Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus mykiss
Abstract
:1. Introduction
2. Results
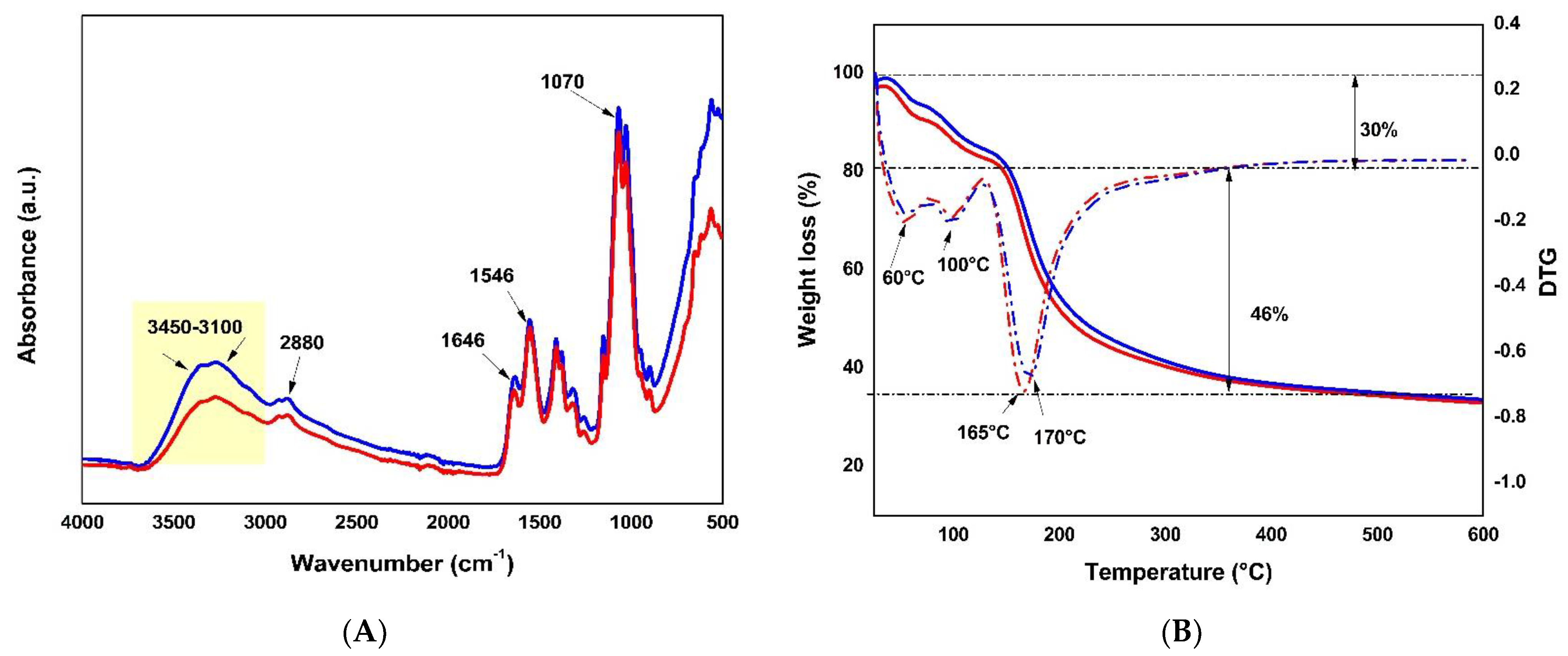
2.1. Microparticle Generation
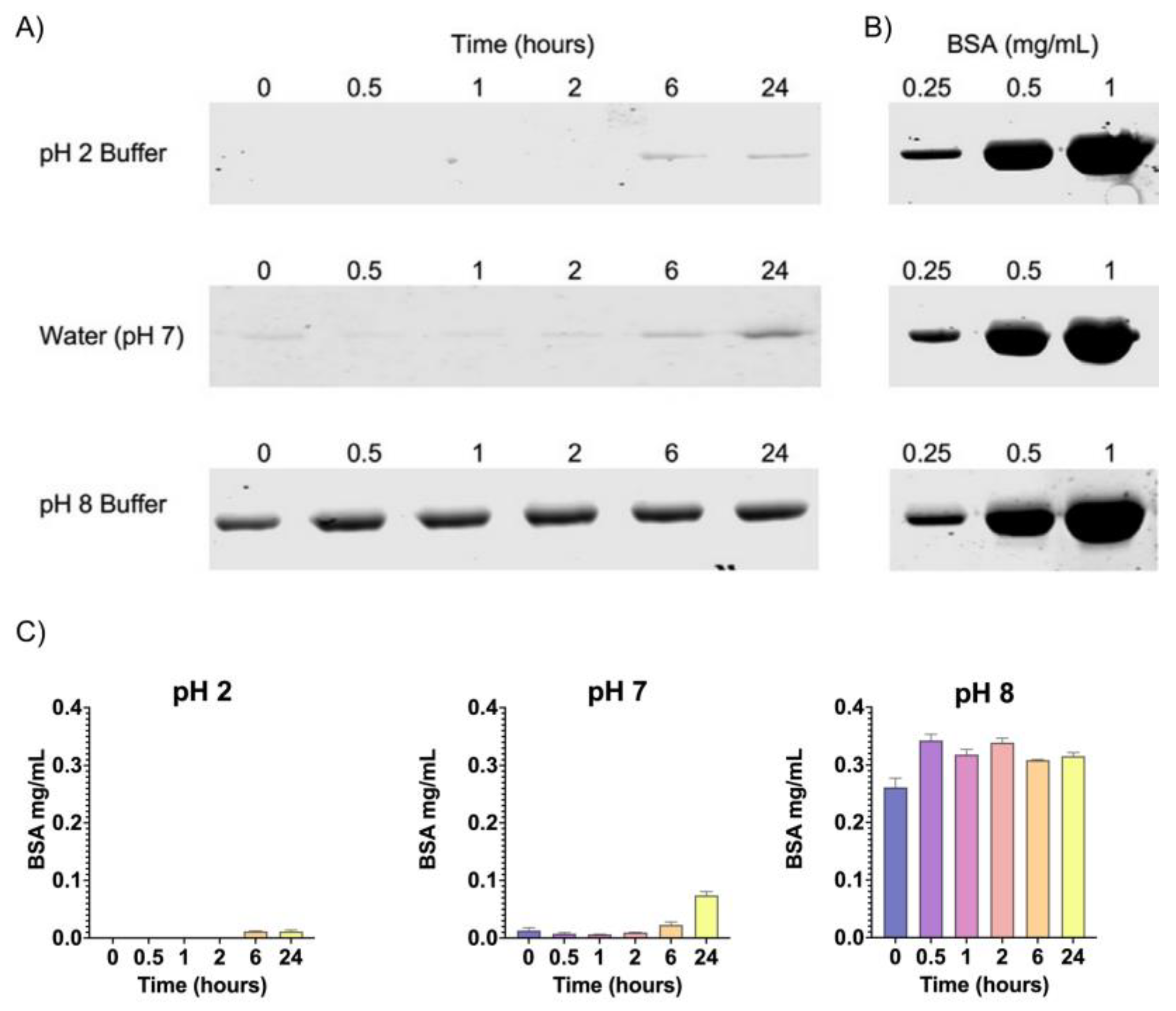
2.2. In Vitro Release of Chitosan/Peptide–BSA in Gastric and Intestinal pH
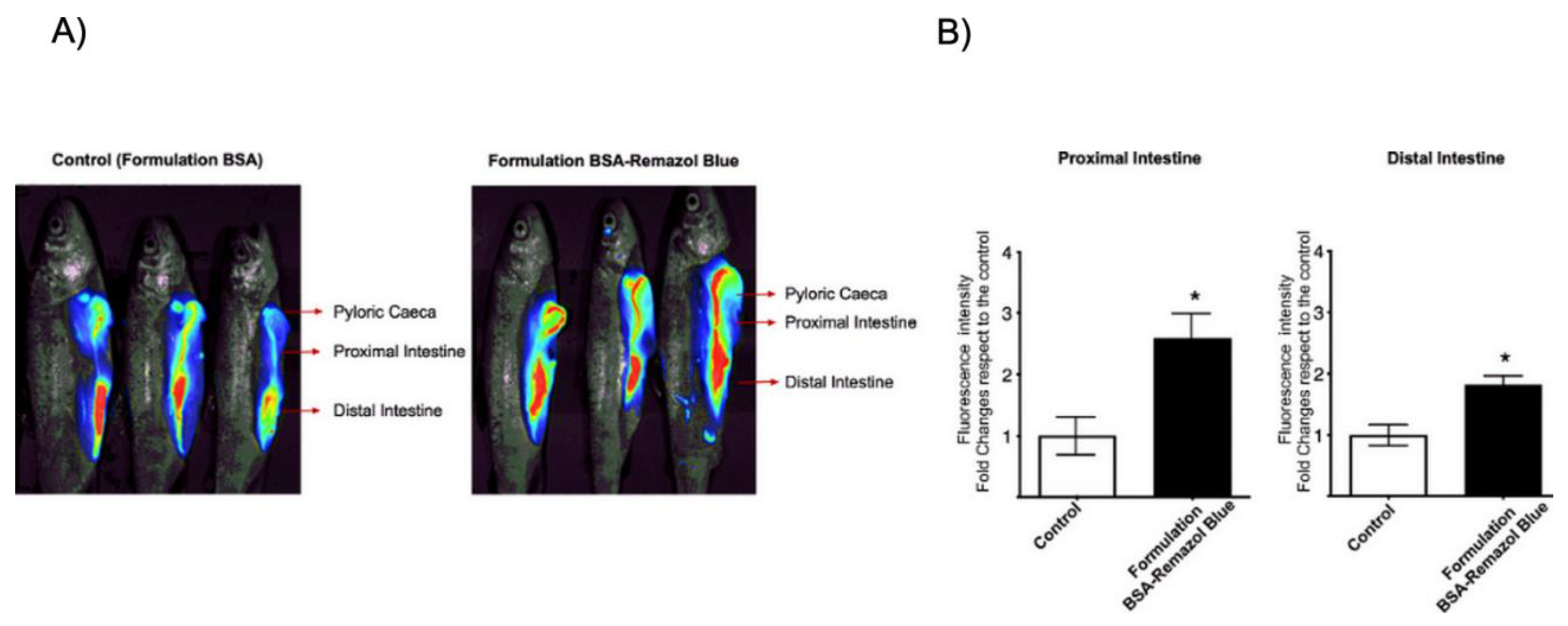
2.3. In Vivo Analysis of CATH–FLA Peptide Release from Chitosan Microparticles
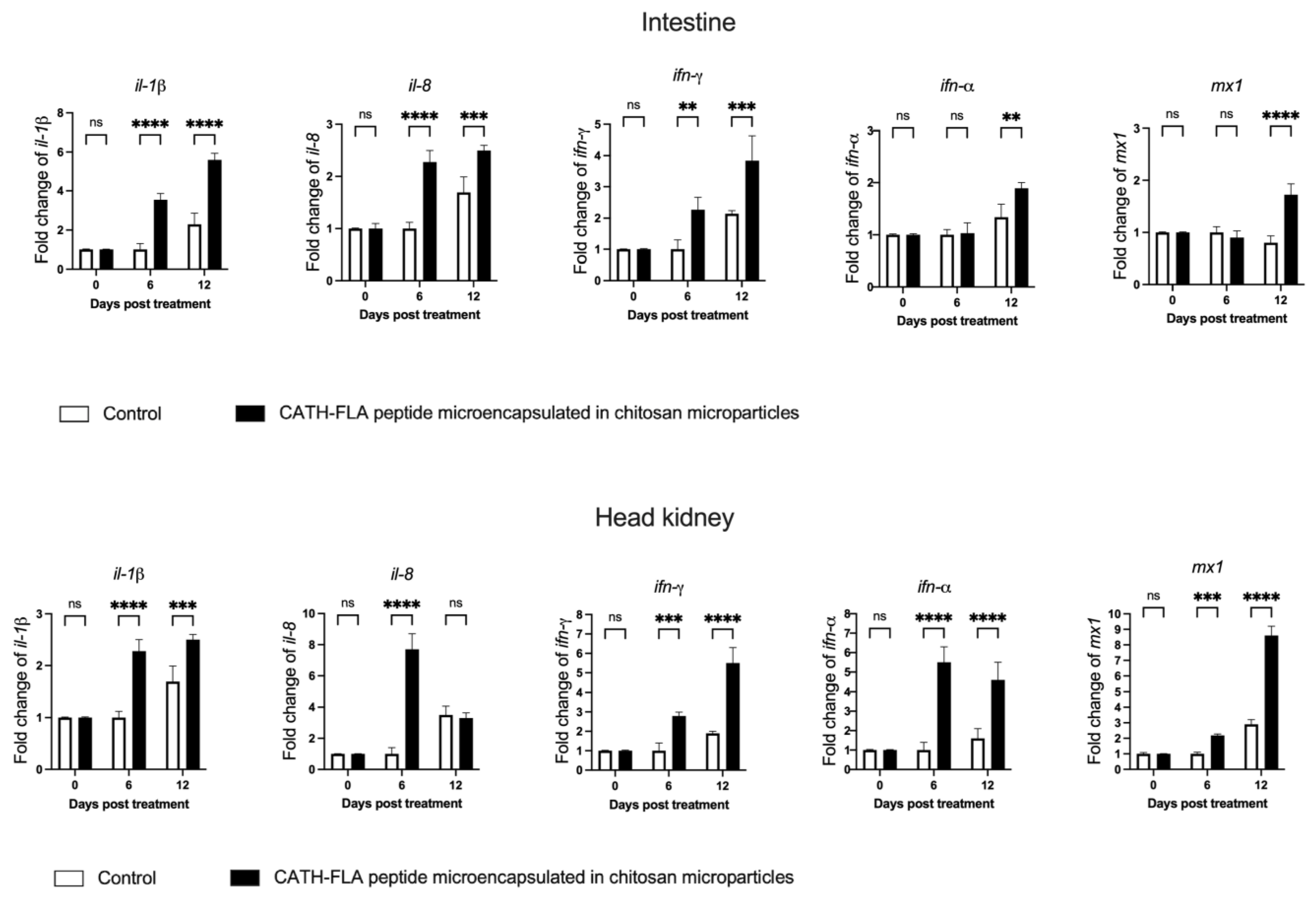
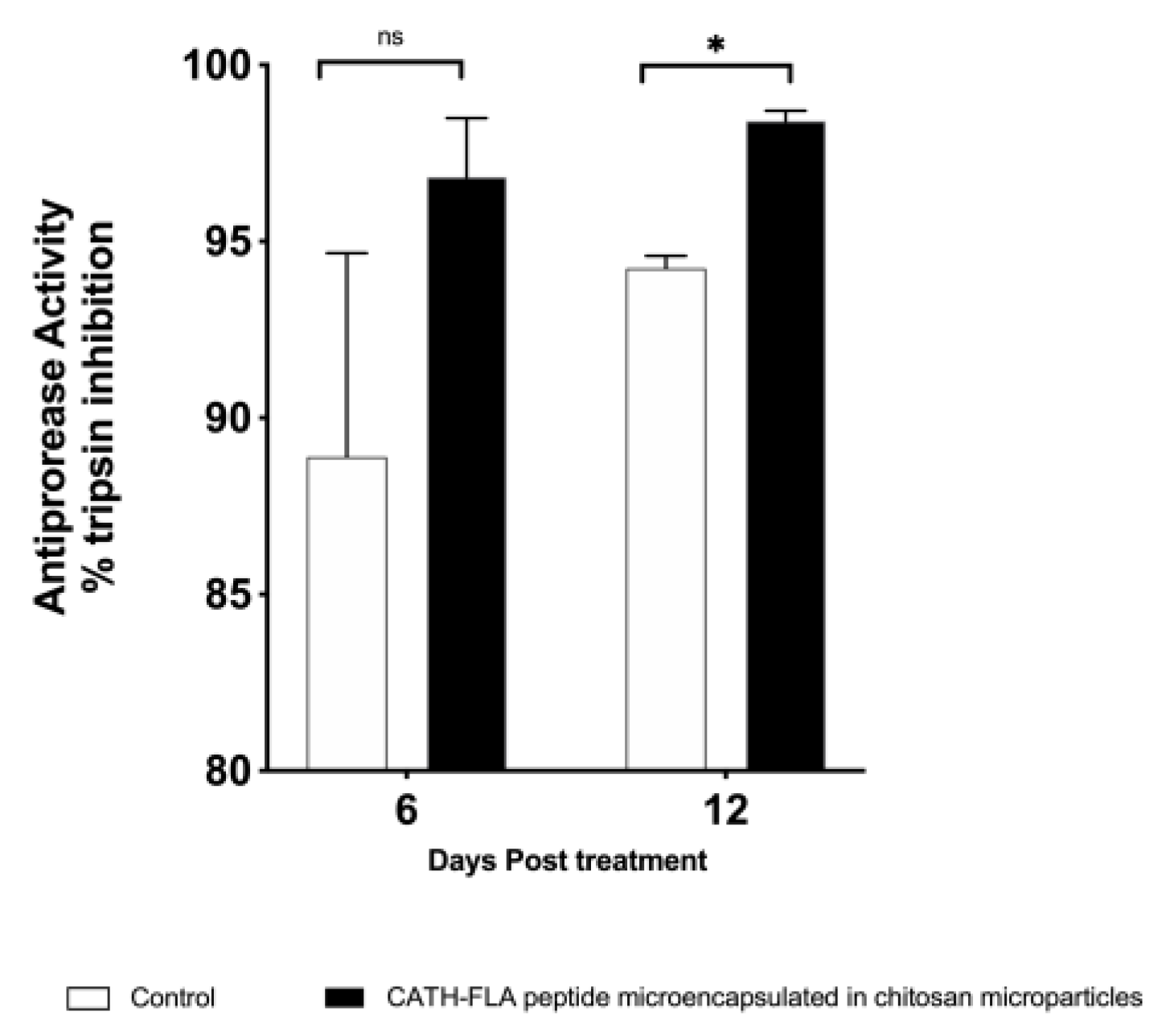
2.4. Antiprotease Activity in the Serum of Fishes Treated with the Formulation of the Microencapsulated Chitosan Immunostimulant Peptide
3. Discussion
4. Materials and Methods
4.1. Microencapsulation of Immunostimulant Peptide
4.2. Microparticle Characterization
4.3. Release Determination of Chitosan Peptide–BSA Formulation in Gastric and Intestinal pH
4.4. In Vivo Release Analysis of Chitosan Peptide–BSA Formulation in Rainbow Trout
4.5. Analysis of In Vivo Biological Activity of CATH–FLA Microparticles
4.6. Antiprotease Activity
4.7. Statistical Analyses
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subasinghe, R.; Soto, D.; Jia, J.S. Global aquaculture and its role in sustainable development. Rev. Aquacult. 2009, 1, 2–9. [Google Scholar] [CrossRef]
- Flores-Kossack, C.; Montero, R.; Kollner, B.; Maisey, K. Chilean aquaculture and the new challenges: Pathogens, immune response, vaccination and fish diversification. Fish Shellfish Immunol. 2020, 98, 52–67. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious diseases affect marine fisheries and aquaculture economics. Ann. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Balcazar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Muzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006, 114, 173–186. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2022, 14, 130–157. [Google Scholar] [CrossRef] [PubMed]
