An Integrative ATAC-Seq and RNA-Seq Analysis of the Endometrial Tissues of Meishan and Duroc Pigs
Abstract
1. Introduction
2. Results
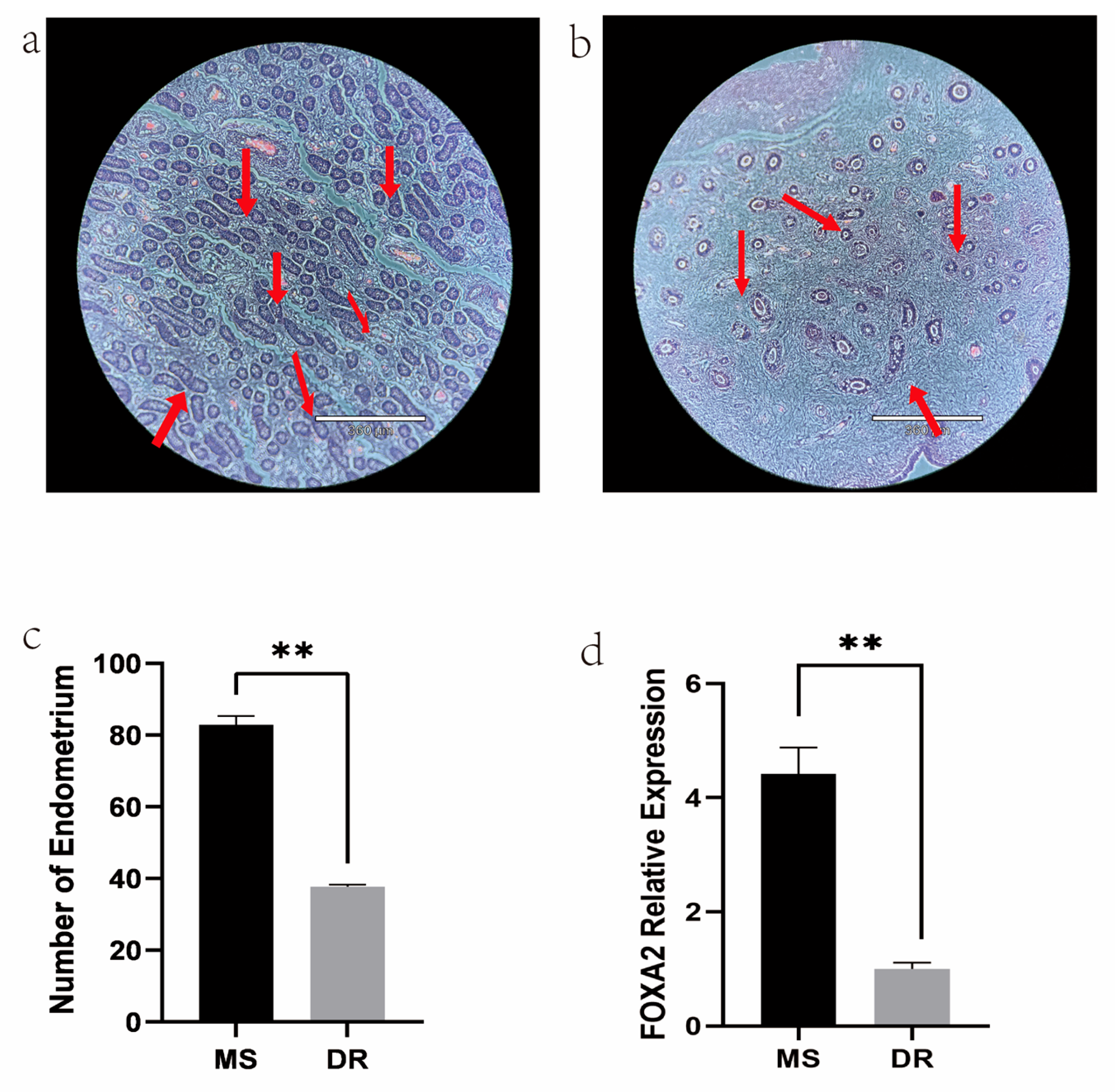
2.1. Phenotype of Meishan and Duroc Pig Endometrial Tissues
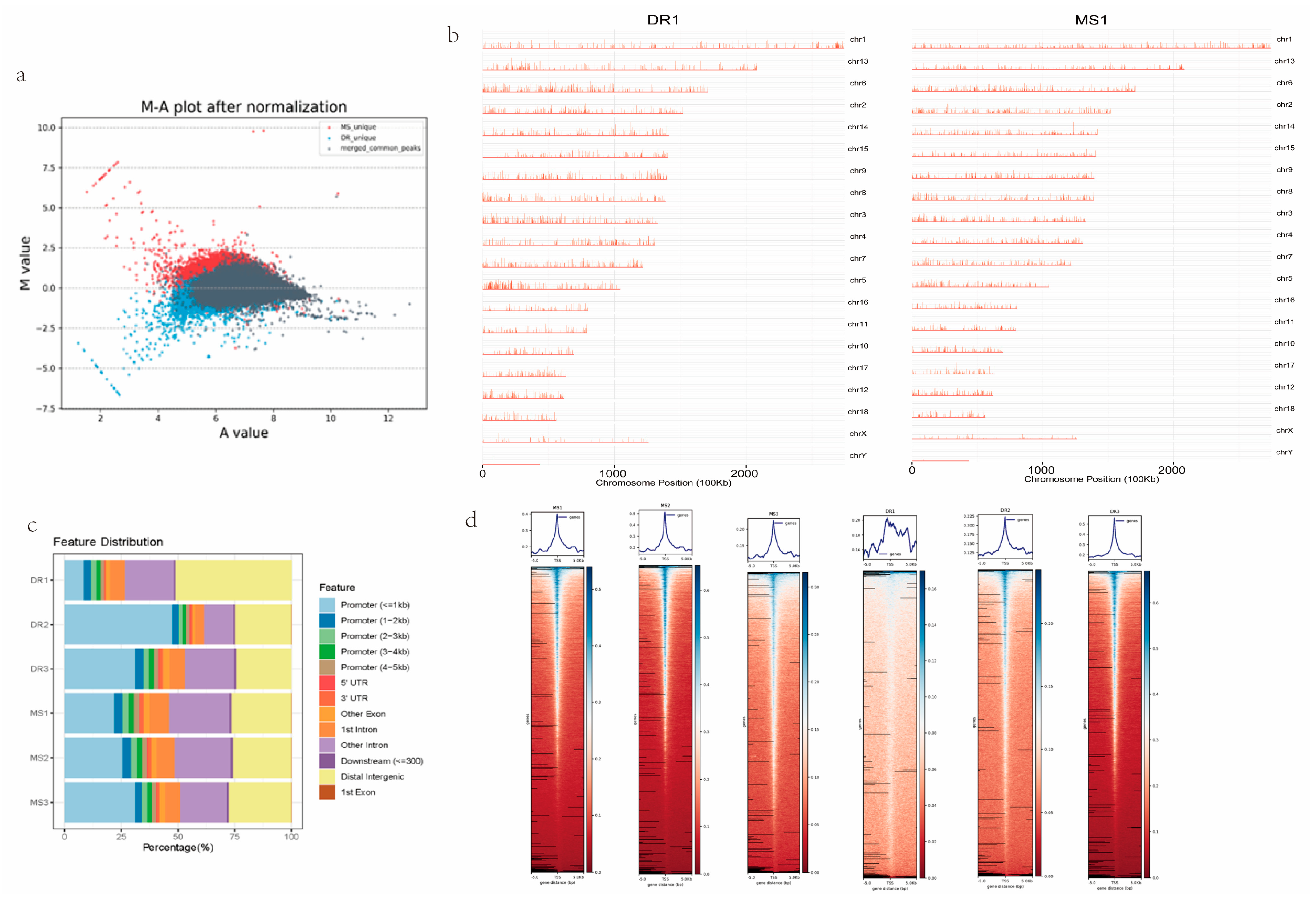
2.2. Quality Assessment of ATAC-Seq Data for Meishan and Duroc Pig Endometrial Tissues
2.3. Chromatin Accessibility in Endometrial Tissues of Meishan and Duroc Pigs
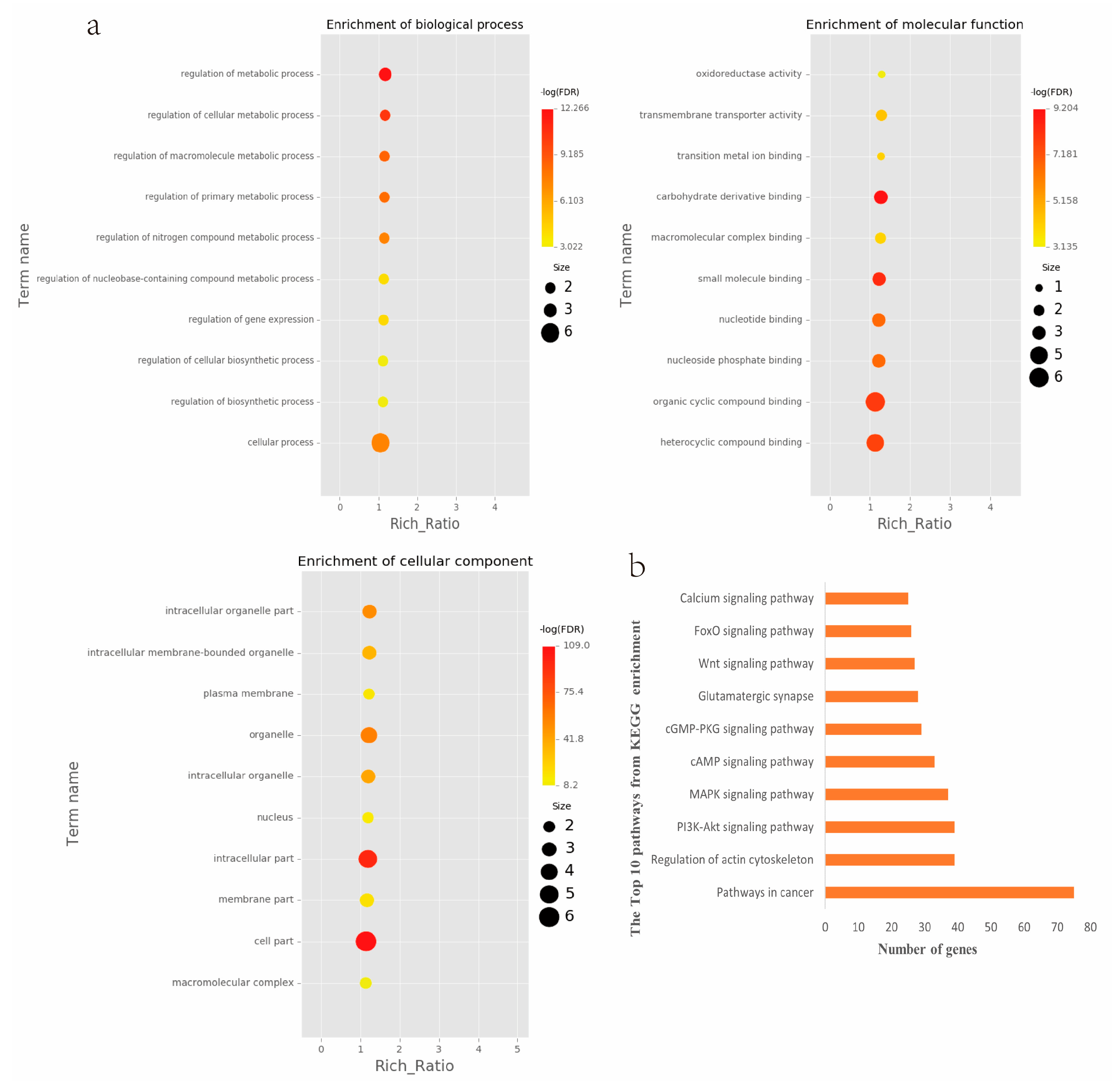
2.4. Analyses of Genes and Motifs in Different Peaks
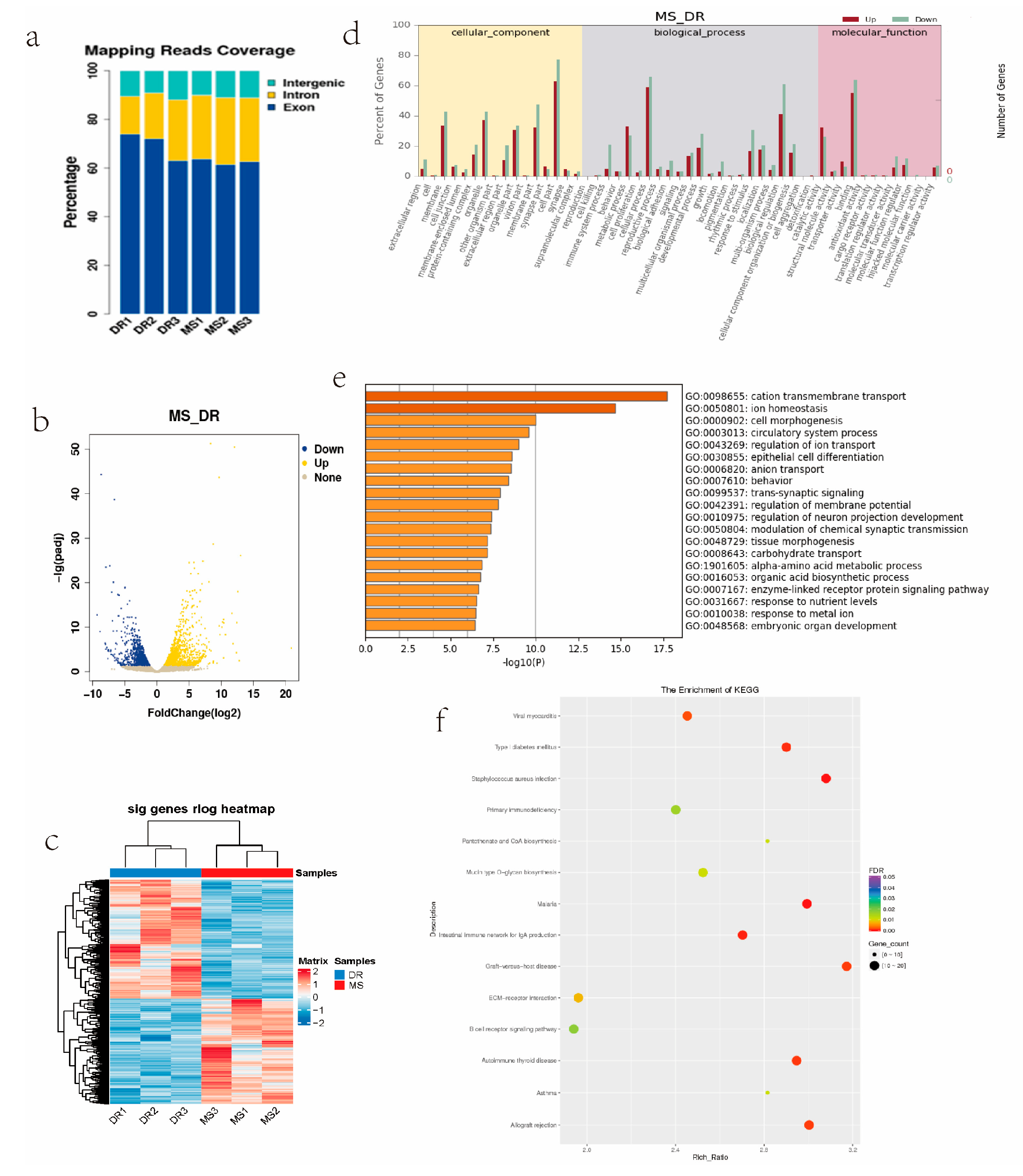
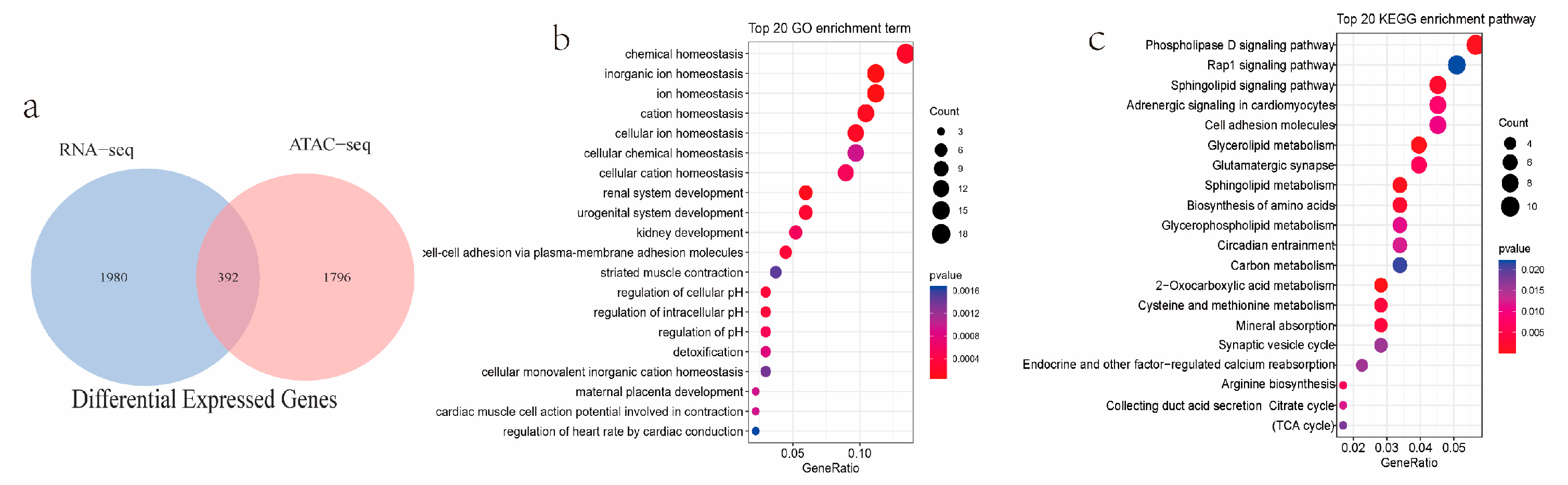
2.5. RNA-Seq Data from Endometrial Tissues of Meishan and Duroc Pigs
2.6. Integration of ATAC-Seq and RNA-Seq Results
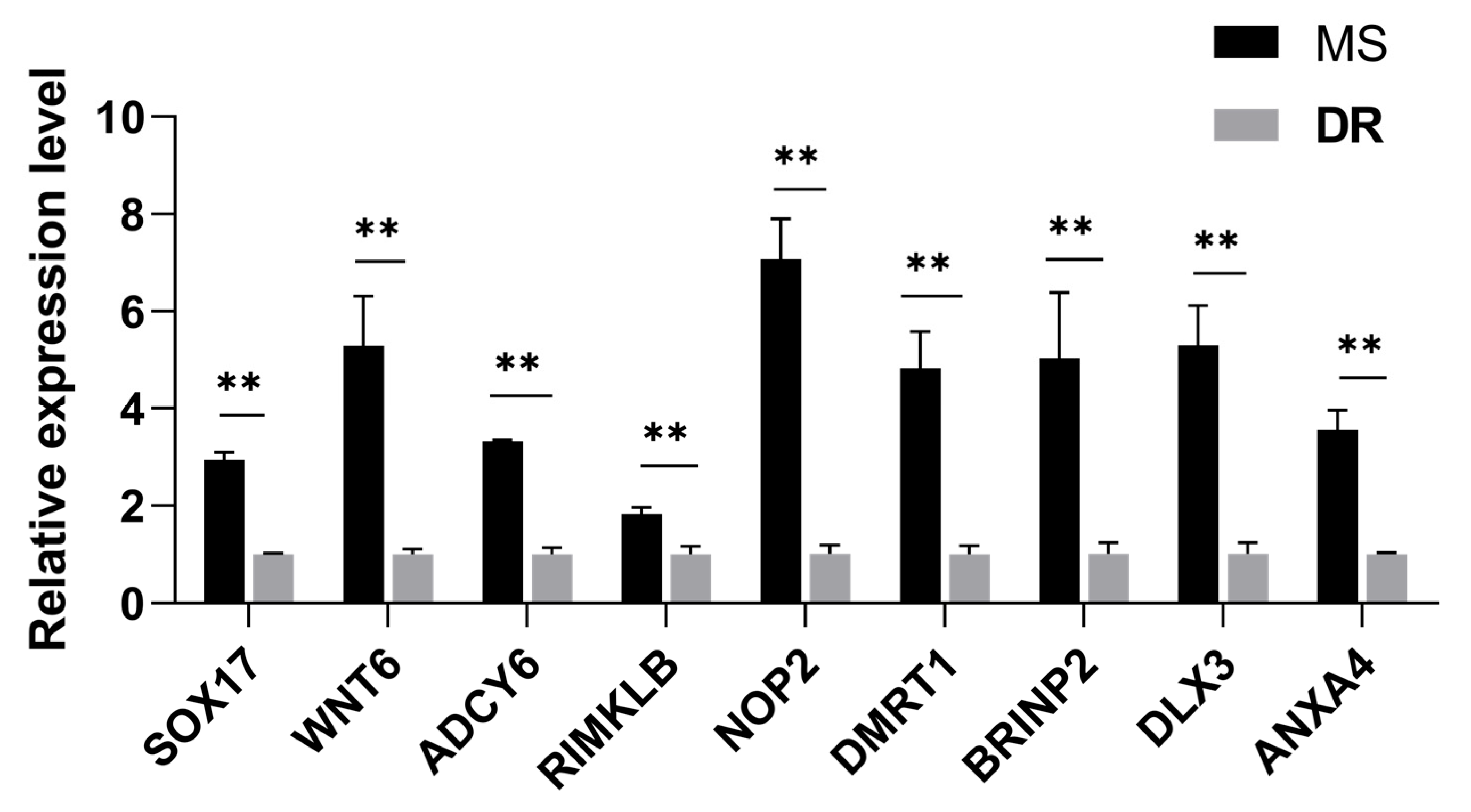
2.7. Validation of RNA-Seq Results Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Histological Analysis
4.3. ATAC-Seq
4.4. RNA-Seq
4.5. Integration of ATAC-Seq and RNA-Seq
4.6. Gene Expression and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ran, X.; Niu, X.; Huang, S.; Li, S.; Wang, J. Whole-genome sequence analysis reveals selection signatures for important economic traits in Xiang pigs. Sci. Rep. 2022, 12, 11823. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.P. Embryonic and fetal development in different genotypes in pigs. J. Reprod. Fertil. Suppl. 1997, 52, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Young, L.D. Reproduction of F1 Meishan, Fengjing, Minzhu, and Duroc gilts and sows. J. Anim. Sci. 1995, 73, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Biensen, N.J.; Wilson, M.E.; Ford, S.P. The impact of either a Meishan or Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J. Anim. Sci. 1998, 76, 2169–2176. [Google Scholar] [CrossRef]
- Young, L.D. Effects of Duroc, Meishan, Fengjing, and Minzhu boars on productivity of mates and growth of first-cross progeny. J. Anim. Sci. 1992, 70, 2020–2029. [Google Scholar] [CrossRef][Green Version]
