Characterization and Localization of Sol g 2.1 Protein from Solenopsis geminata Fire Ant Venom in the Central Nervous System of Injected Crickets (Acheta domestica)
Abstract
1. Introduction
2. Results
2.1. Expression, Purification, and Identification of rSol g 2.1 Protein
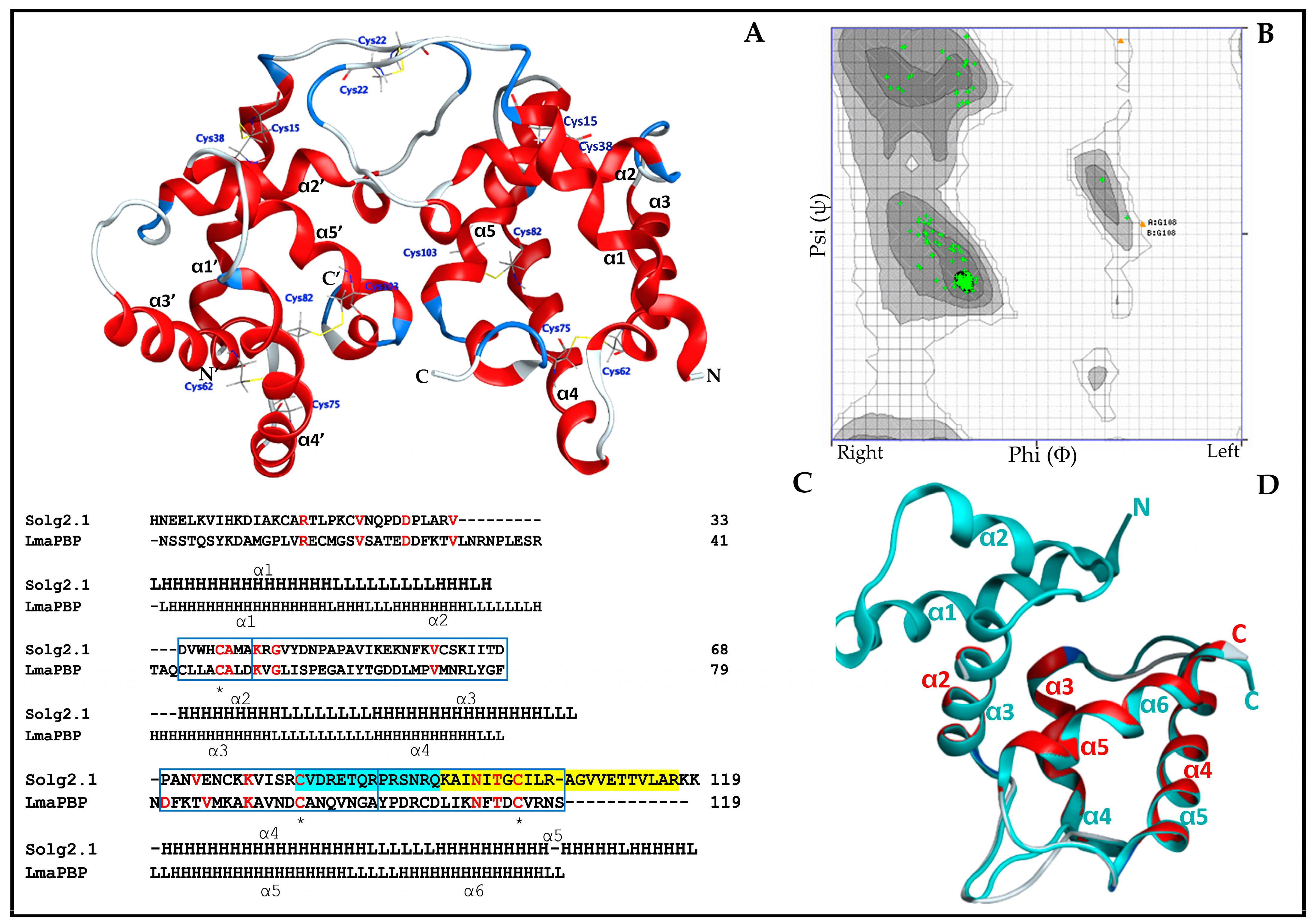
2.2. Secondary Structure of Sol g 2.1
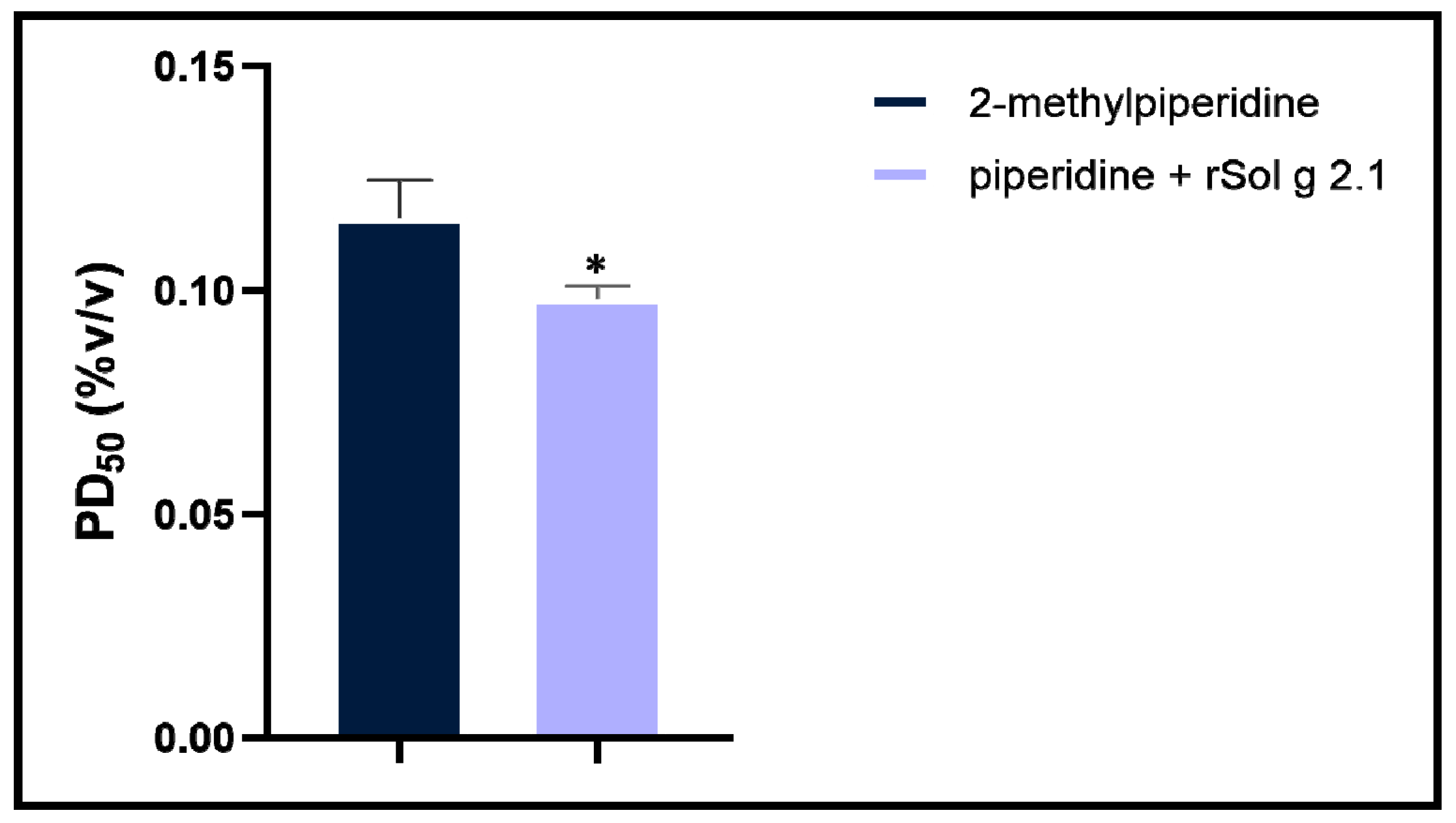
2.3. Paralysis Symptoms and Aggressive Behavior
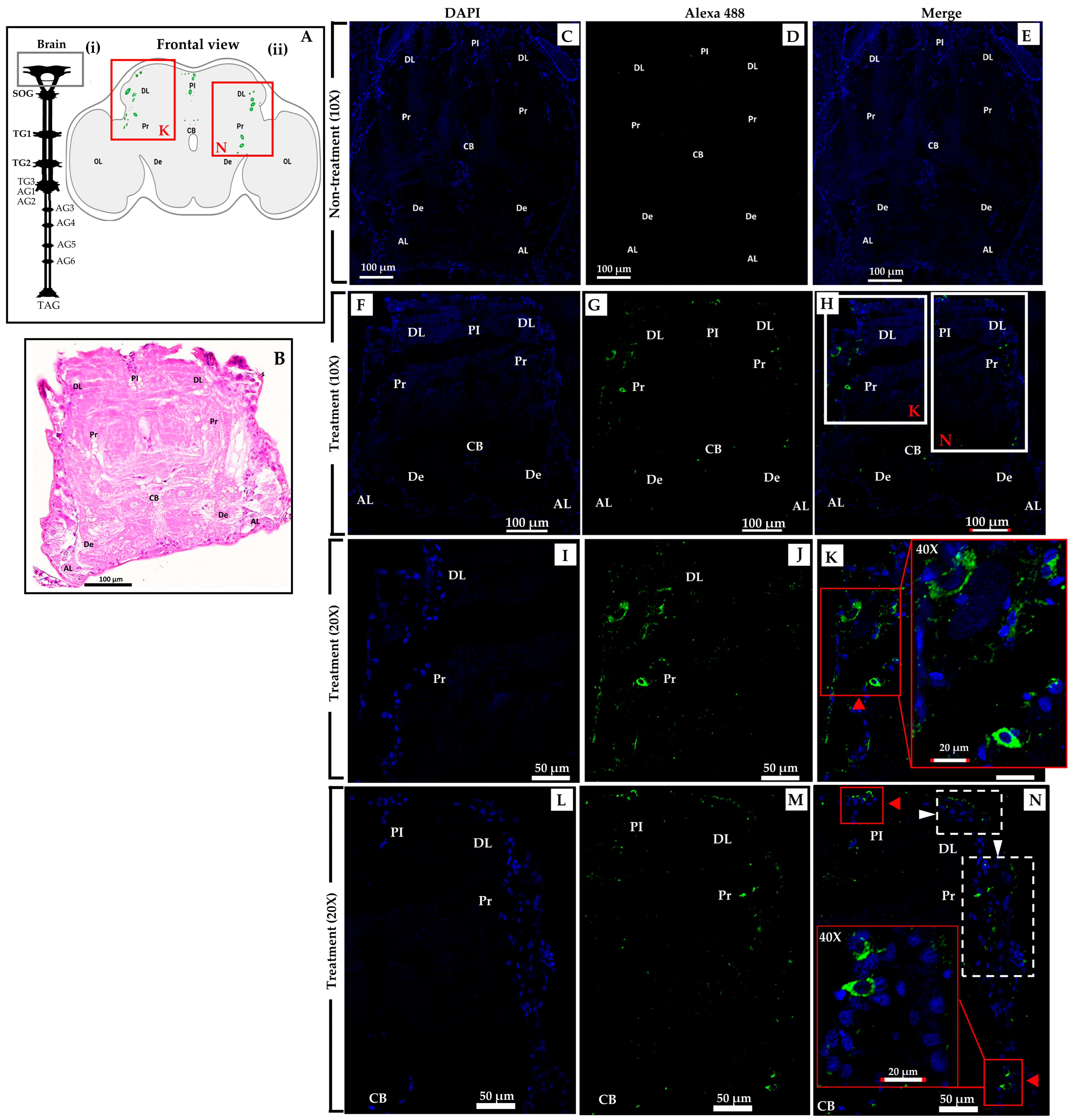
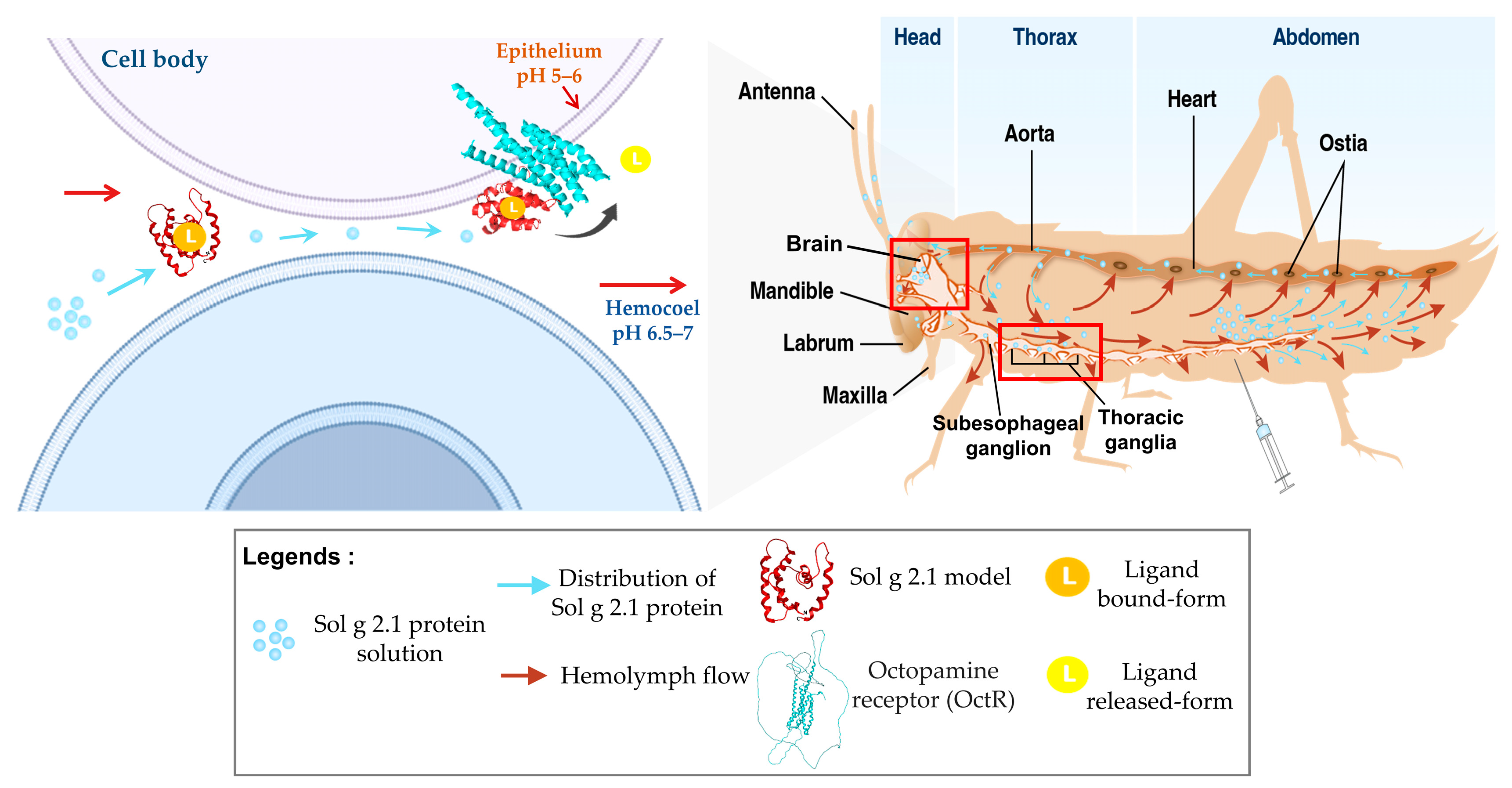
2.4. Specific Localization of Sol g 2.1 Protein with Immunofluorescence Analysis
2.5. Immunoblotting
2.6. The Overall Structure and Structural Homology of Sol g 2.1 Protein
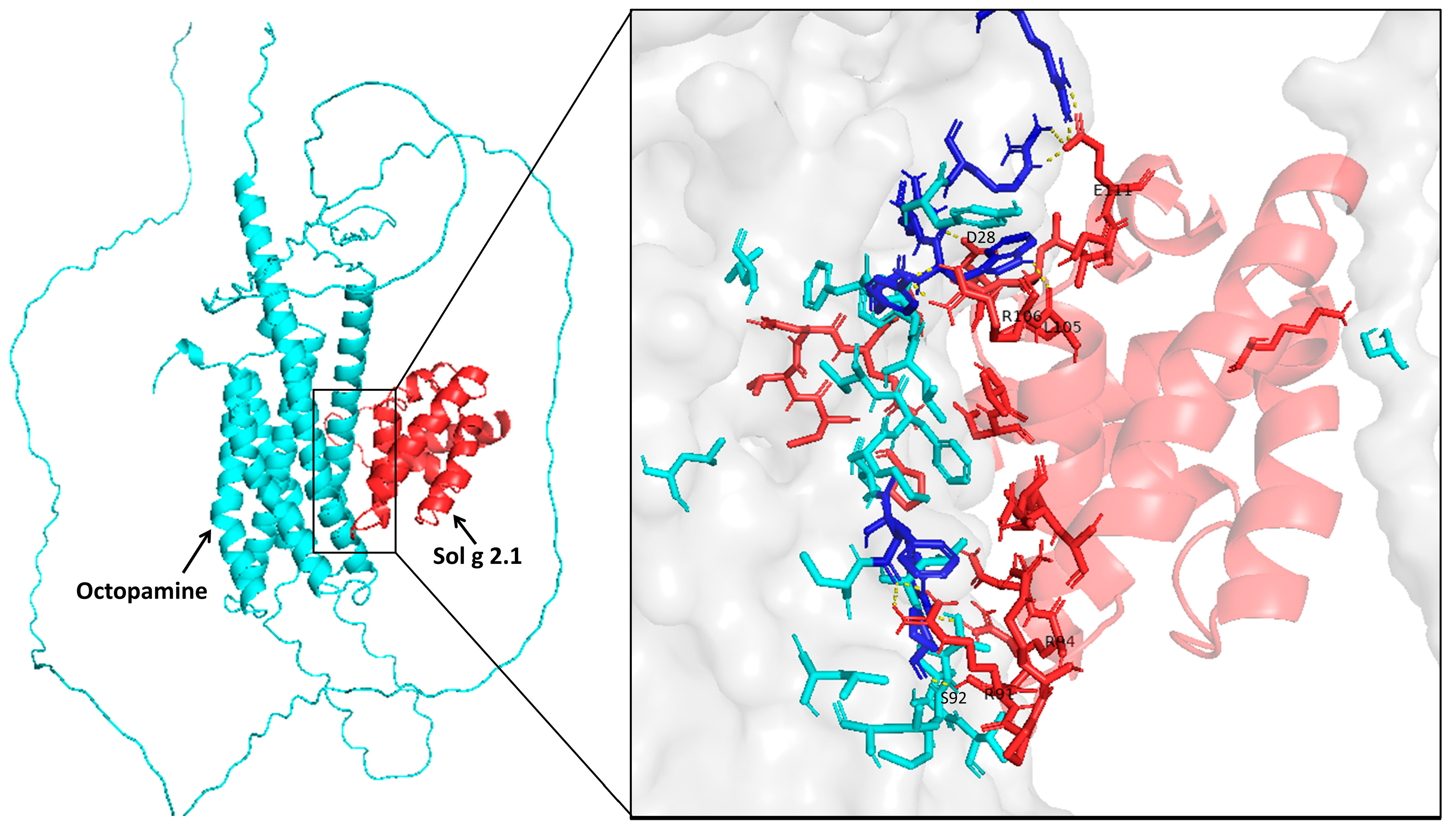
2.7. Sol g 2.1 Protein—Octopamine Receptor Docking
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of Recombinant Sol g 2.1
4.2. Identification of rSol g 2.1 Protein Using LC-MS/MS
4.3. Characterization of rSol g 2.1 Protein
4.4. Paralysis Activity
4.5. Specific Localization of Sol g 2.1 Protein with Immunofluorescence
4.6. Immunoblotting
4.7. Homology Modeling
4.8. Protein—Protein Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- dos Santos-Pinto, J.R.; Fox, E.G.; Saidemberg, D.M.; Santos, L.D.; da Silva Menegasso, A.R.; Costa-Manso, E.; Machado, E.A.; Bueno, O.C.; Palma, M.S. Proteomic view of the venom from the fire ant Solenopsis invicta Buren. J. Proteome Res. 2012, 11, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Borer, A.S.; Wassmann, P.; Schmidt, M.; Hoffman, D.R.; Zhou, J.J.; Wright, C.; Schirmer, T.; Markovic-Housley, Z. Crystal structure of Sol i 2: A major allergen from fire ant venom. J. Mol. Biol. 2012, 415, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Alabi, I.; Colley, M.; Yan, F.; Griffith, W.; Bach, S.; Weintraub, S.T.; Renthal, R. Major venom proteins of the fire ant Solenopsis invicta: Insights into possible pheromone-binding function from mass spectrometric analysis. Insect Mol. Biol. 2018, 27, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Hu, L.; Wang, W.; Vander Meer, R.; Porter, S.; Chen, L. Workers and alate queens of Solenopsis geminata share qualitatively similar but quantitatively different venom alkaloid chemistry. Front. Ecol. Evol. 2015, 3, 46. [Google Scholar] [CrossRef]