- Noor, Z.; Noor, M.; Khan, I.; Khan, S.A. Evaluating the lucrative role of probiotics in the aquaculture using microscopic and biochemical techniques. Microsc. Res. Tech. 2020, 83, 310–317. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Altintzoglou, T.; Mendes, J.; Nunes, M.L.; Dinis, M.T.; Dias, J. Farmed fish as a functional food: Perception of fish fortification and the influence of origin—Insights from Portugal. Aquaculture 2019, 501, 22–31. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, H.A.; Labib, E.M.H.; Zaki, M.A. Response of Nile Tilapia mono-sex (Oreochromis niloticus) Fingerlings to Different Sources and Levels of Protein Using Garlic and Onion as Feed Phytophytoadditives. Arab. Gulf. J. Sci. Res. 2011, 29, 146–159. [Google Scholar] [CrossRef]
- de Oliveira, S.T.L.; Soares, R.A.N.; Sousa, S.M.D.; Fernandes, A.W.C.; Gouveia, G.V.; da Costa, M.M. Natural products as functional food ingredients for Nile tilapia challenged with Aeromonas hydrophila. Aquacult. Int. 2020, 28, 913–926. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Hayes, M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016, 32, 15–45. [Google Scholar] [CrossRef]
- Farooqi, F.S.; Qureshi, W.U.H. Immunostimulants for aquaculture health management. J. Pharmacogn. Phytochem. 2018, 7, 1441–1447. [Google Scholar]
