Involvement of IL-1β-Mediated Necroptosis in Neurodevelopment Impairment after Neonatal Sepsis in Rats
Abstract
1. Introduction
2. Results
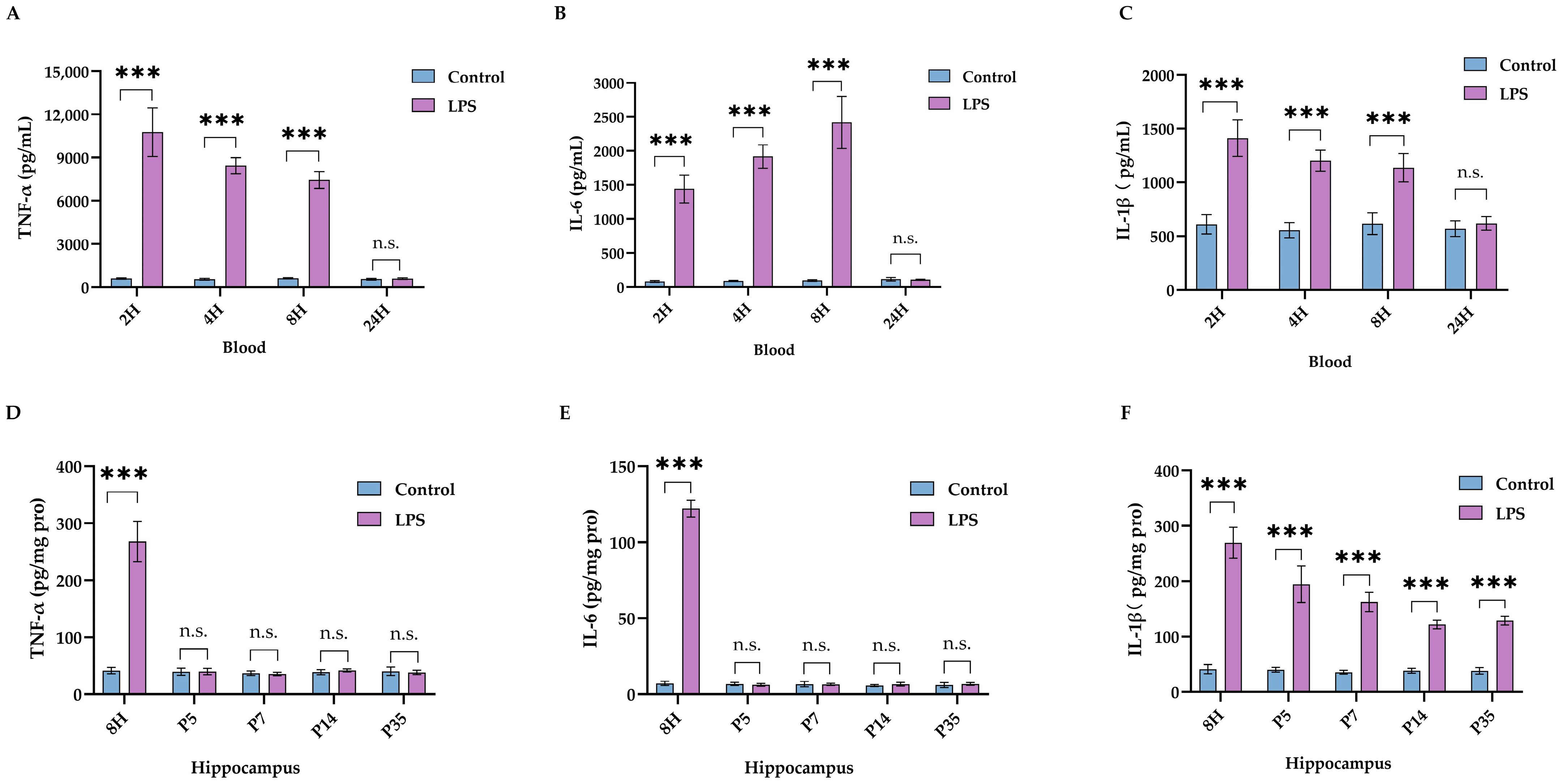
2.1. LPS Led to Impaired Neurodevelopment and Long-Lasting Activation of IL-1β in Brain
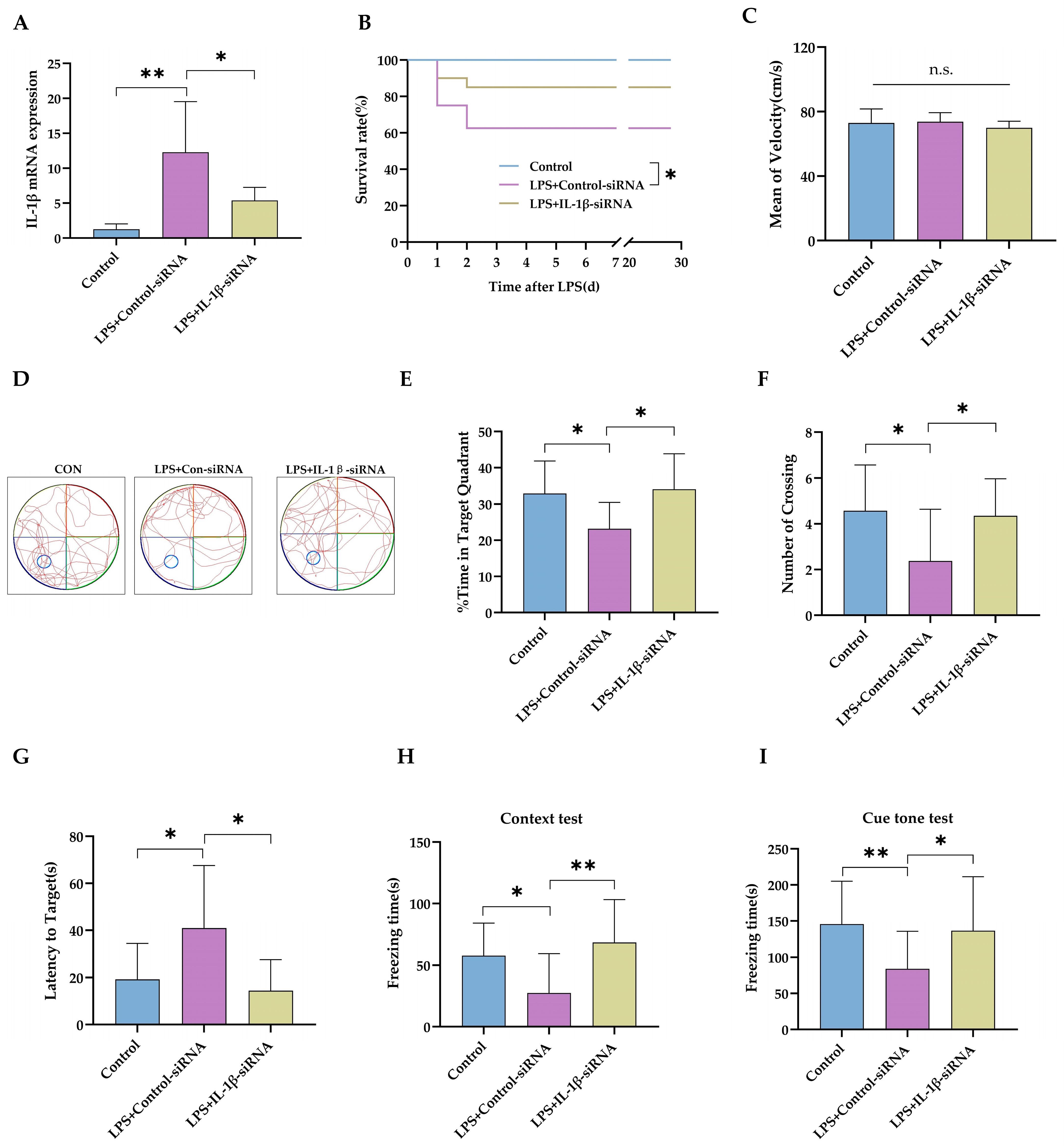
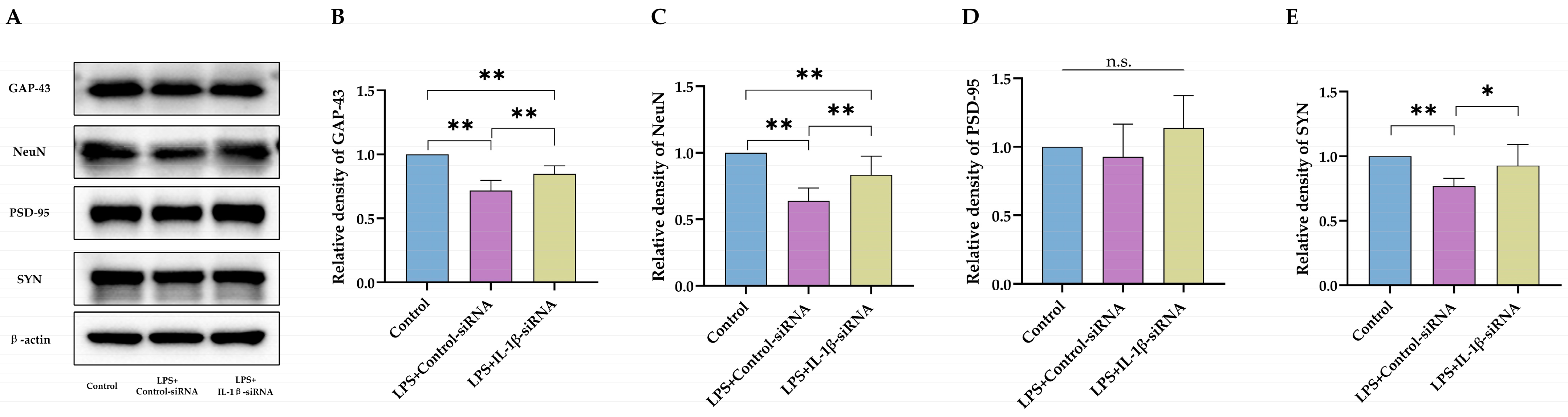
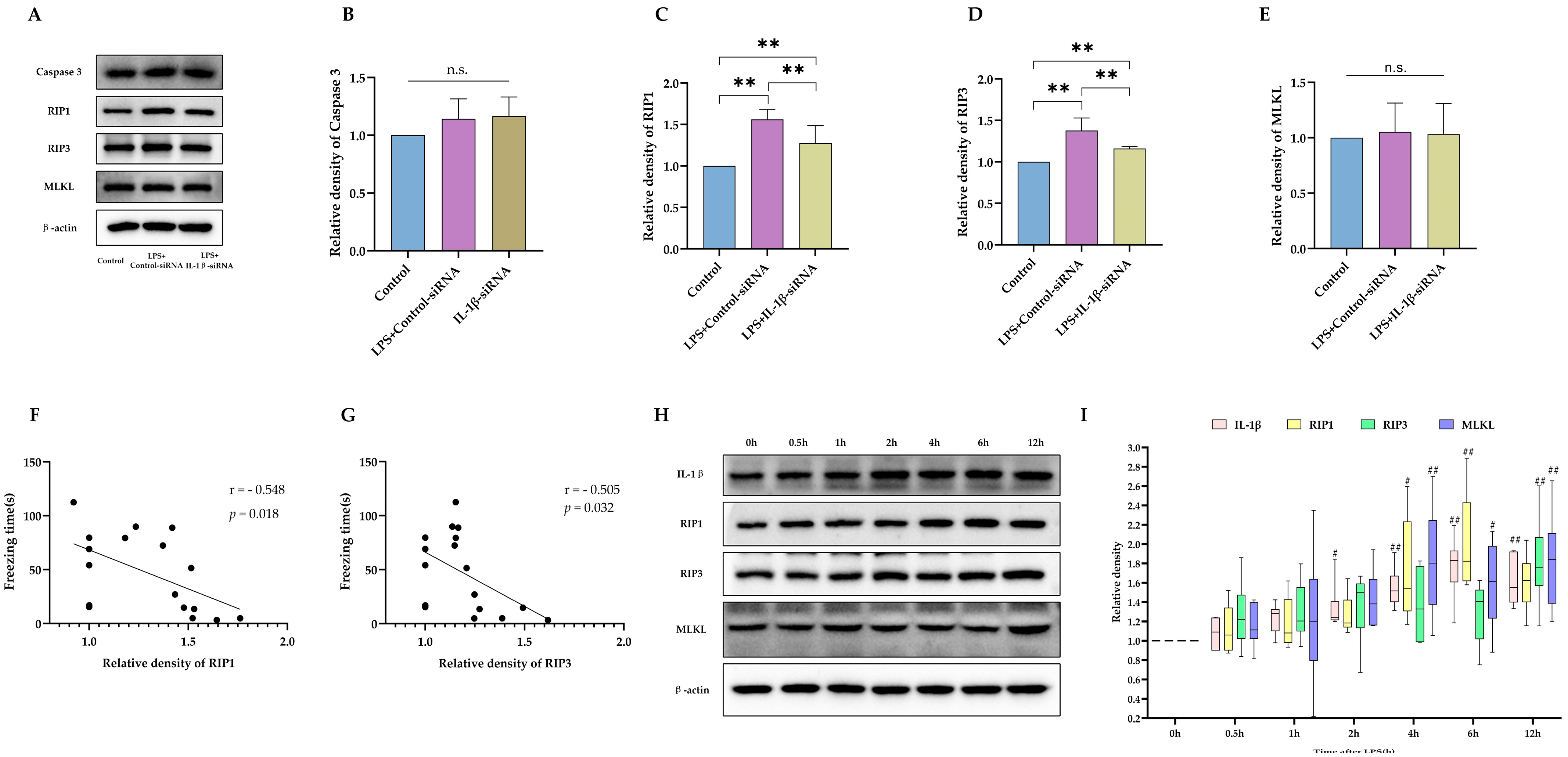
2.2. IL-1β Served as a Critical Cytokine Promoting Impaired Neurodevelopment after Neonatal LPS Exposure
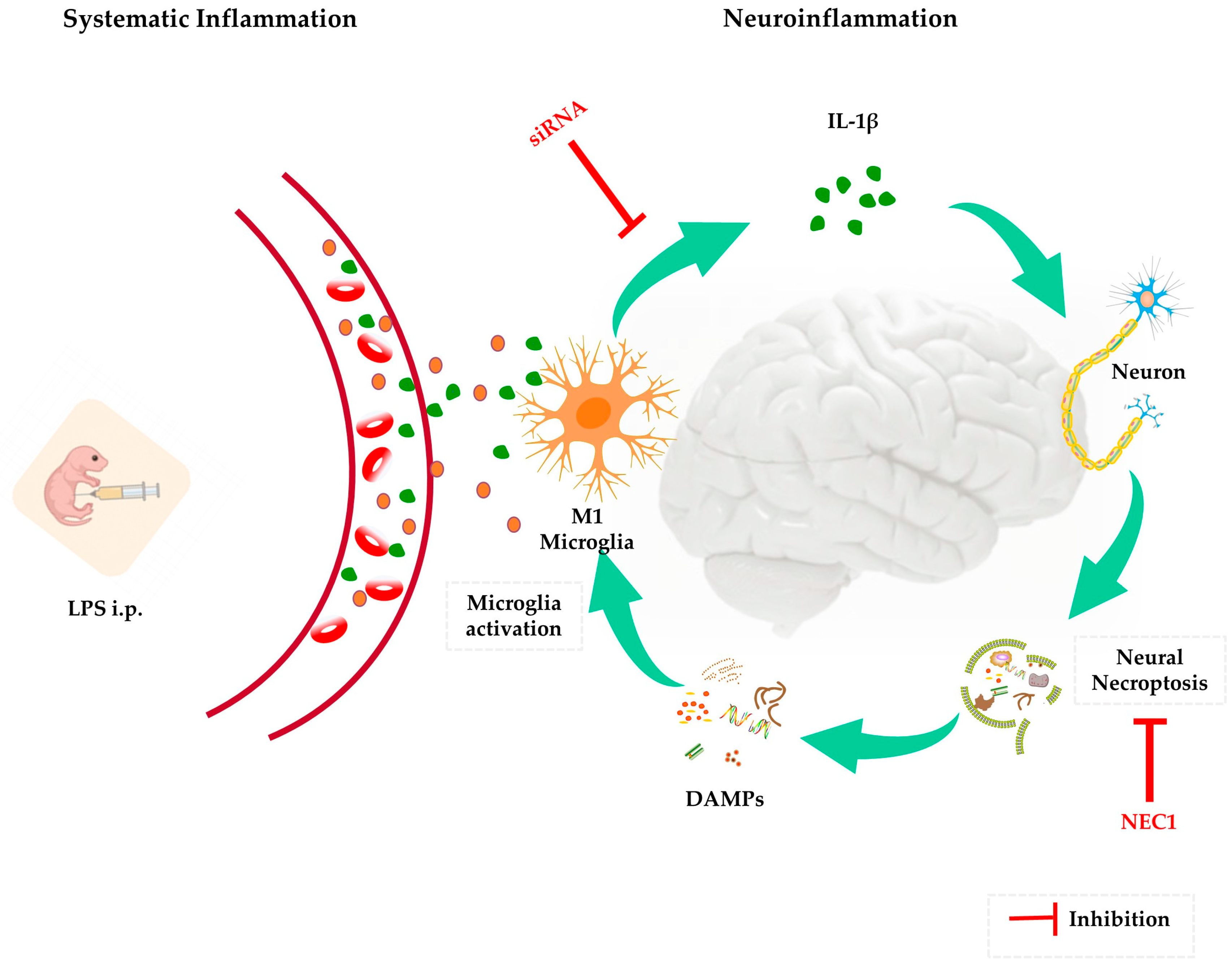
2.3. Necroptosis as the Potential Downstream Executer following Sustained IL-1β Activation
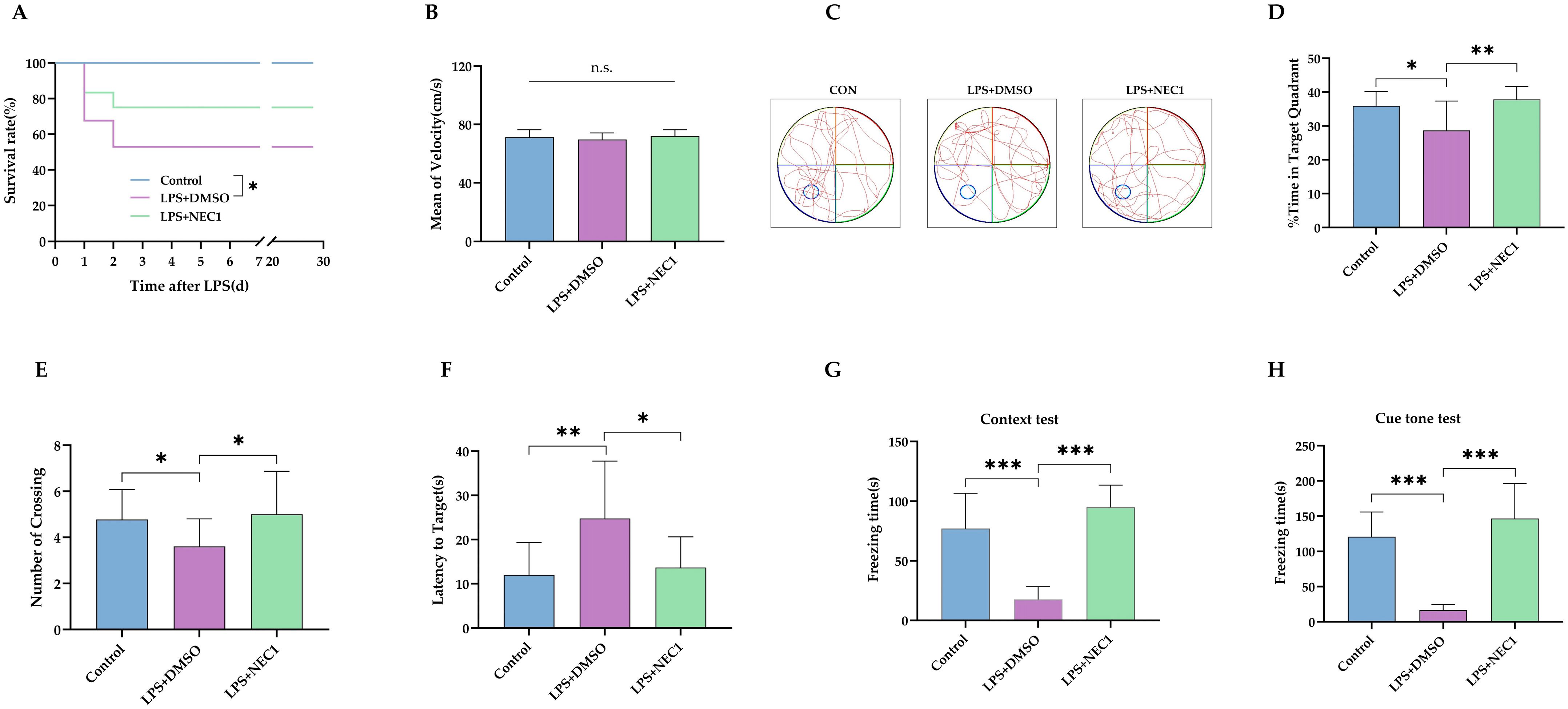
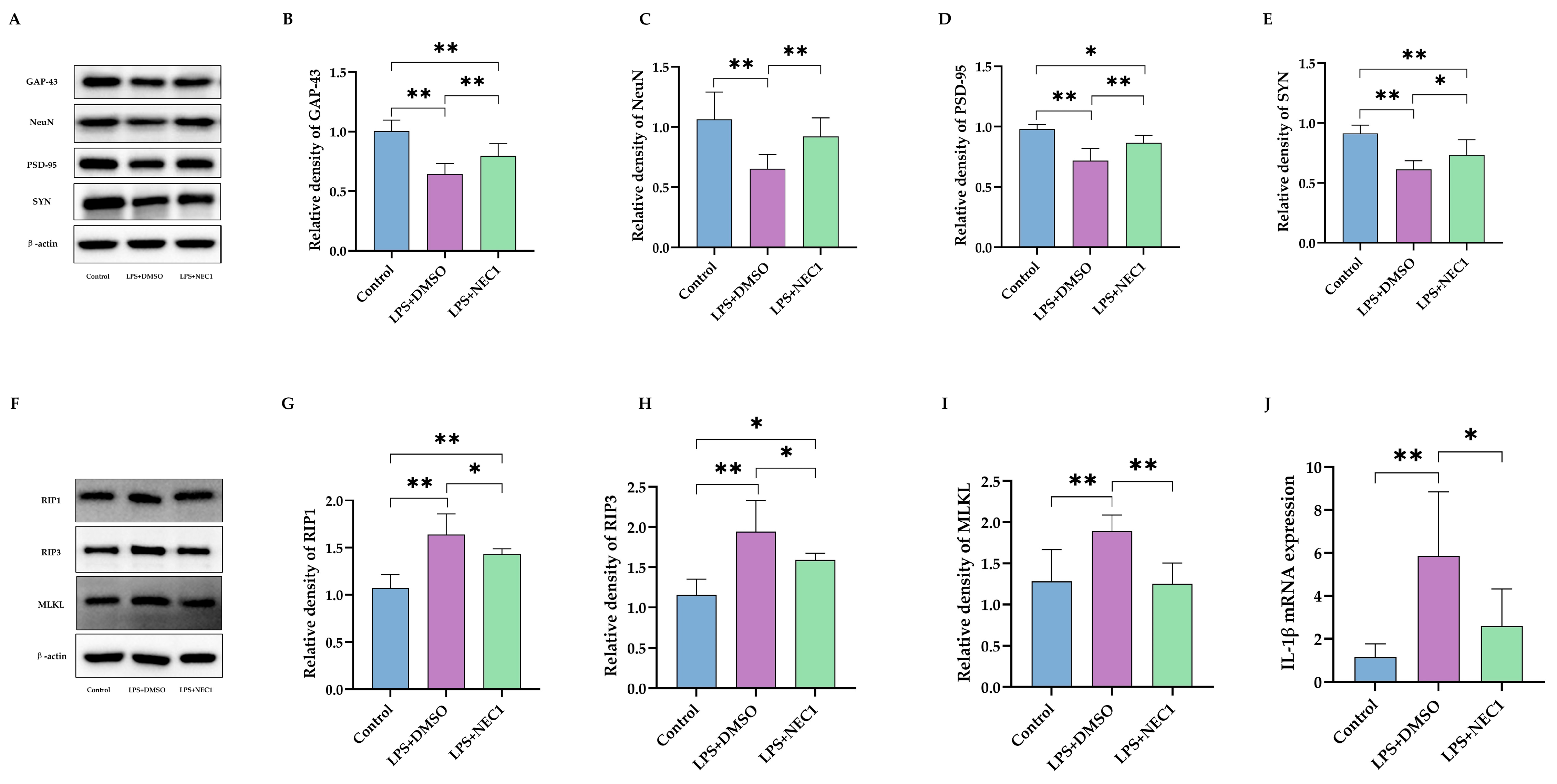
2.4. Impaired Neurodevelopment Caused by Neonatal LPS Injection Could Be Improved by Inhibiting Necroptosis
3. Discussion
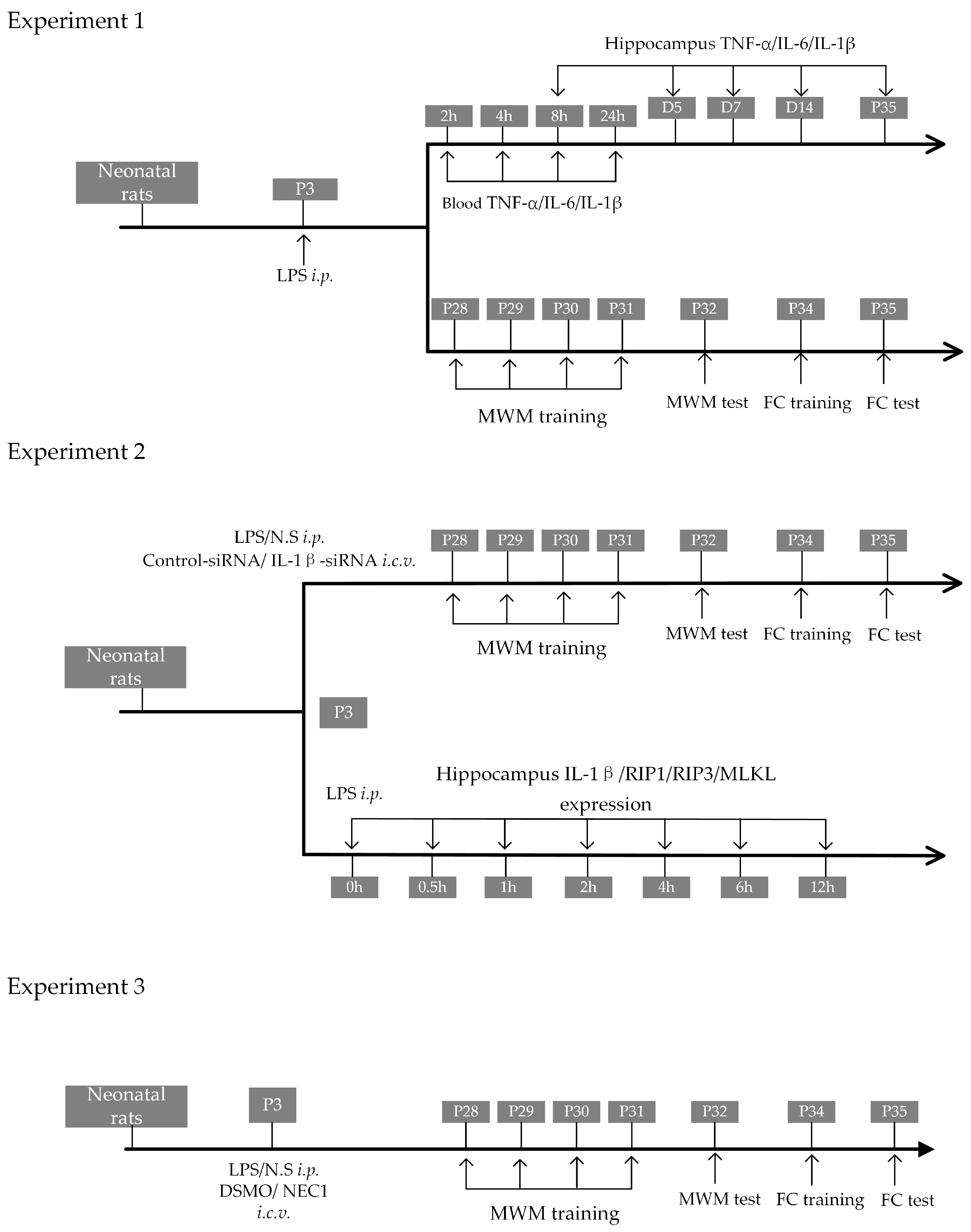
4. Materials and Methods
4.1. Experimental Animals
4.2. Reagents
4.3. Experimental Groups
4.4. Morris Water Maze Test
4.5. Fear-Conditioning Test
4.6. Western Blot
4.7. Quantitative Real-Time PCR
- ·
- β-actin forward:5′-GAAGATCAAGATCATTGCTCCT-3′;
- ·
- β-actin reverse: 5′-TACTCCTGCTTGCTGATCCA-3′;
- ·
- IL-1 β forward: 5′-ATCCTCCAGTCTCCTTGTG-3′;
- ·
- IL-1 β reverse: 5′-AGCTCTTGTGTCGCTGTGA-3′.
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| FADD | Fas-associated death domain |
| FC | Fear conditioning |
| GAP-43 | Nerve growth-associated protein |
| i.c.v. | intracerebroventricular |
| i.p. | intraperitoneal |
| IL-1β | Interleukin-1β |
| INF-γ | Interferon-γ |
| LPS | Lipopolysaccharide |
| MLKL | Mixed lineage kinase domain-like protein |
| MWM | Morris water maze |
| NEC1 | Necrostatin-1 |
