The Resistance of Maize to Ustilago maydis Infection Is Correlated with the Degree of Methyl Esterification of Pectin in the Cell Wall
Abstract
:1. Introduction
2. Results
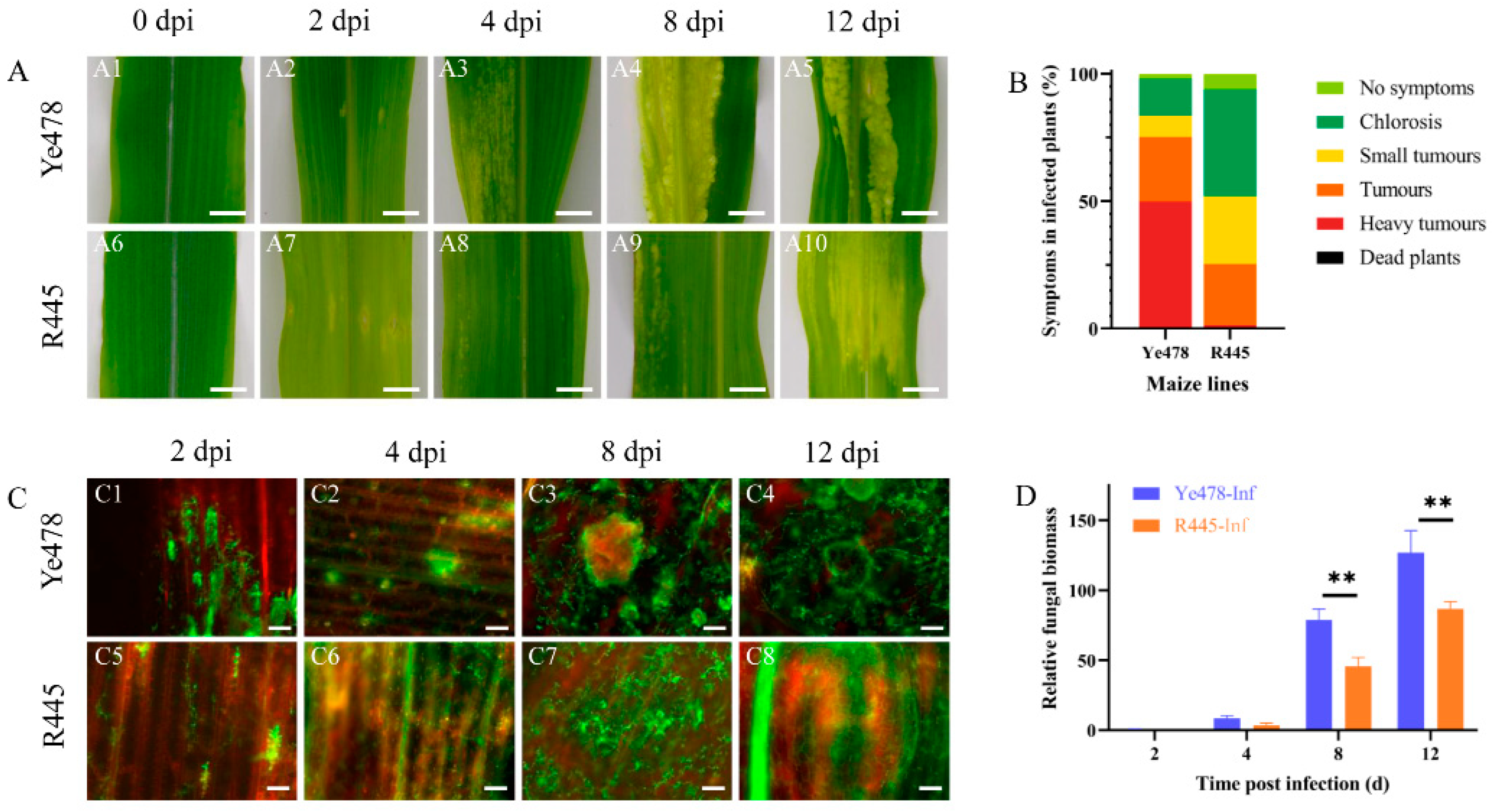
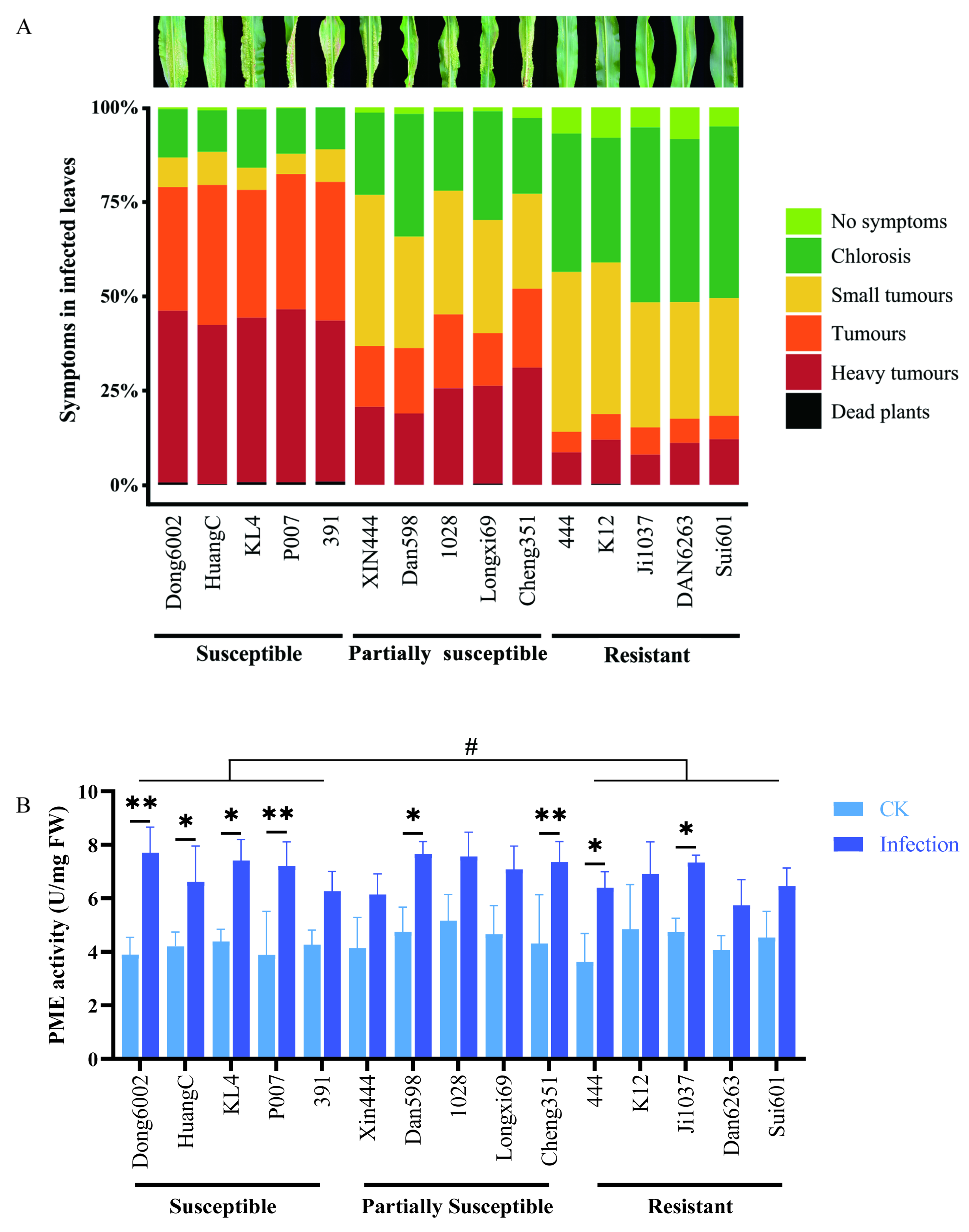
2.1. Identification of Disease-Resistant Phenotype in the Resistant and Susceptible Maize Plants Infected with U. maydis
2.2. Analysis of Cell Wall Components and CWDEs after U. maydis Infection
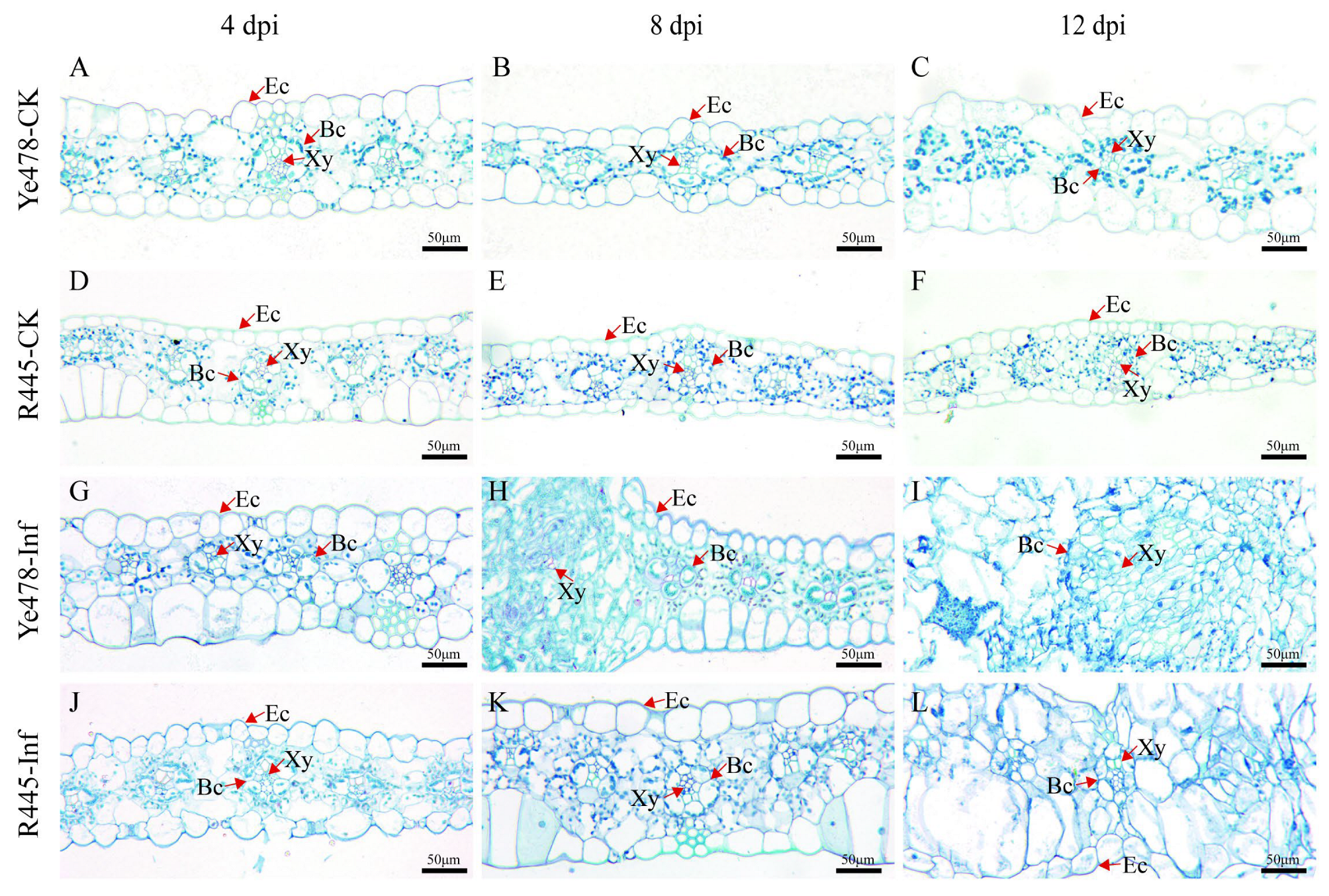
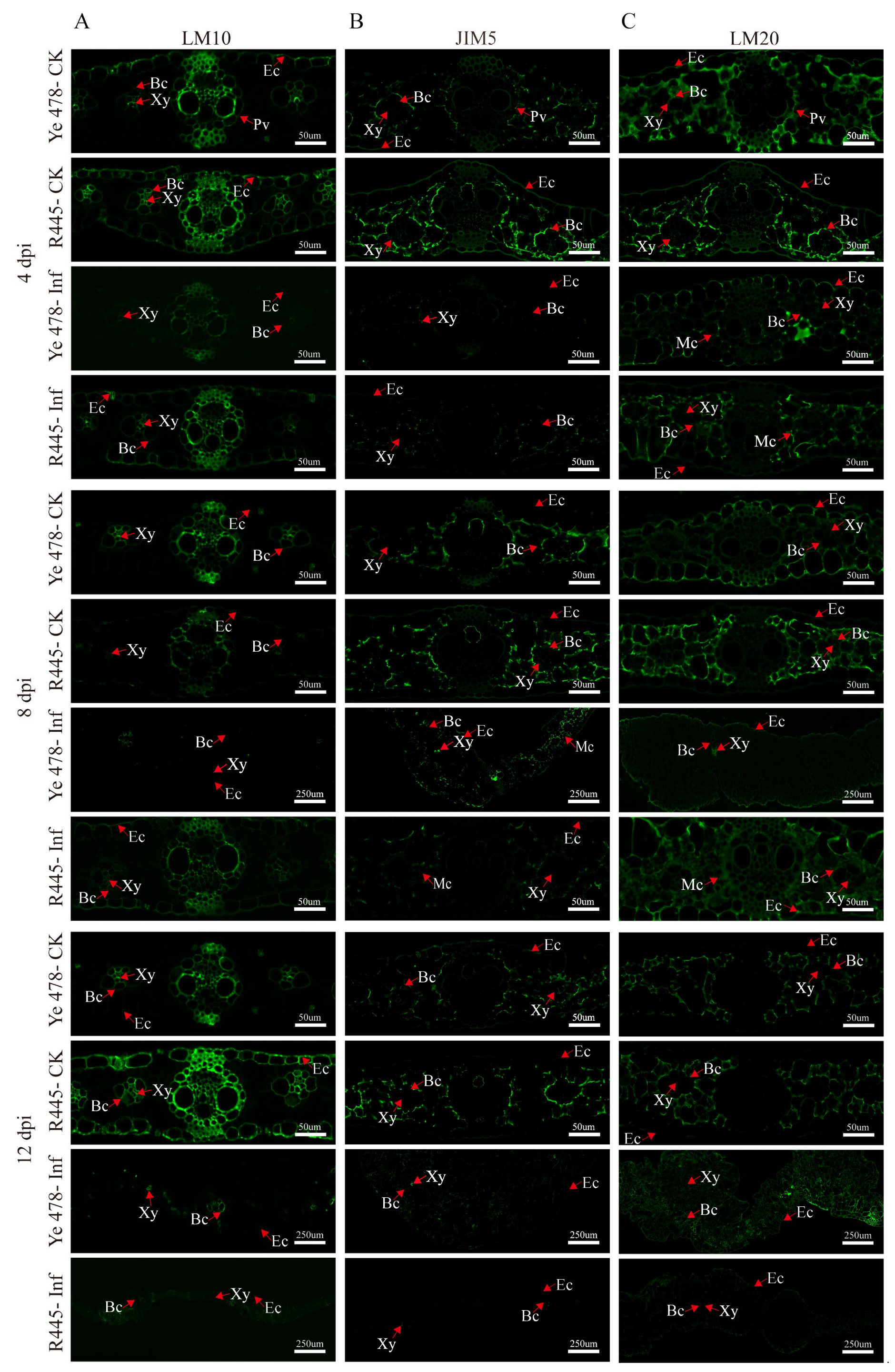
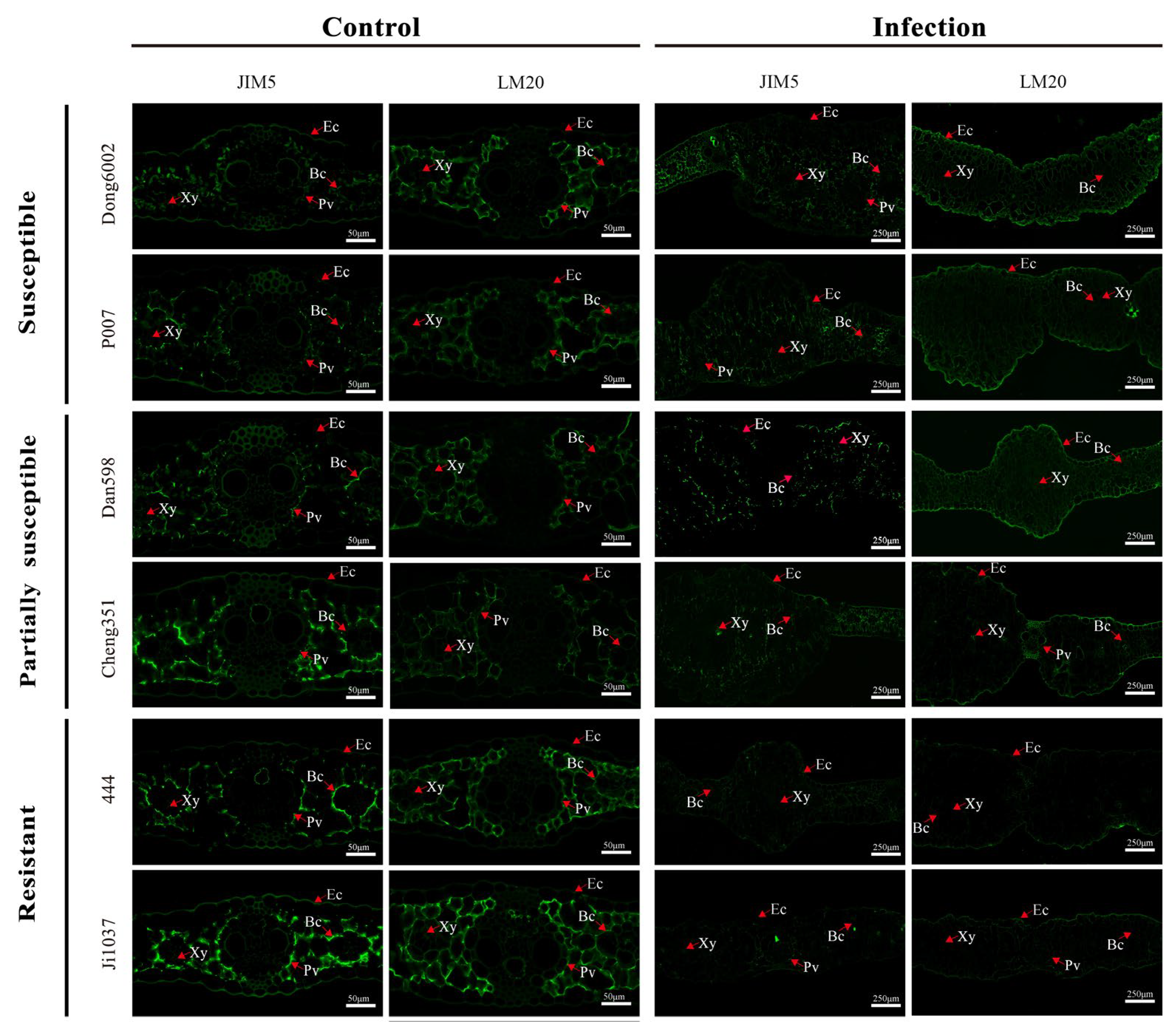
2.3. Assessment of Changes in Cell Wall Components and Structure via Immunofluorescence Analysis
2.4. Analysis of Phenotype and PME Activity in the Maize Seedlings with U. maydis Infection
2.5. Changes in Pectin Methyl Esterification in Maize Lines during Host–Pathogen Interactions
2.6. Changes in the Expression of PME-synthesis-related Genes in Maize Lines Infected with U. maydis
3. Discussion
3.1. U. maydis Induced Changes in Maize Leaf Cell Structure
3.2. The Changes in Cell Wall Components after U. maydis Infection
3.3. The Function of PME in the Plant–Pathogen Interaction
3.4. Resistance to Pathogens Correlates with the Degree of Pectin Methyl Esterification
4. Materials and Methods
4.1. Plant Materials and U. maydis Inoculation
4.2. Analysis of Cell Wall Components, PME, and CWDEs
4.3. Microscopic and Immunofluorescence Analyses
4.4. RNA Extraction and qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, K.K.; Li, Y.; Zhang, W.J.; Jia, Y.F.; Wang, Y.; Ma, Y.T.; Lv, X.L.; Xuan, Y.H.; Du, W.L. Early infection response of fungal biotroph Ustilago maydis in maize. Front. Plant Sci. 2022, 13, 970897. [Google Scholar] [CrossRef]
- Schurack, S. Mechanisms of Quantitative Disease Resistance in the Maize-Ustilago maydis Interaction. Ph.D. Thesis, Universität zu Köln, Köln, Germany, 2021; pp. 1–133. [Google Scholar]