- Wang, J.; Wilson, A.E.; Su, B.; Dunham, R.A. Functionality of dietary antimicrobial peptides in aquatic animal health: Multiple meta-analyses. Anim. Nutr. 2023, 12, 200–214. [Google Scholar] [CrossRef]
- Brunner, S.R.; Varga, J.F.A.; Dixon, B. Antimicrobial Peptides of Salmonid Fish: From Form to Function. Biology 2020, 9, 233. [Google Scholar] [CrossRef]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Deshayes, C.; Arafath, M.N.; Apaire-Marchais, V.; Roger, E. Drug Delivery Systems for the Oral Administration of Antimicrobial Peptides: Promising Tools to Treat Infectious Diseases. Front. Med. Technol. 2021, 3, 778645. [Google Scholar] [CrossRef]
- Munoz-Flores, C.; Gonzalez-Chavarria, I.; Sandoval, F.; Roa, F.J.; Palacios, P.; Astuya, A.; Fernandez, K.; Altamirano, C.; Romero, A.; Acosta, J.; et al. New strategy for the design, production and pre-purification of chimeric peptide with immunomodulatory activity in Salmosalar. Fish Shellfish Immunol. 2022, 125, 120–127. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Brandelli, A. Nanostructures as promising tools for delivery of antimicrobial peptides. Mini. Rev. Med. Chem. 2012, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Delivery Systems for Antimicrobial Peptides and Proteins: Towards Optimization of Bioavailability and Targeting. Curr. Pharm. Biotechnol. 2017, 18, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Drayton, M.; Kizhakkedathu, J.N.; Straus, S.K. Towards Robust Delivery of Antimicrobial Peptides to Combat Bacterial Resistance. Molecules 2020, 25, 3048. [Google Scholar] [CrossRef]