| PSD-95 | Postsynaptic density protein-95 |
| RIP1 | Receptor-interacting protein 1 |
| RIP3 | Receptor-interacting protein 3 |
| RT-PCR | Quantitative real-time polymerase chain reaction |
| SYN | Synapsin |
| TNF-α | Tumor necrosis factor-α |
| TRADD | TNF-receptor-associated death domain |
References
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Kurul, Ş.; Beckers, F.L.M.; Vermeulen, M.J.; Suurland, J.; Hasbek, J.E.; Ramakers, C.R.B.; Simons, S.H.P.; Reiss, I.K.M.; Taal, H.R. Inflammation, sepsis severity and neurodevelopmental outcomes of late-onset sepsis in preterm neonates. Pediatric. Res. 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Deindl, P.; Cassini, A.; Suetens, C.; Zingg, W.; Abu Sin, M.; Velasco, E.; Weiss, B.; Ducomble, T.; Sixtensson, M.; et al. Neurological sequelae of healthcare-associated sepsis in very-low-birthweight infants: Umbrella review and evidence-based outcome tree. Eurosurveillance 2016, 21, 30143. [Google Scholar] [CrossRef] [PubMed]
- Sewell, E.; Roberts, J.; Mukhopadhyay, S. Association of Infection in Neonates and Long-Term Neurodevelopmental Outcome. Clin. Perinatol. 2021, 48, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Sforza, F.; Baroni, L.; Spada, C.; Ambretti, S.; Biasucci, G.; Bolognesi, S.; Capretti, M.; Carretto, E.; Ciccia, M.; et al. Epidemiology and complications of late-onset sepsis: An Italian area-based study. PLoS ONE 2019, 14, e0225407. [Google Scholar] [CrossRef]