- Ruan, X.S.; Ma, L.; Zhang, Y.Y.; Wang, Q.; Gao, X.Q. Dissection of the complex transcription and metabolism regulation networks associated with maize resistance to Ustilago maydis. Genes 2021, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Qi, J.Z.; Han, H.Y.; Wang, P.C.; Liu, C.W. Progress in pathogenesis research of Ustilago maydis, and the metabolites involved along with their biosynthesis. Mol. Plant Pathol. 2023, 24, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Huerta, A.I.; Sancho-Andrés, G.; Montesinos, J.C.; Silva-Navas, J.; Bassard, S.; Pau-Roblot, C.; Kesten, C.; Schlechter, R.; Dora, S.; Ayupov, T.; et al. The WAK-like protein RFO1 acts as a sensor of the pectin methylation status in Arabidopsis cell walls to modulate root growth and defense. Mol. Plant 2023, 16, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.S.; Yang, B.; Xu, Z.D.; Li, F.C.; Guo, K.; Zhang, M.L.; Wang, L.Q.; Zou, W.H.; Wang, Y.T.; Peng, L.C. Global identification of multiple OsGH9 family members and their involvement in cellulose crystallinity modification in rice. PLoS ONE 2013, 8, e50171. [Google Scholar] [CrossRef]
- Tang, Y.J.; Wang, M.H.; Cao, L.Y.; Dang, Z.J.; Ruan, N.; Wang, Y.; Huang, Y.N.; Wu, J.Y.; Zhang, M.F.; Xu, Z.J.; et al. OsUGE3-mediated cell wall polysaccharides accumulation improves biomass production, mechanical strength, and salt tolerance. Plant Cell Environ. 2022, 45, 2492–2507. [Google Scholar] [CrossRef]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef]
- Du, J.; Anderson, C.T.; Xiao, C.W. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 2022, 8, 332–340. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Hückelhoven, R. Cell wall-associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 2007, 45, 101–127. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T. We be jammin’: An update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot. 2016, 67, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T.; Kieber, J.J. Dynamic construction, perception, and remodeling of plant cell walls. Annu. Rev. Plant Boil. 2020, 71, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Kohorn, B.D.; Kobayashi, M.; Johansen, S.; Friedman, H.P.; Fischer, A.; Byers, N. Wall-associated kinase 1 (WAK1) is crosslinked in endomembranes, and transport to the cell surface requires correct cell-wall synthesis. J. Cell Sci. 2006, 119, 2282–2290. [Google Scholar] [CrossRef]
- Benedetti, M.; Pontiggia, D.; Raggi, S.; Cheng, Z.Y.; Scaloni, F.; Ferrari, S.; Ausubel, F.M.; Cervone, F.; De Lorenzo, G. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 5533–5538. [Google Scholar] [CrossRef]
- Savatin, D.V.; Bisceglia, N.G.; Marti, L.; Fabbri, C.; Cervone, F.; De Lorenzo, G. The Arabidopsis nucleus-and phragmoplast-localized KINASE1-related protein kinases are required for elicitor-induced oxidative burst and immunity. Plant Physiol. 2014, 165, 1188–1202. [Google Scholar] [CrossRef]
- Davidsson, P.; Broberg, M.; Kariola, T.; Sipari, N.; Pirhonen, M.; Palva, E.T. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef]
- Escudero, V.; Jordá, L.; Sopeña-Torres, S.; Mélida, H.; Miedes, E.; Muñoz-Barrios, A.; Swami, S.; Alexander, D.; McKee, L.S.; Sánchez-Vallet, A.; et al. Alteration of cell wall xylan acetylation triggers defense responses that counterbalance the immune deficiencies of plants impaired in the β-subunit of the heterotrimeric G-protein. Plant J. 2017, 92, 386–399. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Hoy, J.W.; Appezzato-da-Glória, B.; Viveros, A.F.G.; Vieira, M.L.C.; Baisakh, N. Sugarcane cell wall-associated defense responses to infection by Sporisorium scitamineum. Front. Plant Sci. 2018, 9, 698. [Google Scholar] [CrossRef]