- Zhao, Q.-B.; López-Cortegano, E.; Oyelami, F.O.; Zhang, Z.; Ma, P.-P.; Wang, Q.-S.; Pan, Y.-C. Conservation Priorities Analysis of Chinese Indigenous Pig Breeds in the Taihu Lake Region. Front. Genet. 2021, 12, 558873. [Google Scholar] [CrossRef]
- McLaren, D.G. Potential of Chinese pig breeds to improve pork production efficiency in the USA. Anim. Breed. Abstr. 1990, 58, 347–369. [Google Scholar]
- Zhang, H.; Wang, S.; Liu, M.; Zhang, A.; Wu, Z.; Zhang, Z.; Li, J. Differential gene expression in the endometrium on gestation day 12 provides insight into sow prolificacy. BMC Genom. 2013, 14, 45. [Google Scholar] [CrossRef]
- Christenson, R.K.; Vallet, J.L.; Leymaster, K.A.; Young, L.D. Uterine function in Meishan pigs. J. Reprod. Fertil. Suppl. 2020, 14, 279–289. [Google Scholar] [CrossRef]
- Spencer, T.E.; Kelleher, A.M.; Bartol, F.F. Development and Function of Uterine Glands in Domestic Animals. Annu Rev. Anim. Biosci. 2019, 7, 125–147. [Google Scholar] [CrossRef]
- Gray, C.A.; Bartol, F.F.; Tarleton, B.J.; Wiley, A.A.; Johnson, G.A.; Bazer, F.W.; Spencer, T.E. Developmental Biology of Uterine Glands. Biol. Reprod. 2001, 65, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Bazer, F.W. Uterine and placental factors regulating conceptus growth in domestic animals. J. Anim. Sci. 2004, 82, E4–E13. [Google Scholar] [PubMed]
- Carson, D.D.; Bagchi, I.; Dey, S.K.; Enders, A.C.; Fazleabas, A.T.; Lessey, B.A.; Yoshinaga, K. Embryo implantation. Dev. Biol. 2000, 223, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Bednar, J.; Horowitz, R.A.; Grigoryev, S.A.; Carruthers, L.M.; Hansen, J.C.; Koster, A.J.; Woodcock, C.L. Nucleosomes, linker DNA, and linker histone form a unique structural motif that directs the higher-order folding and compaction of chromatin. Proc. Natl. Acad. Sci. USA 1998, 95, 14173–14178. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, J.D.; Wu, B.; Chang, H.Y.; Greenleaf, W.J. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr. Protoc. Mol. Biol. 2015, 109, 21.29.1–21.29.9. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Hou, X.; Liu, X.; Wang, L.; Gao, H.; Zhao, F.; Shi, L.; Shi, L.; Yan, H.; Deng, T.; et al. The landscape of chromatin accessibility in skeletal muscle during embryonic development in pigs. J. Anim. Sci. Biotechnol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Luo, L.; Gribskov, M.; Wang, S. Bibliometric review of ATAC-Seq and its application in gene expression. Briefings Bioinform. 2022, 23, bbac061. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, I.J.; Allen, M.A.; Dowell, R.D. Detecting Differential Transcription Factor Activity from ATAC-Seq Data. Molecules 2018, 23, 1136. [Google Scholar] [CrossRef]
- Vrljicak, P.; Lucas, E.S.; Lansdowne, L.; Lucciola, R.; Muter, J.; Dyer, N.P.; Brosens, J.J.; Ott, S. Analysis of chromatin accessibility in decidualizing human endometrial stromal cells. FASEB J. 2017, 32, 2467–2477. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef]
- Lowe, E.K.; Cuomo, C.; Voronov, D.; Arnone, M.I. Using ATAC-seq and RNA-seq to increase resolution in GRN connectivity. Methods Cell Biol. 2018, 151, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, J.; Zhou, J.; Zhang, Y.; Qiao, M.; Sun, H.; Li, Z.; Li, L.; Chen, N.; Oyelami, F.O.; et al. Integration of ATAC-seq and RNA-seq analysis identifies key genes affecting intramuscular fat content in pigs. Front. Nutr. 2022, 9, 1016956. [Google Scholar] [CrossRef] [PubMed]
- Salavati, M.; Woolley, S.A.; Araya, Y.C.; Halstead, M.M.; Stenhouse, C.; Johnsson, M.; Ashworth, C.J.; Archibald, A.L.; Donadeu, F.X.; Hassan, M.A.; et al. Profiling of open chromatin in developing pig (Sus scrofa) muscle to identify regulatory regions. G3 2021, 12, jkab424. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Ma, Z.; Tang, Z.; Yu, L.; Liu, S.; Huang, T.; Wang, P.; Wu, T.; Song, Z.; Zhang, H.; et al. Integrative ATAC-seq and RNA-seq Analysis of the Longissimus Muscle of Luchuan and Duroc Pigs. Front. Nutr. 2021, 8, 742672. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.M.; Behura, S.K.; Burns, G.W.; Young, S.L.; Demayo, F.J.; Spencer, T.E. Integrative analysis of the forkhead box A2 (FOXA2) cistrome for the human endometrium. FASEB J. 2019, 33, 8543–8554. [Google Scholar] [CrossRef]