- Mitha, A.; Foix-L’Hélias, L.; Arnaud, C.; Marret, S.; Vieux, R.; Aujard, Y.; Thiriez, G.; Larroque, B.; Cambonie, G.; Burguet, A.; et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 2013, 132, e372–e380. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Aebischer, M.; Adams, M.; Natalucci, G.; Bonhoeffer, J.; Latzin, P.; Nelle, M.; Bucher, H.U.; Latal, B.; the Swiss Neonatal Network and Follow-Up Group. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011, 128, e348–e357. [Google Scholar] [CrossRef]
- Smith, D.D.; Sagaram, D.; Miller, R.; Gyamfi-Bannerman, C. Risk of cerebral palsy by gestational age among pregnancies at-risk for preterm birth. J. Matern. Fetal Neonatal Med. 2020, 33, 2059–2063. [Google Scholar] [CrossRef]
- Cai, S.; Thompson, D.K.; Anderson, P.J.; Yang, J.Y. Short- and Long-Term Neurodevelopmental Outcomes of Very Preterm Infants with Neonatal Sepsis: A Systematic Review and Meta-Analysis. Children 2019, 6, 131. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zimbron, J.; Lewis, G.; Jones, P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013, 43, 239–257. [Google Scholar] [CrossRef]
- Cheslack-Postava, K.; Brown, A.S. Prenatal infection and schizophrenia: A decade of further progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Blomström, Å.; Karlsson, H.; Svensson, A.; Frisell, T.; Lee, B.K.; Dal, H.; Magnusson, C.; Dalman, C. Hospital admission with infection during childhood and risk for psychotic illness--a population-based cohort study. Schizophr. Bull. 2014, 40, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Comim, C.M.; Constantino, L.S.; Petronilho, F.; Quevedo, J.; Dal-Pizzol, F. Aversive memory in sepsis survivor rats. J. Neural. Transm. 2011, 118, 213–217. [Google Scholar] [CrossRef]
- Pang, Y.; Dai, X.; Roller, A.; Carter, K.; Paul, I.; Bhatt, A.J.; Lin, R.C.; Fan, L.W. Early postnatal lipopolysaccharide exposure leads to enhanced neurogenesis and impaired communicative functions in rats. PLoS ONE 2016, 11, e0164403. [Google Scholar] [CrossRef]