- Shukla, S.; Nakano-Baker, O.; Godin, D.; MacKenzie, D.; Sarikaya, M. iOBPdb A Database for Experimentally Determined Functional Characterization of Insect Odorant Binding Proteins. Sci. Data 2023, 10, 295. [Google Scholar] [CrossRef]
- de March, C.A.; Kim, S.K.; Antonczak, S.; Goddard, W.A., III; Golebiowski, J. G protein-coupled odorant receptors: From sequence to structure. Protein Sci. 2015, 24, 1543–1548. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Wang, M.; Song, X.; Tang, J.; Feng, F.; Li, B. Identification and Expression Analysis of G Protein-Coupled Receptors in the Miridae Insect Apolygus lucorum. Front. Endocrinol. 2021, 12, 773669. [Google Scholar] [CrossRef]
- Eyun, S.I.; Soh, H.Y.; Posavi, M.; Munro, J.B.; Hughes, D.S.T.; Murali, S.C.; Qu, J.; Dugan, S.; Lee, S.L.; Chao, H.; et al. Evolutionary History of Chemosensory-Related Gene Families across the Arthropoda. Mol. Biol. Evol. 2017, 34, 1838–1862. [Google Scholar] [CrossRef]
- Bonazza, C.; Zhu, J.; Hasler, R.; Mastrogiacomo, R.; Pelosi, P.; Knoll, W. Responses of the Pheromone-Binding Protein of the Silk Moth Bombyx mori on a Graphene Biosensor Match Binding Constants in Solution. Sensors 2021, 21, 499. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Adams, R.M.M. On the Biological Diversity of Ant Alkaloids. Annu. Rev. Entomol. 2022, 67, 367–385. [Google Scholar] [CrossRef]
- Sukprasert, S.; Uawonggul, N.; Jamjanya, T.; Thammasirirak, S.; Daduang, J.; Daduang, S. Characterization of the allergen Sol gem 2 from the fire ant venom, Solenopsis geminata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 325–334. [Google Scholar] [CrossRef]
- Srisong, H.; Sukprasert, S.; Klaynongsruang, S.; Daduang, J.; Daduang, S. Identification, expression and characterization of the recombinant Sol g 4.1 protein from the venom of the tropical fire ant Solenopsis geminata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Dacks, A.M.; Christensen, T.A.; Agricola, H.J.; Wollweber, L.; Hildebrand, J.G. Octopamine-immunoreactive neurons in the brain and subesophageal ganglion of the hawkmoth Manduca sexta. J. Comp. Neurol. 2005, 488, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Awata, H.; Wakuda, R.; Ishimaru, Y.; Matsuoka, Y.; Terao, K.; Katata, S.; Matsumoto, Y.; Hamanaka, Y.; Noji, S.; Mito, T.; et al. Roles of OA1 octopamine receptor and Dop1 dopamine receptor in mediating appetitive and aversive reinforcement revealed by RNAi studies. Sci. Rep. 2016, 6, 29696. [Google Scholar] [CrossRef] [PubMed]