- Matei, A.; Ernst, C.; Günl, M.; Thiele, B.; Altmüller, J.; Walbot, V.; Usadel, B.; Doehlemann, G. How to make a tumour: Cell type specific dissection of Ustilago maydis induced tumour development in maize leaves. New Phytol. 2018, 217, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Happel, P.; Kahmann, R. Nuclear status and leaf tumor formation in the Ustilago maydis-maize pathosystem. New Phytol. 2021, 231, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Gao, Y.H.; Zhang, L.J.; Zhou, Y.H. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Song, D.L.; Shen, J.H.; Li, L.G. Cellulose synthesis in the cell walls of higher plants. Plant Physiol. Commun. 2008, 44, 791–796. [Google Scholar]
- Hérnandez-Blanco, C.; Feng, D.X.; Hu, J.; Sánchez-Vallet, A.; Deslandes, L.; Llorente, F.; Berrocal-Lobo, M.; Keller, H.; Barlet, X.; Sánchez-Rodríguez, C.; et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 2007, 19, 890–903. [Google Scholar] [CrossRef]
- Douchkov, D.; Lueck, S.; Hensel, G.; Kumlehn, J.; Rajaraman, J.; Johrde, A.; Doblin, M.S.; Beahan, C.T.; Kopischke, M.; Fuchs, R.; et al. The barley (Hordeum vulgare) cellulose synthase-like D2 gene (HvCslD2) mediates penetration resistance to host-adapted and nonhost isolates of the powdery mildew fungus. New Phytol. 2016, 212, 421–433. [Google Scholar] [CrossRef]
- Delgado-Cerezo, M.; Sánchez-Rodríguez, C.; Escudero, V.; Miedes, E.; Fernández, P.V.; Jordá, L.; Hernández-Blanco, C.; Sánchez-Vallet, A.; Bednarek, P.; Schulze-Lefert, P.; et al. Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol. Plant 2012, 5, 98–114. [Google Scholar] [CrossRef]
- Rogers, L.A.; Dubos, C.; Surman, C.; Willment, J.; Cullis, I.F.; Mansfield, S.D.; Campbell, M.M. Comparison of lignin deposition in three ectopic lignification mutants. New Phytol. 2005, 168, 123–140. [Google Scholar] [CrossRef]
- Brown, D.M.; Zeef, L.A.H.; Ellis, J.; Goodacre, R.; Turner, S.R. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 2005, 17, 2281–2295. [Google Scholar] [CrossRef]
