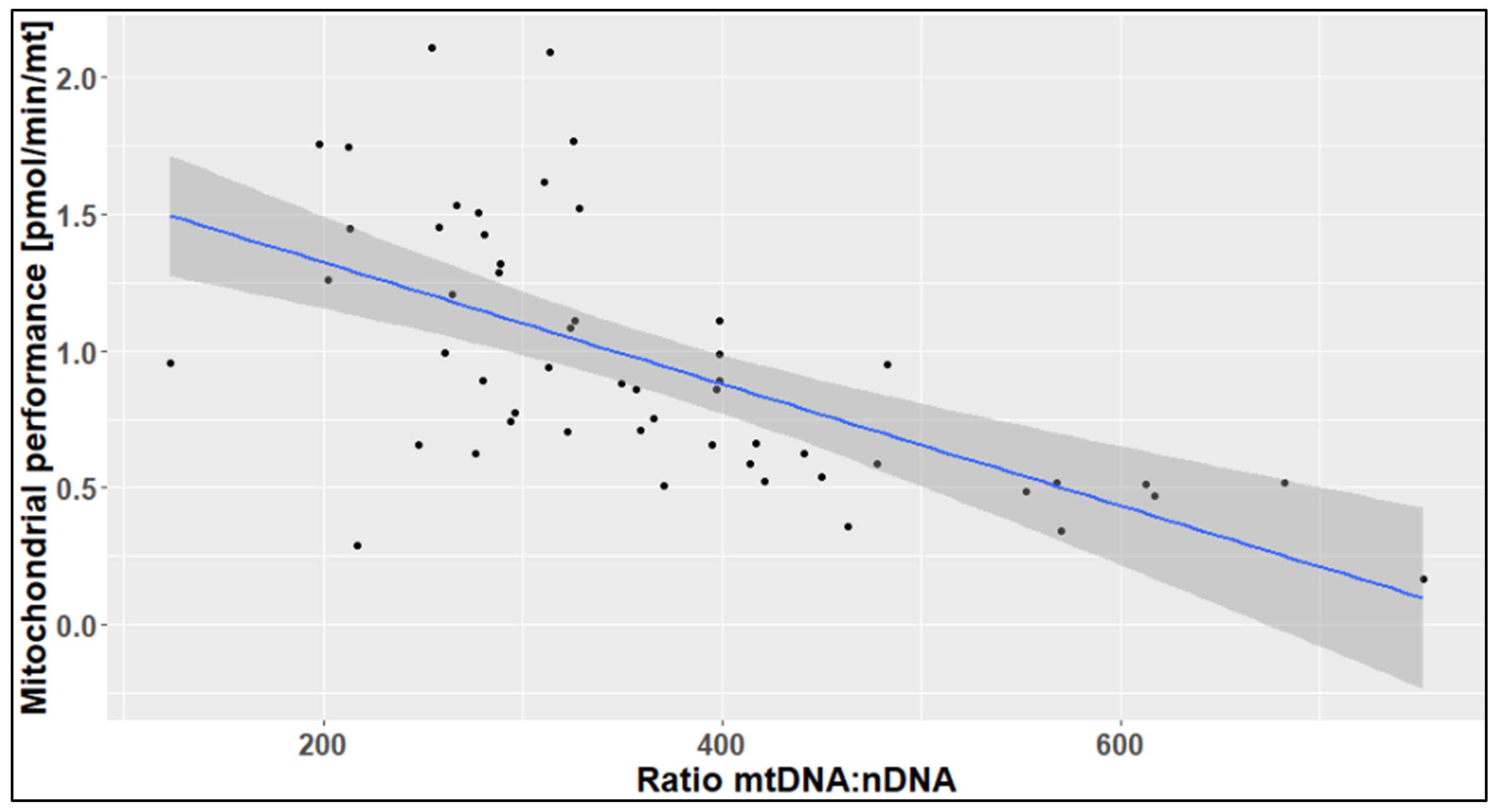
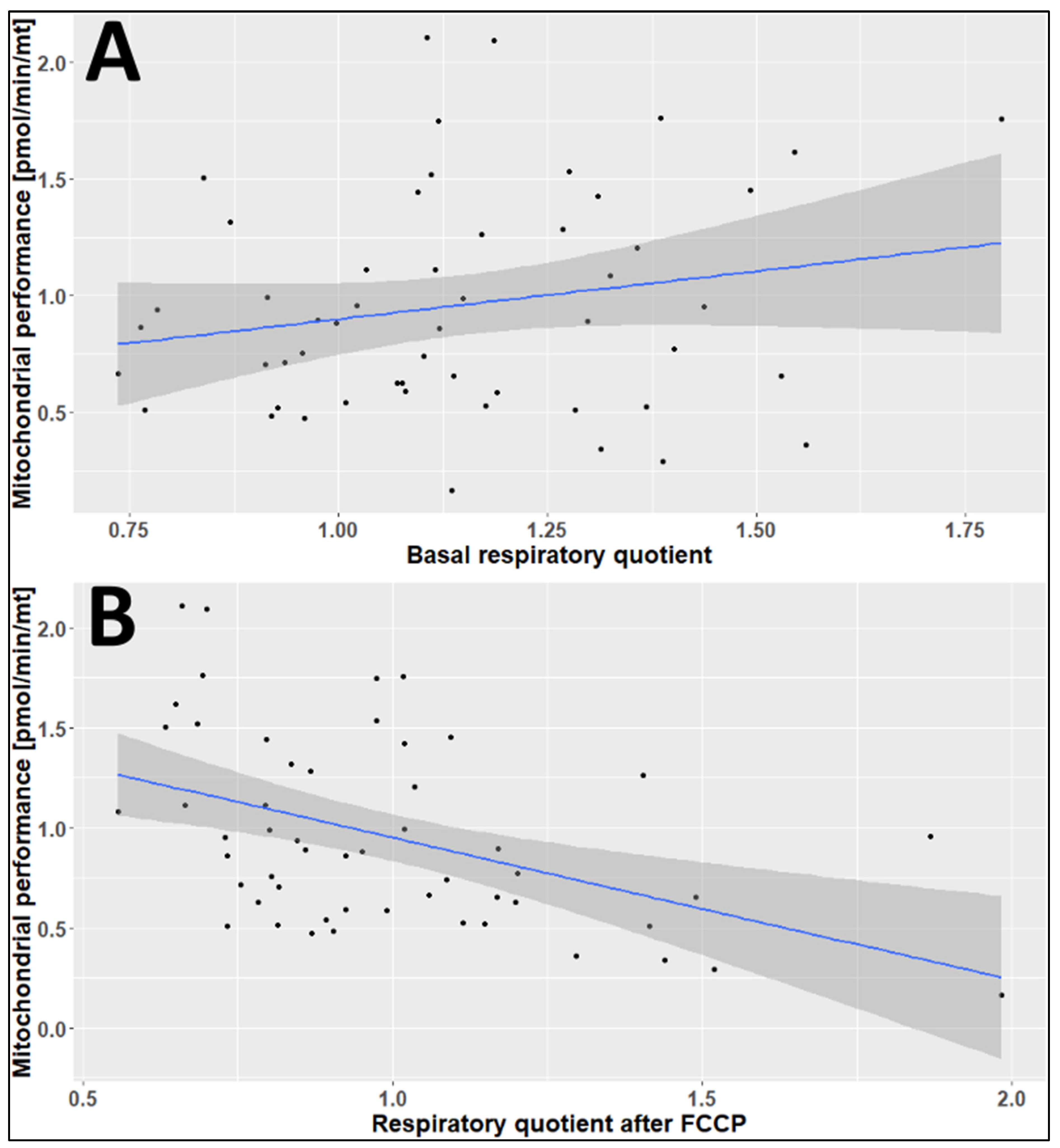
2.3. The Effects of Age, Season, and Mitochondrial Mass on Mitochondrial Performance (Respiration Per Mitochondrion)
3. Discussion
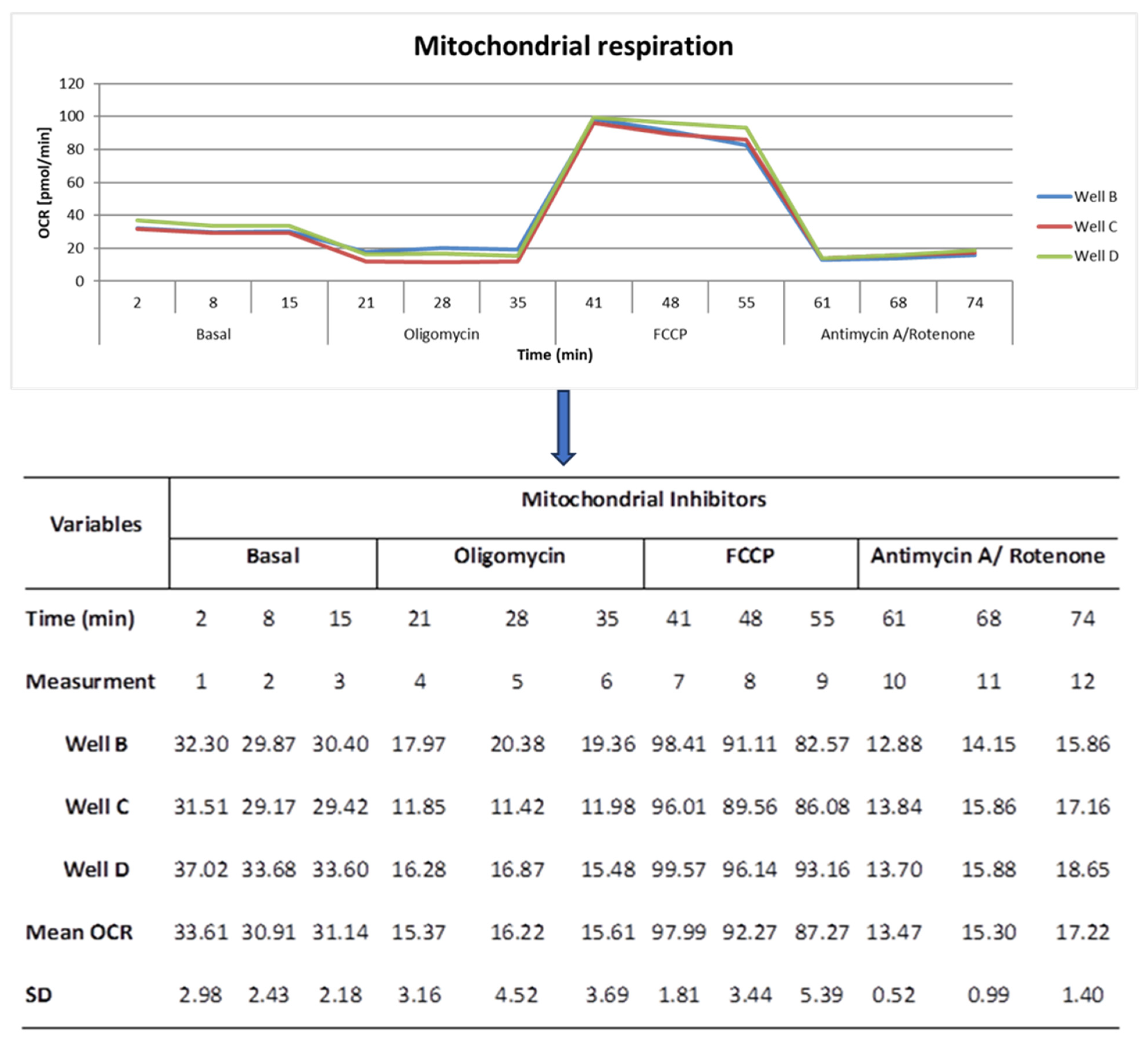
4. Materials and Methods
4.1. Study Design, Period and Settings
4.2. Study Participants
4.3. Blood Sample Collection and PBMCs Isolation
4.4. Cellular Stress Assay
4.5. Measurement of Mitochondrial Mass
4.6. Determination of Mitochondrial Performance (MP)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chance, B.; Sies, H.; Walker, C.L.; Pomatto, L.C.D.; Tripathi, D.N.; Davies, K.J.A.; Ninsontia, C.; Phiboonchaiyanan, P.P.; Kiratipaiboon, C.; Chanvorachote, P.; et al. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Papa, S. Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim. Biophys. Acta (BBA) Bioenerg. 1996, 1276, 87–105. [Google Scholar] [CrossRef]
- Wei, Y.-H. Oxidative Stress and Mitochondrial DNA Mutations in Human Aging. Proc. Soc. Exp. Boil. Med. 1998, 217, 53–63. [Google Scholar] [CrossRef]
- Medina-Gómez, G. Mitochondria and endocrine function of adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Buras, E.D.; Balasubramanyam, A. The Role of the Immune System in Obesity and Insulin Resistance. J. Obes. 2013, 2013, 616193. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, J.L.; Pompilius, M.; Westbrook, D.G.; Uyeminami, D.; Brown, J.; Pinkerton, K.E.; Ballinger, S.W. Developmental Exposure to Sec-ond-Hand Smoke Increases Adult Atherogenesis and Alters Mitochondrial DNA Copy Number and Deletions in apoE−/− Mice. PLoS ONE 2013, 8, e66835. [Google Scholar] [CrossRef]