- Michels, M.; Vieira, A.S.; Vuolo, F.; Zapelini, H.G.; Mendonça, B.; Mina, F.; Dominguini, D.; Steckert, A.; Schuck, P.F.; Quevedo, J.; et al. The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav. Immun. 2015, 43, 54–59. [Google Scholar] [CrossRef]
- Williamson, L.L.; Sholar, P.W.; Mistry, R.S.; Smith, S.H.; Bilbo, S.D. Microglia and memory: Modulation by early-life infection. J. Neurosci. 2011, 31, 15511–15521. [Google Scholar] [CrossRef]
- Wang, K.C.; Fan, L.W.; Kaizaki, A.; Pang, Y.; Cai, Z.; Tien, L.T. Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience 2013, 234, 146–157. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Yang, Y.; Liu, J.; Zhu, T.; Huang, H.; Zhou, C. Severe inflammation in new-borns induces long-term cognitive impairment by activation of IL-1β/KCC2 signaling during early development. BMC Med. 2022, 20, 235. [Google Scholar] [CrossRef]
- Visan, I. Mapping IL-1 in the brain. Nat. Immunol. 2019, 20, 245. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef]
- Walton, N.M.; Sutter, B.M.; Laywell, E.D.; Levkoff, L.H.; Kearns, S.M.; Marshall, G.P., 2nd; Scheffler, B.; Steindler, D.A. Microglia instruct subven-tricular zone neurogenesis. Glia 2006, 54, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.A.; Zaverucha-do-Valle, C.; Fleurance, R.; Sharshar, T.; Bozza, F.A.; d’Avila, J.C. Neuroinflammation in Sepsis: Molecular Pathways of Microglia Activation. Pharmaceuticals 2021, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Pathak, D.; Sriram, K. Molecular Mechanisms Underlying Neuroinflammation Elicited by Occupational Injuries and Toxicants. Int. J. Mol. Sci. 2023, 24, 2272. [Google Scholar] [CrossRef]