- Rillich, J.; Stevenson, P.A. Releasing stimuli and aggression in crickets: Octopamine promotes escalation and maintenance but not initiation. Front. Behav. Neurosci. 2015, 9, 95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aoki, K.; Kosakai, K.; Yoshino, M. Monoaminergic Modulation of the Na+-Activated K+ Channel in Kenyon Cells Isolated From the Mushroom Body of the Cricket (Gryllus bimaculatus) Brain. J. Neurophysiol. 2008, 100, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.A.; Schildberger, K. Mechanisms of experience dependent control of aggression in crickets. Curr. Opin. Neurobiol. 2013, 23, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Mizunami, M.; Matsumoto, Y. Roles of Octopamine and Dopamine Neurons for Mediating Appetitive and Aversive Signals in Pavlovian Conditioning in Crickets. Front. Physiol. 2017, 8, 1027. [Google Scholar] [CrossRef]
- Leal, W.S.; Parra-Pedrazzoli, A.L.; Kaissling, K.E.; Morgan, T.I.; Zalom, F.G.; Pesak, D.J.; Dundulis, E.A.; Burks, C.S.; Higbee, B.S. Unusual pheromone chemistry in the navel orange worm: Novel sex attractants and a behavioral antagonist. Naturwissenschaften 2005, 92, 139–146. [Google Scholar] [CrossRef]
- Yi, G.B.; McClendon, D.; Desaiah, D.; Goddard, J.; Lister, A.; Moffitt, J.; Vander Meer, R.K.; deShazo, R.; Lee, K.S.; Rockhold, R.W. Fire ant venom alkaloid, isosolenopsin A, a potent and selective inhibitor of neuronal nitric oxide synthase. Int. J. Toxicol. 2003, 22, 81–86. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, M.; Dong, K.; Zhang, J.; Wang, H.; Xie, M.; Wu, W.; Zhang, Y.J.; Chen, Z. Structural Insights into the Ligand-Binding and -Releasing Mechanism of Helicoverpa armigera Pheromone-Binding Protein PBP1. Int. J. Mol. Sci. 2022, 23, 1190. [Google Scholar] [CrossRef] [PubMed]
- MacConnell, J.G.; Blum, M.S.; Fales, H.M. The chemistry of fire ant venom. Tetrahedron 1971, 27, 1129–1139. [Google Scholar] [CrossRef]
- Jefford, C.W.; Wang, J.B. An enantiospecific synthesis of solenopsin A. Tetrahedron Lett. 1993, 34, 2911–2914. [Google Scholar] [CrossRef]
- Wu, X.; Wang, G.; Xu, G.; Chen, L. Synthesis and Insecticidal Activity of Fire Ant Venom Alkaloid-Based 2-Methyl-6-alkyl- ∆1,6-piperideines. Molecules 2022, 27, 1107. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Price, N.C. The Use of Circular Dichroism in the Investigation of Protein Structure and Function. Curr. Protein Pept. Sci. 2000, 1, 349–384. [Google Scholar] [CrossRef] [PubMed]