- Vaishnav, S.; Chauhan, A.; Ajay, A.; Saini, B.L.; Kumar, S.; Kumar, A.; Bhushan, B.; Gaur, G.K. Allelic to genome wide perspectives of swine genetic variation to litter size and its component traits. Mol. Biol. Rep. 2023, 50, 3705–3721. [Google Scholar] [CrossRef] [PubMed]
- Zak, L.J.; Gaustad, A.H.; Bolarin, A.; Broekhuijse, M.L.W.J.; Walling, G.A.; Knol, E.F. Genetic control of complex traits, with a focus on reproduction in pigs. Mol. Reprod. Dev. 2017, 84, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.S.; Puonti, M.; Rydhmer, L.; Jensen, J. Relationship between litter size and perinatal and pre-weaning survival in pigs. J. Anim. Sci. 2002, 74, 217–222. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Hondo, E.; Stewart, C.L. Profiling gene expression in growth-arrested mouse embryos in diapause. Genome Biol. 2005, 6, 202. [Google Scholar] [CrossRef][Green Version]
- Hondo, E.; Phichitrasilp, T.; Kokubu, K.; Kusakabe, K.; Nakamuta, N.; Oniki, H.; Kiso, Y. Distribution Patterns of Uterine Glands and Embryo Spacing in the Mouse. Anat. Histol. Embryol. 2007, 36, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; Zhang, M.-M.; Li, Q.-G.; Tang, H.; Zhang, L.-F.; Wang, K.-J.; Zhu, M.-Z.; Lu, Y.-F.; Bao, H.-G.; Zhang, Y.-M.; et al. Whole-genome resequencing reveals candidate mutations for pig prolificacy. Proc. R. Soc. B Boil. Sci. 2017, 284, 20172437. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Wang, Y.; Li, X.; Wan, Z.; Wang, X.; Zhu, H.; Wang, C.; Zhao, S.; Chen, H.; Liu, Y. Effect of flaxseed oil on muscle protein loss and carbohydrate oxidation impairment in a pig model after lipopolysaccharide challenge. Br. J. Nutr. 2019, 123, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Wei, Y.; Zhang, Z.; Li, C.; Song, C.; Liu, Y.; Qi, K.; Li, X.; Li, X.; Qiao, R.; et al. Transcriptome-wide analysis of RNA m6A methylation regulation of muscle development in Queshan Black pigs. BMC Genom. 