- Lalani, R.; Misra, A.; Amrutiya, J.; Patel, H.; Bhatt, P.; Patel, V. Challenges in Dermal Delivery of Therapeutic Antimicrobial Protein and Peptides. Curr. Drug Metab. 2017, 18, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Han, Y.; Guan, R.; Liu, S.; Bi, X.; Liu, S.; Cui, W.; Zhang, T.; He, T. Inorganic hollow mesoporous spheres-based delivery for antimicrobial agents. Front. Mater. Sci. 2023, 17, 230631. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Gomara, M.J.; Haro, I. Nanotechnology-Based Platforms for Vaginal Delivery of Peptide Microbicides. Curr. Med. Chem. 2021, 28, 4356–4379. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef]
- Urban, P.; Valle-Delgado, J.J.; Moles, E.; Marques, J.; Diez, C.; Fernandez-Busquets, X. Nanotools for the delivery of antimicrobial peptides. Curr. Drug Targets 2012, 13, 1158–1172. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Dey, B. Chitosan microspheres in novel drug delivery systems. Indian J. Pharm. Sci. 2011, 73, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Durand, A.; Desobry, S. Chitosan-Based Particulate Carriers: Structure, Production and Corresponding Controlled Release. Pharmaceutics 2023, 15, 1455. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Meton, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Ucak, I.; Afreen, M.; Montesano, D.; Carrillo, C.; Tomasevic, I.; Simal-Gandara, J.; Barba, F.J. Functional and Bioactive Properties of Peptides Derived from Marine Side Streams. Mar. Drugs 2021, 19, 71. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the stability challenges of bioactive peptides and improvement strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef]