- Wojtasek, H.; Leal, W.S. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J. Biol. Chem. 1999, 274, 30950–30956. [Google Scholar] [CrossRef] [PubMed]
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Arkaravichien, T.; Bunyatratchata, W.; Wipaporn, R.; Jearranaiprepame, P.; Nakamura, T.; Matsuda, M.; Kobayashi, M.; et al. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon 2007, 49, 19–29. [Google Scholar] [CrossRef]
- Shao, Q.M.; Sehadová, H.; Ichihara, N.; Sehnal, F.; Takeda, M. Immunoreactivities to three circadian clock proteins in two ground crickets suggest interspecific diversity of the circadian clock structure. J. Biol. Rhythms. 2006, 21, 118–131. [Google Scholar] [CrossRef]
- Ghosal, K.; Gupta, M.; Killian, K.A. Agonistic behavior enhances adult neurogenesis in male Acheta domesticus crickets. J. Exp. Biol. 2009, 212, 2045–2056. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Mizunami, M. Tyrosine hydroxylase-immunoreactive neurons in the mushroom body of the field cricket, Gryllus bimaculatus. Cell Tissue Res. 2019, 376, 97–111. [Google Scholar] [CrossRef]
- Zacouteguy, A.M.B.; Limberger, G.M.; de Oliveira, P.S.C.; da Fonseca, D.B.; Bruch, G.E.; Barros, D.M. The adverse effects of injected functionalized multi-walled carbon nanotube (f-MWCNT) on in vivo neurosecretory brain cells of Jamaican field cricket, Gryllus assimilis. Environ. Sci. Pollut. Res. Int. 2021, 28, 66968–66977. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Nagase, M.; Takaki, K.; Watanabe, H.; Yamawaki, Y. Three-dimensional atlas of thoracic ganglia in the praying mantis, Tenodera aridifolia. J. Comp. Neurol. 2020, 528, 1599–1615. [Google Scholar] [CrossRef] [PubMed]
- Bevan, S.; Burrows, M. Localisation of Even-skipped in the mature CNS of the locust, Schistocerca gregaria. Cell Tissue Res. 2003, 313, 237–244. [Google Scholar] [CrossRef]
- Schöneich, S.; Hedwig, B. Corollary discharge inhibition of wind-sensitive cercal giant interneurons in the singing field cricket. J. Neurophysiol. 2015, 113, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Teajaroen, W.; Phimwapi, S.; Daduang, J.; Klaynongsruang, S.; Tipmanee, V.; Daduang, S. A Role of Newly Found Auxiliary Site in Phospholipase A1 from Thai Banded Tiger Wasp (Vespa affinis) in Its Enzymatic Enhancement: In Silico Homology Modeling and Molecular Dynamics Insights. Toxins 2020, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Rungsa, P.; Peigneur, S.; Jangpromma, N.; Klaynongsruang, S.; Tytgat, J.; Daduang, S. In Silico and In Vitro Structure-Activity Relationship of Mastoparan and Its Analogs. Molecules 2022, 27, 561. [Google Scholar] [CrossRef] [PubMed]