- Sampedro, J.; Pardo, B.; Gianzo, C.; Guitián, E.; Revilla, G.; Zarra, I. Lack of α-xylosidase activity in Arabidopsis alters xyloglucan composition and results in growth defects. Plant Physiol. 2010, 154, 1105–1115. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants, 2nd ed.; John Wiley & Sons Publishing Company: New York, NY, USA, 2020; pp. 43–107. [Google Scholar]
- Imeson, A. Food Stabilisers, Thickeners and Gelling Agents; Blackwell Publishing Ltd.: London, UK, 2009; pp. 237–265. [Google Scholar]
- Bethke, G.; Thao, A.; Xiong, G.Y.; Li, B.H.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M.; et al. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Raab, T.K.; Schiff, C.; Somerville, S.C. PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 2002, 14, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Raab, T.K.; Somerville, C.R.; Somerville, S.C. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004, 40, 968–978. [Google Scholar] [CrossRef]
- Li, W.T.; Wang, K.; Chern, M.; Liu, Y.C.; Zhu, Z.W.; Liu, J.; Zhu, X.B.; Yin, J.J.; Ran, L.; Xiong, J.; et al. Sclerenchyma cell thickening through enhanced lignification induced by OsMYB30 prevents fungal penetration of rice leaves. New Phytol. 2020, 226, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Giraldo, L.; Jikumaru, Y.; Kamiya, Y.; Tang, Y.H.; Dixon, R.A. Selective lignin downregulation leads to constitutive defense response expression in alfalfa (Medicago sativa L.). New Phytol. 2011, 190, 627–639. [Google Scholar] [CrossRef]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; L’Enfant, M.; Domon, J.M.; Rosiau, E.; Crépeau, M.J.; Surcouf, O.; Esquivel-Rodriguez, J.; Marcelo, P.; Mareck, A.; Guérineau, F.; et al. Tuning of pectin methylesterification pectin methylesterase inhibitor 7 modulates the processive activity of co-expressed pectin methylesterase 3 in a pH-dependent manner. J. Biol. Chem. 2015, 290, 23320–23335. [Google Scholar]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef]