- Huang, Z.B.; Sheng, G.Q. Interleukin-1β with learning and memory. Neurosci. Bull. 2010, 26, 455–468. [Google Scholar] [CrossRef]
- Wang, D.S.; Zurek, A.A.; Lecker, I.; Yu, J.; Abramian, A.M.; Avramescu, S.; Davies, P.A.; Moss, S.J.; Lu, W.Y.; Orser, B.A. Memory deficits induced by inflammation are regulated by α5-subunit-containing GABAA receptors. Cell. Rep. 2012, 2, 488–496. [Google Scholar] [CrossRef]
- Lan, K.M.; Tien, L.T.; Pang, Y.; Bhatt, A.J.; Fan, L.W. IL-1 receptor antagonist attenuates neonatal lipopolysaccharide-induced long-lasting learning impairment and hippocampal injury in adult rats. Toxicol. Lett. 2015, 234, 30–39. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Mázló, A.; Jenei, V.; Burai, S.; Molnár, T.; Bácsi, A.; Koncz, G. Types of necroinflammation, the effect of cell death modalities on sterile inflammation. Cell Death Dis. 2022, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Apaijai, N.; Chattipakorn, N.; Chattipakorn, S.C. The possible roles of necroptosis during cerebral ischemia and ischemia/reperfusion injury. Arch. Biochem. Biophys. 2020, 695, 108629. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Terada, Y.; Tyurina, Y.Y.; Tyurin, V.A.; Bery, A.I.; Gauthier, J.M.; Higashikubo, R.; Tong, A.Y.; Zhou, D.; Nunez-Santana, F.; et al. Necroptosis triggers spatially restricted neutrophil-mediated vascular damage during lung ischemia reperfusion injury. Proc. Natl. Acad. Sci. USA 2022, 119, e2111537119. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Cheng, J.; Yang, X.; Liu, W.; Li, Y. Heliox preconditioning exerts neuroprotective effects on neonatal ischemia/hypoxia injury by inhibiting necroptosis induced by Ca2+ elevation. Transl. Stroke Res. 2023, 14, 409–424. [Google Scholar] [CrossRef]