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J. Chem. Inf. Model. 2009, 49, 444–460. [Google Scholar] [CrossRef]
- Lartigue, A.; Gruez, A.; Spinelli, S.; Rivière, S.; Brossut, R.; Tegoni, M.; Cambillau, C. The crystal structure of a cockroach pheromone-binding protein suggests a new ligand binding and release mechanism. J. Biol. Chem. 2007, 278, 30213–30218. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Li, T.; Feng, X. G-Protein Coupled Receptors (GPCRs): Signaling Pathways, Characterization, and Functions in Insect Physiology and Toxicology. Int. J. Mol. Sci. 2021, 22, 5260. [Google Scholar] [CrossRef]
- Hollingworth, R.M. Chemistry, biological activity, and uses of formamidine pesticides. Environ. Health Perspect. 1976, 14, 57–69. [Google Scholar] [CrossRef]
- Chu, W.T.; Wu, Y.J.; Zhang, J.L.; Zheng, Q.C.; Chen, L.; Xue, Q.; Zhang, H.X. Constant pH Molecular Dynamics (CpHMD) and mutation studies: Insights into AaegOBP1 pH-induced ligand releasing mechanism. Biochim. Biophys. Acta-Proteins Proteom. 2012, 1824, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Honson, N.; Johnson, M.A.; Oliver, J.E.; Prestwich, G.D.; Plettner, E. Structure-activity studies with pheromone-binding proteins of the gypsy moth, Lymantria dispar. Chem. Senses 2003, 28, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Terrado, M.; Okon, M.; McIntosh, L.P.; Plettner, E. Ligand-and pH-Induced structural transition of Gypsy Moth Lymantria dispar pheromone-binding protein 1 (LdisPBP1). Biochemistry 2020, 59, 3411–3426. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Rathmayer, W.; Libersat, F. Neuromodulation of the escape behavior of the cockroach Periplaneta americana by the venom of the parasitic wasp Ampulex compressa. J. Comp. Physiol. 1996, 178, 91–100. [Google Scholar] [CrossRef]
- Nie, L.; Zhao, F.; Chen, Y.; Xiao, Q.; Pan, Z.; Ran, H.; Xu, Y. Prey Status Affects Paralysis Investment in the Ponerine Ant Harpegnathos venator. Insects 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.Z.; Narahashi, T.; Almon, R.R. Characterization of neuromuscular blocking action of piperidine derivatives. J. Pharmacol. Exp. Ther. 1975, 194, 373–383. [Google Scholar] [PubMed]