2023, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yu, Y.; Feng, W.; Du, H.; Yu, J.; Kang, H.; Zheng, X.; Wang, Z.; Liu, G.E.; Ernst, C.W.; et al. Evidence of evolutionary history and selective sweeps in the genome of Meishan pig reveals its genetic and phenotypic characterization. Gigascience 2018, 7, giy058. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, S.; Liu, X.; Liu, H.; Hu, T.; Qiu, X.; Zhang, J.; Lei, M. Analyses of Long Non-Coding RNA and mRNA profiling using RNA sequencing during the pre-implantation phases in pig endometrium. Sci. Rep. 2016, 6, 20238. [Google Scholar] [CrossRef]
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu. Rev. Biochem. 2022, 91, 571–598. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, A.; Verp, S.; Offner, S.; Grun, D.; Trono, D. DUX is a non-essential synchronizer of zygotic genome activation. Development 2020, 147, 177725. [Google Scholar] [CrossRef]
- Ren, W.; Gao, L.; Mou, Y.; Deng, W.; Hua, J.; Yang, F. DUX: One Transcription Factor Controls 2-Cell-like Fate. Int. J. Mol. Sci. 2022, 23, 2067. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Fang, Y.; Gu, X.; Zheng, Q.; Lu, J.; Li, L. The role of LncRNA LBX2-AS1 in cancers: Functions, mechanisms and potential clinical utility. Clin. Transl. Oncol. 2023, 25, 293–305. [Google Scholar] [CrossRef]
- Moisan, V.; Robert, N.M.; Tremblay, J.J. Expression of ladybird-like homeobox 2 (LBX2) during ovarian development and folliculogenesis in the mouse. J. Mol. Histol. 2010, 41, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Moisan, V.; Bomgardner, D.; Tremblay, J.J. Expression of the Ladybird-like homeobox 2 transcription factor in the developing mouse testis and epididymis. BMC Dev. Biol. 2008, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, D.; Modi, D. LIM Homeodomain (LIM-HD) Genes and Their Co-Regulators