- Krzywanski, D.M.; Moellering, D.R.; Fetterman, J.L.; Dunham-Snary, K.J.; Sammy, M.J.; Ballinger, S.W. The mitochondrial paradigm for cardiovascular disease susceptibility and cellular function: A complementary concept to Mendelian genetics. Lab. Investig. 2011, 91, 1122–1135. [Google Scholar] [CrossRef]
- Wallace, D.C. Bioenergetic Origins of Complexity and Disease. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 1–16. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Ballinger, S.W. Mitochondrial genetics and obesity: Evolutionary adaptation and contemporary disease susceptibility. Free Radic. Biol. Med. 2013, 65, 1229–1237. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010, 51, 440–450. [Google Scholar] [CrossRef]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Benavides, G.A.; Mitchell, T.; Dranka, B.; Ferrick, D.; Singal, A.K.; Ballinger, S.W.; Bailey, S.M.; et al. The Bioenergetic Health Index: A new concept in mitochondrial translational research. Clin. Sci. 2014, 127, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R.; Ballinger, S.; Dell’Italia, L.; Zhang, J.; Darley-Usmar, V. Integration of cellular bioenergetics with mi-tochondrial quality control and autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying intracellular rates of glycolytic and oxidative ATP pro-duction and consumption using extracellular flux measurements. J. Biol. Chem. 2017, 292, 7189–7207. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Dranka, B.P.; Zou, L.; Chatham, J.C.; Darley-Usmar, V.M. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 2009, 424, 99–107. [Google Scholar] [CrossRef]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Johnson, M.S.; Hardy, R.W.; Ballinger, S.W.; Darley-Usmar, V.M. Methods for defining distinct bioenergetic pro-files in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Investig. 2013, 93, 690–700. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Soares, M.J.; Rowlands, J.; Newsholme, P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016, 10, 243–250. [Google Scholar] [CrossRef]