- Dora, S.; Terrett, O.M.; Sánchez-Rodríguez, C. Plant–microbe interactions in the apoplast: Communication at the plant cell wall. Plant Cell 2022, 34, 1532–1550. [Google Scholar] [CrossRef]
- Rao, T.B.; Chopperla, R.; Methre, R.; Punniakotti, E.; Venkatesh, V.; Sailaja, B.; Reddy, M.R.; Yugander, A.; Laha, G.S.; Madhav, M.S.; et al. Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol. Biol. 2019, 100, 59–71. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defense responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Bano, N.; Ansari, S.A.; Hashem, A.; Abd Allah, E.F.; Ansari, M.I. Amplification, sequencing and characterization of pectin methyl esterase inhibitor 51 gene in Tectona grandis L.f. Saudi J. Biol. Sci. 2021, 28, 5451–5460. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Raiola, A.; Camardella, L.; Giovane, A.; Obel, N.; Pauly, M.; Favaron, F.; Cervone, F.; Bellincampi, D. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 2007, 143, 1871–1880. [Google Scholar] [CrossRef]
- Del Corpo, D.; Fullone, M.R.; Miele, R.; Lafond, M.; Pontiggia, D.; Grisel, S.; Kieffer-Jaquinod, S.; Giardina, T.; Bellincampi, D.; Lionetti, V. AtPME17 is a functional Arabidopsis thaliana pectin methylesterase regulated by its PRO region that triggers PME activity in the resistance to Botrytis cinerea. Mol. Plant Pathol. 2020, 21, 1620–1633. [Google Scholar] [CrossRef]
- Raiola, A.; Lionetti, V.; Elmaghraby, I.; Immerzeel, P.; Mellerowicz, E.J.; Salvi, G.; Cervone, F.; Bellincampi, D. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant-Microbe Interact. 2011, 24, 432–440. [Google Scholar] [CrossRef]
- Liu, N.N.; Sun, Y.; Pei, Y.K.; Zhang, X.Y.; Wang, P.; Li, X.C.; Li, F.G.; Hou, Y.H. A pectin methylesterase inhibitor enhances resistance to Verticillium Wilt. Plant Physiol. 2018, 176, 2202–2220. [Google Scholar] [CrossRef]