in Developing Reproductive System and Disorders of Sex Development. Sex. Dev. 2022, 16, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Wang, Y.; Wang, H. Identification and Analysis of Regulatory Elements in Porcine Bone Morphogenetic Protein 15 Gene Promoter. Int. J. Mol. Sci. 2015, 16, 25759–25772. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martín, L.; Galay-Burgos, M.; Sweeney, G.; Piferrer, F. Different sox17 transcripts during sex differentiation in sea bass, Dicentrarchus labrax. Mol. Cell Endocrinol. 2009, 299, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, A.; Rogers, P. Cyclic changes and hormonal regulation of annexin IV mRNA and protein in human endometrium. Mol. Hum. Reprod. 2006, 12, 661–669. [Google Scholar] [CrossRef] [PubMed]
- King, J.H.; Kwan, S.T.C.; Yan, J.; Jiang, X.; Fomin, V.G.; Levine, S.P.; Wei, E.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation Modulates Placental Markers of Inflammation, Angiogenesis, and Apoptosis in a Mouse Model of Placental Insufficiency. Nutrients 2019, 11, 374. [Google Scholar] [CrossRef]
- Lu, H.; Huang, X.; Zhang, L.; Guo, Y.; Cheng, H.; Zhou, R. Multiple alternative splicing of mouse Dmrt1 during gonadal differentiation. Biochem. Biophys. Res. Commun. 2007, 352, 630–634. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, F.; Sandzén, J.; Cao, R.; Flaberg, E.; Szekely, L.; Cao, Y.; Ohlsson, C.; Bergo, M.O.; Borén, J.; et al. Filamin B deficiency in mice results in skeletal malformations and impaired microvascular development. Proc. Natl. Acad. Sci. USA 2007, 104, 3919–3924. [Google Scholar] [CrossRef]
- Smith, A.L. Determining the Role of IRF6 in Oogenesis and Extra Embryonic Development. Ph.D. Dissertation, Michigan State University, East Lansing, MI, USA, 2014. [Google Scholar]