- Wang, T.; Perera, N.D.; Chiam, M.D.F.; Cuic, B.; Wanniarachchillage, N.; Tomas, D.; Samson, A.L.; Cawthorne, W.; Valor, E.N.; Murphy, J.M.; et al. Necroptosis is dispensable for motor neuron degeneration in a mouse model of ALS. Cell Death Differ. 2020, 27, 1728–1739. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Lau, A.; Wang, S.; Jiang, J.; Haig, A.; Pavlosky, A.; Linkermann, A.; Zhang, Z.X.; Jevnikar, A.M. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am. J. Transplant. 2013, 13, 2805–2818. [Google Scholar] [CrossRef]
- Shi, S.; Bonaccorsi-Riani, E.; Schurink, I.; van den Bosch, T.; Doukas, M.; Lila, K.A.; Roest, H.P.; Xhema, D.; Gianello, P.; de Jonge, J.; et al. Liver Ischemia and Reperfusion Induce Periportal Expression of Necroptosis Executor pMLKL Which Is Associated with Early Allograft Dysfunction after Transplantation. Front. Immunol. 2022, 13, 890353. [Google Scholar] [CrossRef]
- Chevin, M.; Chabrier, S.; Allard, M.J.; Sébire, G. Necroptosis blockade potentiates the neuroprotective effect of hypothermia in neonatal hypoxic-ischemic encephalopathy. Biomedicines 2022, 10, 2913. [Google Scholar] [CrossRef]
- Liao, Z.; Ou, X.; Zhou, C.; Ma, D.; Zhao, H.; Huang, H. Xenon attenuated neonatal lipopolysaccharide exposure induced neuronal necroptosis and subsequently improved cognition in juvenile rats. Front. Pharmacol. 2022, 13, 1002920. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis-a narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, D.; Honarpour, N.; Wang, X. Apoptosis in neural development and disease. Annu. Rev. Neurosci. 