- Milenkovic, D.; Blaza, J.N.; Larsson, N.-G.; Hirst, J. The Enigma of the Respiratory Chain Supercomplex. Cell Metab. 2017, 25, 765–776. [Google Scholar] [CrossRef]
- Wesselink, E.; Koekkoek, W.; Grefte, S.; Witkamp, R.; van Zanten, A. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Parikh, S.; The Mitochondrial Medicine Society; Saneto, R.; Falk, M.J.; Anselm, I.; Cohen, B.H.; Haas, R. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Options Neurol. 2009, 11, 414–430. [Google Scholar] [CrossRef]
- Giordano, C.; Iommarini, L.; Giordano, L.; Maresca, A.; Pisano, A.; Valentino, M.L.; Caporali, L.; Liguori, R.; Deceglie, S.; Roberti, M.; et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain 2013, 137, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Tessema, B.; Riemer, J.; Sack, U.; König, B. Cellular Stress Assay in Peripheral Blood Mononuclear Cells: Factors Influencing Its Results. Int. J. Mol. Sci. 2022, 23, 13118. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, B.; Mensink, G.B.M.; Thierfelder, W.; Müller, M.J.; Scheidt-Nave, C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Payne, B.A.I.; Chinnery, P.F. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim. Biophys. Acta 2015, 1847, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Bösch, F.; Angele, M.K.; Chaudry, I.H. Gender differences in trauma, shock and sepsis. Mil. Med. Res. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-C.; Frink, M.; Choudhry, M.A.; Bland, K.I.; Chaudry, I.H. Metabolic modulators following trauma sepsis: Sex hormones. Crit. Care Med. 2007, 35, S621–S629. [Google Scholar] [CrossRef]