- Berraquero-Garcia, C.; Perez-Galvez, R.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M.; Garcia-Moreno, P.J. Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying. Foods 2023, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Akbarbaglu, Z.; Peighambardoust, S.H.; Sarabandi, K.; Jafari, S.M. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021, 359, 129965. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bridle, A.; Nosworthy, E.; Polinski, M.; Nowak, B. Evidence of an antimicrobial-immunomodulatory role of Atlantic salmon cathelicidins during infection with Yersinia ruckeri. PLoS ONE 2011, 6, e23417. [Google Scholar] [CrossRef]
- Gonzalez-Stegmaier, R.; Guzman, F.; Albericio, F.; Villarroel-Espindola, F.; Romero, A.; Mulero, V.; Mercado, L. A synthetic peptide derived from the D1 domain of flagellin induced the expression of proinflammatory cytokines in fish macrophages. Fish Shellfish Immunol. 2015, 47, 239–244. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, X.Y.; Zhang, N.; Guo, X.; Peng, K.S.; Wu, H.; Lu, L.F.; Wu, N.; Chen, D.D.; Li, S.; et al. Distinctive structural hallmarks and biological activities of the multiple cathelicidin antimicrobial peptides in a primitive teleost fish. J. Immunol. 2015, 194, 4974–4987. [Google Scholar] [CrossRef]
- Tian, H.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Cytokine networks provide sufficient evidence for the differentiation of CD4(+) T cells in teleost fish. Dev. Comp. Immunol. 2023, 141, 104627. [Google Scholar] [CrossRef]
- Acosta, J.; Roa, F.; Gonzalez-Chavarria, I.; Astuya, A.; Maura, R.; Montesino, R.; Munoz, C.; Camacho, F.; Saavedra, P.; Valenzuela, A.; et al. In vitro immunomodulatory activities of peptides derived from Salmo salar NK-lysin and cathelicidin in fish cells. Fish Shellfish Immunol. 2019, 88, 587–594. [Google Scholar] [CrossRef]