- David, J.A.; Crowley, P.J.; Hall, S.G.; Battersby, M.; Sattelle, D.B. Actions of synthetic piperidine derivatives on an insect acetylcholine receptor/ion channel complex. J. Insect Physiol. 1984, 30, 191–196. [Google Scholar] [CrossRef]
- Lai, L.; Kuo, T.; Huang, R.; Wu, W. The Insecticidal Activities of Fire Ant (Hymenoptera: Formicidae) Venoms Against Plutella xylostella (Lepidoptera: Plutellidae) Larvae. J. Econ. Entomol. 2012, 105, 1591–1596. [Google Scholar] [CrossRef]
- Alkam, T.; Nabeshima, T. Molecular mechanisms for nicotine intoxication. Neurochem. Int. 2019, 125, 117–126. [Google Scholar] [CrossRef]
- Stevenson, P.A.; Spörhase-Eichmann, U. Localization of octopaminergic neurones in insects. Comp. Biochem. Physiol. A Physiol. 1995, 110, 203–215. [Google Scholar] [CrossRef]
- Spörhase-Eichmann, U.; Vullings, H.G.B.; Buijs, R.M.; Hörner, M.; Schürmann, F. Octopamine-immunoreactive neurons in the central nervous system of the cricket, Gryllus bimaculatus. Cell Tissue Res. 1992, 268, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Kasture, A.S.; Hummel, T.; Sucic, S.; Freissmuth, M. Big Lessons from Tiny Flies: Drosophila melanogaster as a Model to Explore Dysfunction of Dopaminergic and Serotonergic Neurotransmitter Systems. Int. J. Mol. Sci. 2018, 19, 1788. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.; McNeil, J.N.; Donly, C. Octopamine receptor gene expression in three lepidopteran species of insect. Peptides 2013, 41, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Benton, R. Molecular mechanisms of olfactory detection in insects: Beyond receptors. Open Biol. 2020, 10, 200252. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Palma, M.S. Polybitoxins: A group of phospholipases A2 from the venom of the neotropical social wasp paulistinha (Polybia paulista). Toxicon 1998, 36, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hana, S.; Lange, A.B. Cloning and Functional Characterization of Octβ2-Receptor and Tyr1-Receptor in the Chagas Disease Vector, Rhodnius prolixus. Front. Physiol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Srisong, H. Cloning, Expression and Characterization of Sol gem 1, 2, 3 and 4 in Venom from Tropical Red Fire Ants, Solenopsis grminata. Ph.D. Thesis, Khon Kaen University, Khon Kaen, Thailand, 2016. [Google Scholar]