- Tu, S.; Narendra, V.; Yamaji, M.; Vidal, S.E.; Rojas, L.A.; Wang, X.; Kim, S.Y.; Garcia, B.A.; Tuschl, T.; Stadtfeld, M.; et al. Co-repressor CBFA2T2 regulates pluripotency and germline development. Nature 2016, 534, 387–390. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Liu, D.; Ying, Q.-L.; Ye, S. Tfcp2l1 represses multiple lineage commitment of mouse embryonic stem cells through MTA1 and LEF1. J. Cell Sci. 2017, 130, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Worku, T.; Wang, K.; Ayers, D.; Wu, D.; Rehman, Z.U.; Zhou, H.; Yang, L. Regulatory roles of ephrinA5 and its novel signaling pathway in mouse primary granulosa cell apoptosis and proliferation. Cell Cycle 2018, 17, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Peng, H.; Lu, W.H.; Shuai, H.L.; Zha, Q.B.; Yeung, C.K.; Li, H.; Wang, L.J.; Ho Lee, K.K.; Zhu, W.J.; et al. Role of Slit2/Robo1 in trophoblast invasion and vascular remodeling during ectopic tubal pregnancy. Placenta 2015, 36, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, H.; Lee, Y.; Kim, Y.; Lee, B.; Kim, J.Y.; Jin, C.; Kim, S.; Kim, H.; Han, K. Smaller Body Size, Early Postnatal Lethality, and Cortical Extracellular Matrix-Related Gene Expression Changes of Cyfip2-Null Embryonic Mice. Front. Mol. Neurosci. 2019, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Shi, J.; Huang, R.; Wu, Z.; Wu, S.; Bao, W. Transcriptome-Wide lncRNA and mRNA Profiling of Spleens from Meishan Pigs at Different Development Stages. Animals 2022, 12, 2676. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Yoshihara, S.; Akita, T.; Goto, N. Immunological Characteristics and Skin Structure of the Meishan Pig: Serum Complement Activity, Serum C3 Level and Immune Response. Anim. Sci. J. 1999, 70, 393–398. [Google Scholar] [CrossRef][Green Version]
- Tayade, C.; Fang, Y.; Croy, B.A. A Review of Gene Expression in Porcine Endometrial Lymphocytes, Endothelium and Trophoblast During Pregnancy Success and Failure. J. Reprod. Dev. 2007, 53, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, J.; Lv, L.; Gou, Y.; Wang, B.; Hao, T. Integration of ATAC-seq and RNA-seq identifies active G-protein coupled receptors functioning in molting process in muscle of Eriocheir sinensis. Front. Mar. Sci. 2022, 9, 900160. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, R.; Zhang, H.; Wang, D.; Wang, J.; Wu, K. A unique 15-bp InDel in the first intron of BMPR1B regulates its expression in Taihu pigs. BMC Genom. 2022, 23, 799. [Google Scholar] [CrossRef]