- Chen, M.H.; Citovsky, V. Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J. 2003, 35, 386–392. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Balestrieri, C.; Castaldo, D.; Giovane, A.; Quagliuolo, L.; Servillo, L. A glycoprotein inhibitor of pectin methylesterase in kiwi fruit (Actinidia chinensis). Eur. J. Biochem. 1990, 193, 183–187. [Google Scholar] [CrossRef]
- Camardella, L.; Carratore, V.; Ciardiello, M.A.; Servillo, L.; Balestrieri, C.; Giovane, A. Kiwi protein inhibitor of pectin methylesterase. Eur. J. Biochem. 2000, 267, 4561–4565. [Google Scholar] [CrossRef] [PubMed]
- Denès, J.M.; Baron, A.; Renard, C.M.; Péan, C.; Drilleau, J.F. Different action pattern for apple pectin methylesterase at pH 7.0 and 4.5. Carbohydr. Res. 2000, 327, 385–393. [Google Scholar] [CrossRef]
- Limberg, G.; Körner, R.; Buchholt, H.C.; Christensen, T.M.; Roepstorff, P.; Mikkelsen, J.D. Analysis of different deesterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. Niger. Carbohydr. Res. 2000, 327, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Wiethölter, N.; Graeßner, B.; Mierau, M.; Mort, A.J.; Moerschbacher, B.M. Differences in the methyl ester distribution of homogalacturonans from near-isogenic wheat lines resistant and susceptible to the wheat stem rust fungus. Mol. Plant Microbe Interact. 2003, 16, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Shen, X.P.; Cao, J.S. The metabolism and function of homogalacturonan in plant cell wall. Chin. J. Cell Biol. 2014, 36, 93–98. (In Chinese) [Google Scholar]
- An, S.H.; Choi, H.W.; Hong, J.K.; Hwang, B.K. Regulation and function of the pepper pectin methylesterase inhibitor (CaPMEI1) gene promoter in defense and ethylene and methyl jasmonate signaling in plants. Planta 2009, 230, 1223–1237. [Google Scholar] [CrossRef]
- Le Cam, B.; Massiot, P.; Campion, C.; Rouxel, F. Susceptibility of carrot cultivars to Mycocentrospora acerina and the structure of cell wall polysaccharides. Physiol. Mol. Plant Pathol. 1994, 45, 139–151. [Google Scholar] [CrossRef]
- Redkar, A.; Doehlemann, G. Ustilago maydis virulence assays in maize. Bio-Protocol 2016, 6, e1760. [Google Scholar] [CrossRef]