- Batys, P.; Morga, M.; Bonarek, P.; Sammalkorpi, M. pH-Induced Changes in Polypeptide Conformation: Force-Field Comparison with Experimental Validation. J. Phys. Chem. B. 2020, 124, 2961–2972. [Google Scholar] [CrossRef]
- Vossen, L.E.; Nilsson, E.; Jansson, A.; Roman, E. Open field behavior in the house cricket (Acheta domesticus): Effect of illumination, sex differences and individual consistency. J. Insects Food Feed 2023, 9, 317–324. [Google Scholar] [CrossRef]
- Ji, Y.H.; Mansuelle, P.; Terakawa, S.; Kopeyan, C.; Yanaihara, N.; Hsu, K.; Rochat, H. Two neurotoxins (BmK I and BmK II) from the venom of the scorpion Buthus martensi Karsch: Purification, amino acid sequences and assessment of specific activity. Toxicon 1996, 34, 987–1001. [Google Scholar] [CrossRef]
- Zobel-Thropp, P.A.; Mullins, J.; Kristensen, C.; Kronmiller, B.A.; David, C.L.; Breci, L.A.; Binford, G.J. Not so Dangerous After All? Venom Composition and Potency of the Pholcid (Daddy Long-Leg) Spider Physocyclus mexicanus. Front. Ecol. Evol. 2019, 7, 256. [Google Scholar] [CrossRef]
- Sekimizu, N.; Paudel, A.; Hamamoto, H. Animal welfare and use of silkworm as a model animal. Drug Discov Ther. 2012, 6, 226–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kornthong, N.; Phanaksri, T.; Saetan, J.; Duangprom, S.; Lekskul, B.; Vivattanasarn, T.; Songkoomkrong, S.; Jattujan, P.; Cummins, S.F.; Sobhon, P.; et al. Identification and localization of growth factor genes in the sea cucumber, Holothuria scabra. Heliyon 2021, 7, e08370. [Google Scholar] [CrossRef] [PubMed]
- Kruangkum, T.; Duangprom, S.; Songkoomkrong, S.; Chotwiwatthanakun, C.; Vanichviriyakit, R.; Sobhon, P.; Kornthong, N. Discovery of a hidden form of neuropeptide F and its presence throughout the CNS-gut axis in the mud crab, Scylla olivacea. Front. Mar. Sci. 2022, 9, 951648. [Google Scholar] [CrossRef]
- Sheldon, A.L.; Zhang, J.; Fei, H.; Levitan, I.B. SLOB, a SLOWPOKE channel binding protein, regulates insulin pathway signaling and metabolism in Drosophila. PLoS ONE 2011, 6, e23343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahid, F.; Zaheer, T.; Ashraf, S.T.; Shehroz, M.; Anwer, F.; Naz, A.; Ali, A. Chimeric vaccine designs against Acinetobacter baumannii using pan genome and reverse vaccinology approaches. Sci. Rep. 2021, 11, 13213. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Lensink, M.F.; Méndez, R.; Wodak, S.J. Docking and scoring protein complexes: CAPRI 3rd Edition. Proteins 2007, 69, 704–718. [Google Scholar] [CrossRef]