| Number | Motifs | TF ID | E-Value |
|---|---|---|---|
| 1 | 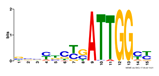 | NFYA | 0.000071539 |
| 2 |  | NFYB | 0.081957 |
| 3 | 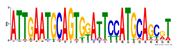 | Dux | 0.166414 |
| 4 |  | LBX2 | 0.934459 |
| 5 | 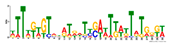 | MSX1 | 1.61321 |
| 6 |  | Nkx2-5(var.2) | 1.62006 |
| 7 |  | EN1 | 2.37482 |
| 8 |  | Lhx8 | 2.50521 |
| 9 |  | Msx3 | 2.93158 |
| 10 |  | GBX2 | 2.93158 |
| Upregulated Gene Number | Downregulated Gene Number | Total | |
|---|---|---|---|
| ATAC-seq | 1960 | 228 | 2188 |
| RNA-seq | 1158 | 1214 | 2372 |
| Overlap | 223 | 169 | 392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, Z.; Wang, J.; Zeng, T.; Ai, X.; Wu, K. An Integrative ATAC-Seq and RNA-Seq Analysis of the Endometrial Tissues of Meishan and Duroc Pigs. Int. J. Mol. Sci. 2023, 24, 14812. https://doi.org/10.3390/ijms241914812
Zhang H, Liu Z, Wang J, Zeng T, Ai X, Wu K. An Integrative ATAC-Seq and RNA-Seq Analysis of the Endometrial Tissues of Meishan and Duroc Pigs. International Journal of Molecular Sciences. 2023; 24(19):14812. https://doi.org/10.3390/ijms241914812
Chicago/Turabian StyleZhang, Han, Zhexi Liu, Ji Wang, Tong Zeng, Xiaohua Ai, and Keliang Wu. 2023. "An Integrative ATAC-Seq and RNA-Seq Analysis of the Endometrial Tissues of Meishan and Duroc Pigs" International Journal of Molecular Sciences 24, no. 19: 14812. https://doi.org/10.3390/ijms241914812
APA StyleZhang, H., Liu, Z., Wang, J., Zeng, T., Ai, X., & Wu, K. (2023). An Integrative ATAC-Seq and RNA-Seq Analysis of the Endometrial Tissues of Meishan and Duroc Pigs. International Journal of Molecular Sciences, 24(19), 14812. https://doi.org/10.3390/ijms241914812