- Robin, E.D.; Wong, R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J. Cell. Physiol. 1988, 136, 507–513. [Google Scholar] [CrossRef]
| Mitochondrial Performance | Spring | Summer | Autumn | Winter | Overall |
|---|---|---|---|---|---|
| (n = 8) | (n = 15) | (n = 18) | (n = 12) | (n = 53) | |
| Mean (SD) | 0.680 (0.222) | 1.14 (0.456) | 1.09 (0.551) | 0.735 (0.318) | 0.960 (0.471) |
| Median [Min, Max] | 0.588 (0.485, 1.11) | 1.21 (0.290, 1.76) | 0.905 (0.358, 2.11) | 0.798 (0.164, 1.26) | 0.882 (0.164, 2.11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, B.; Haag, J.; Sack, U.; König, B. The Determination of Mitochondrial Mass Is a Prerequisite for Accurate Assessment of Peripheral Blood Mononuclear Cells’ Oxidative Metabolism. Int. J. Mol. Sci. 2023, 24, 14824. https://doi.org/10.3390/ijms241914824
Tessema B, Haag J, Sack U, König B. The Determination of Mitochondrial Mass Is a Prerequisite for Accurate Assessment of Peripheral Blood Mononuclear Cells’ Oxidative Metabolism. International Journal of Molecular Sciences. 2023; 24(19):14824. https://doi.org/10.3390/ijms241914824
Chicago/Turabian StyleTessema, Belay, Janine Haag, Ulrich Sack, and Brigitte König. 2023. "The Determination of Mitochondrial Mass Is a Prerequisite for Accurate Assessment of Peripheral Blood Mononuclear Cells’ Oxidative Metabolism" International Journal of Molecular Sciences 24, no. 19: 14824. https://doi.org/10.3390/ijms241914824
APA StyleTessema, B., Haag, J., Sack, U., & König, B. (2023). The Determination of Mitochondrial Mass Is a Prerequisite for Accurate Assessment of Peripheral Blood Mononuclear Cells’ Oxidative Metabolism. International Journal of Molecular Sciences, 24(19), 14824. https://doi.org/10.3390/ijms241914824