- Kozakov, D.; Brenke, R.; Comeau, S.R.; Vajda, S. PIPER: An FFT-based protein docking program with pairwise potentials. Proteins 2006, 65, 392–406. [Google Scholar] [CrossRef]
- Comeau, S.R.; Gatchell, D.W.; Vajda, S.; Camacho, C.J. ClusPro: A fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004, 32, W96–W99. [Google Scholar] [CrossRef]



| Band | Theoretical MW 1 | MW 2 | Experiment MW 3 | Matched Peptide Sequences 4 | Score XC 5 | Homologous Molecule |
|---|---|---|---|---|---|---|
| Purified band | 15,966 Da | 13,386.68 Da | 17 kDa | R.AGVVETTVLAR.E K.AINITGCILR.A | 155 | Venom allergen 2; S. invicta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonkhwao, S.; Rungsa, P.; Buraphaka, H.; Klaynongsruang, S.; Daduang, J.; Kornthong, N.; Daduang, S. Characterization and Localization of Sol g 2.1 Protein from Solenopsis geminata Fire Ant Venom in the Central Nervous System of Injected Crickets (Acheta domestica). Int. J. Mol. Sci. 2023, 24, 14814. https://doi.org/10.3390/ijms241914814
Nonkhwao S, Rungsa P, Buraphaka H, Klaynongsruang S, Daduang J, Kornthong N, Daduang S. Characterization and Localization of Sol g 2.1 Protein from Solenopsis geminata Fire Ant Venom in the Central Nervous System of Injected Crickets (Acheta domestica). International Journal of Molecular Sciences. 2023; 24(19):14814. https://doi.org/10.3390/ijms241914814
Chicago/Turabian StyleNonkhwao, Siriporn, Prapenpuksiri Rungsa, Hathairat Buraphaka, Sompong Klaynongsruang, Jureerut Daduang, Napamanee Kornthong, and Sakda Daduang. 2023. "Characterization and Localization of Sol g 2.1 Protein from Solenopsis geminata Fire Ant Venom in the Central Nervous System of Injected Crickets (Acheta domestica)" International Journal of Molecular Sciences 24, no. 19: 14814. https://doi.org/10.3390/ijms241914814
APA StyleNonkhwao, S., Rungsa, P., Buraphaka, H., Klaynongsruang, S., Daduang, J., Kornthong, N., & Daduang, S. (2023). Characterization and Localization of Sol g 2.1 Protein from Solenopsis geminata Fire Ant Venom in the Central Nervous System of Injected Crickets (Acheta domestica). International Journal of Molecular Sciences, 24(19), 14814. https://doi.org/10.3390/ijms241914814