- Gonzalez-Stegmaier, R.; Pena, A.; Villarroel-Espindola, F.; Aguila, P.; Oliver, C.; MacLeod-Carey, D.; Rozas-Serri, M.; Enriquez, R.; Figueroa, J. Full recombinant flagellin B from Vibrio anguillarum (rFLA) and its recombinant D1 domain (rND1) promote a pro-inflammatory state and improve vaccination against P. salmonis in Atlantic salmon (S. salar). Dev. Comp. Immunol. 2021, 117, 103988. [Google Scholar] [CrossRef]
- Govindharajan, S.; Vairakannu, T. Impacts on the immune system of Cyprinus carpio exposure with a mixed algal extract against Aeromonas hydrophila. Nat. Resour. Hum. Health 2022, 2, 107–113. [Google Scholar] [CrossRef]
- Priyadarshini, S.K.; Subramani, P.A.; Michael, R.D. Modulation of the innate immune responses in the striped snakehead murrel, Channa striata upon experimental infection with live and heat killed Aeromonas hydrophila. Open Vet. J. 2017, 7, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.M.I.; Bulfon, C.; Galeotti, M.; Acutis, P.L.; Altinok, I.; Kotzamanidis, C.; Vela, A.I.; Fariano, L.; Prearo, M.; Colussi, S.; et al. Immune profiling of rainbow trout (Oncorhynchus mykiss) exposed to Lactococcus garvieae: Evidence in asymptomatic versus symptomatic or vaccinated fish. J. Fish Dis. 2023, 46, 731–741. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward (5′→ 3′) | Reverse (5′→3′) |
|---|---|---|
| ifn-α | TGGGAGGAGATATCACAAAGC | TCCCAGGTGACAGATTTCAT |
| il-1β | GCTGGAGAGTGCTGTGGAAGA | TGCTTCCCTCCTGCTCGTAG |
| il-8 | ATTGAGACAGAAAGCAGACG | CGCTGACATCCAGACAAATCT |