2000, 23, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, A.C.; Yang, W.L.; Hansen, L.W.; Denning, N.L.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Inhibition of necroptosis attenuates lung injury and improves survival in neonatal sepsis. Surgery 2018, 164, 110–116. [Google Scholar] [CrossRef]
- Zhou, R.; Ying, J.; Qiu, X.; Yu, L.; Yue, Y.; Liu, Q.; Shi, J.; Li, X.; Qu, Y.; Mu, D. A new cell death program regulated by toll-like receptor 9 through p38 mitogen-activated protein kinase signaling pathway in a neonatal rat model with sepsis associated encephalopathy. Chin. Med. J. 2022, 135, 1474–1485. [Google Scholar] [CrossRef]
- Greengard, P.; Valtorta, F.; Czernik, A.J.; Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 1993, 259, 780–785. [Google Scholar] [CrossRef]
- Dore, K.; Malinow, R. Elevated PSD-95 Blocks Ion-flux Independent LTD: A potential new Role for PSD-95 in synaptic plasticity. Neuroscience 2021, 456, 43–49. [Google Scholar] [CrossRef]
- Zhang, M.; Augustine, G.J. Synapsins and the Synaptic Vesicle Reserve Pool: Floats or Anchors? Cells 2021, 10, 658. [Google Scholar] [CrossRef]
- Levy, A.M.; Gomez-Puertas, P.; Tümer, Z. Neurodevelopmental disorders associated with PSD-95 and its interaction partners. Int. J. Mol. Sci. 2022, 23, 4390. [Google Scholar] [CrossRef]
- Han, Q.; Lin, Q.; Huang, P.; Chen, M.; Hu, X.; Fu, H.; He, S.; Shen, F.; Zeng, H.; Deng, Y. Microglia-derived IL-1β contributes to axon development disorders and synaptic deficit through p38-MAPK signal pathway in septic neonatal rats. J. Neuroinflamm. 2017, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Carulli, D.; Buffo, A.; Strata, P. Reparative mechanisms in the cerebellar cortex. Prog. Neurobiol. 2004, 72, 373–398. [Google Scholar] [CrossRef]