- Gao, L.; Kelliher, T.; Nguyen, L.; Walbot, V. Ustilago maydis reprograms cell proliferation in maize anthers. Plant J. 2013, 75, 903–914. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, W.J.; Yang, Y.; Ma, Y.T.; Li, X.Y.; Meng, D.X.; Luo, H.S.; Xue, W.; Lv, X.L.; et al. BSA-Seq and transcriptomic analysis provide candidate genes associated with inflorescence architecture and kernel orientation by phytohormone homeostasis in maize. Int. J. Mol. Sci. 2023, 24, 10728. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, Y.; Zou, K.; Wang, Y.; Ma, Y.; Meng, D.; Luo, H.; Qu, J.; Li, F.; Xuan, Y.; et al. The Resistance of Maize to Ustilago maydis Infection Is Correlated with the Degree of Methyl Esterification of Pectin in the Cell Wall. Int. J. Mol. Sci. 2023, 24, 14737. https://doi.org/10.3390/ijms241914737
Huang Y, Li Y, Zou K, Wang Y, Ma Y, Meng D, Luo H, Qu J, Li F, Xuan Y, et al. The Resistance of Maize to Ustilago maydis Infection Is Correlated with the Degree of Methyl Esterification of Pectin in the Cell Wall. International Journal of Molecular Sciences. 2023; 24(19):14737. https://doi.org/10.3390/ijms241914737
Chicago/Turabian StyleHuang, Yingni, Yang Li, Kunkun Zou, Yang Wang, Yuting Ma, Dexuan Meng, Haishan Luo, Jianzhou Qu, Fengcheng Li, Yuanhu Xuan, and et al. 2023. "The Resistance of Maize to Ustilago maydis Infection Is Correlated with the Degree of Methyl Esterification of Pectin in the Cell Wall" International Journal of Molecular Sciences 24, no. 19: 14737. https://doi.org/10.3390/ijms241914737
APA StyleHuang, Y., Li, Y., Zou, K., Wang, Y., Ma, Y., Meng, D., Luo, H., Qu, J., Li, F., Xuan, Y., & Du, W. (2023). The Resistance of Maize to Ustilago maydis Infection Is Correlated with the Degree of Methyl Esterification of Pectin in the Cell Wall. International Journal of Molecular Sciences, 24(19), 14737. https://doi.org/10.3390/ijms241914737