| ifn-γ | CCGTACACCGATTGAGGACT | GCGGCATTACTCCATCCTAA |
| mx1 | TGCCTCGTCTAGAAGAGCA | CTTTCCAGCTCGGCATGTG |
| ef1-α | GTGACACCGAAACTAAGCGAC | TGTAGATCAGATGGCCGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Chavarría, I.; Roa, F.J.; Sandoval, F.; Muñoz-Flores, C.; Kappes, T.; Acosta, J.; Bertinat, R.; Altamirano, C.; Valenzuela, A.; Sánchez, O.; et al. Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus mykiss. Int. J. Mol. Sci. 2023, 24, 14685. https://doi.org/10.3390/ijms241914685
González-Chavarría I, Roa FJ, Sandoval F, Muñoz-Flores C, Kappes T, Acosta J, Bertinat R, Altamirano C, Valenzuela A, Sánchez O, et al. Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus mykiss. International Journal of Molecular Sciences. 2023; 24(19):14685. https://doi.org/10.3390/ijms241914685
Chicago/Turabian StyleGonzález-Chavarría, Iván, Francisco J. Roa, Felipe Sandoval, Carolina Muñoz-Flores, Tomas Kappes, Jannel Acosta, Romina Bertinat, Claudia Altamirano, Ariel Valenzuela, Oliberto Sánchez, and et al. 2023. "Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus mykiss" International Journal of Molecular Sciences 24, no. 19: 14685. https://doi.org/10.3390/ijms241914685
APA StyleGonzález-Chavarría, I., Roa, F. J., Sandoval, F., Muñoz-Flores, C., Kappes, T., Acosta, J., Bertinat, R., Altamirano, C., Valenzuela, A., Sánchez, O., Fernández, K., & Toledo, J. R. (2023). Chitosan Microparticles Enhance the Intestinal Release and Immune Response of an Immune Stimulant Peptide in Oncorhynchus mykiss. International Journal of Molecular Sciences, 24(19), 14685. https://doi.org/10.3390/ijms241914685