- Chung, D.; Shum, A.; Caraveo, G. GAP-43 and BASP1 in axon regeneration: Implications for the treatment of neurodegenerative diseases. Front. Cell Dev. Biol. 2020, 8, 567537. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Grunke, S.D.; Levites, Y.; Golde, T.E.; Jankowsky, J.L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J. Vis. Exp. 2014, 91, e51863. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Contextual and cued fear conditioning test using a video analyzing system in mice. J. Vis. Exp. 2014, 85, 50871. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.; Zhu, Q.; Huang, H. Involvement of IL-1β-Mediated Necroptosis in Neurodevelopment Impairment after Neonatal Sepsis in Rats. Int. J. Mol. Sci. 2023, 24, 14693. https://doi.org/10.3390/ijms241914693
Liao Z, Zhu Q, Huang H. Involvement of IL-1β-Mediated Necroptosis in Neurodevelopment Impairment after Neonatal Sepsis in Rats. International Journal of Molecular Sciences. 2023; 24(19):14693. https://doi.org/10.3390/ijms241914693
Chicago/Turabian StyleLiao, Zhimin, Qing Zhu, and Han Huang. 2023. "Involvement of IL-1β-Mediated Necroptosis in Neurodevelopment Impairment after Neonatal Sepsis in Rats" International Journal of Molecular Sciences 24, no. 19: 14693. https://doi.org/10.3390/ijms241914693
APA StyleLiao, Z., Zhu, Q., & Huang, H. (2023). Involvement of IL-1β-Mediated Necroptosis in Neurodevelopment Impairment after Neonatal Sepsis in Rats. International Journal of Molecular Sciences, 24(19), 14693. https://doi.org/10.3390/ijms241914693




