Design, Synthesis, and Potent Anticancer Activity of Novel Indole-Based Bcl-2 Inhibitors
Abstract
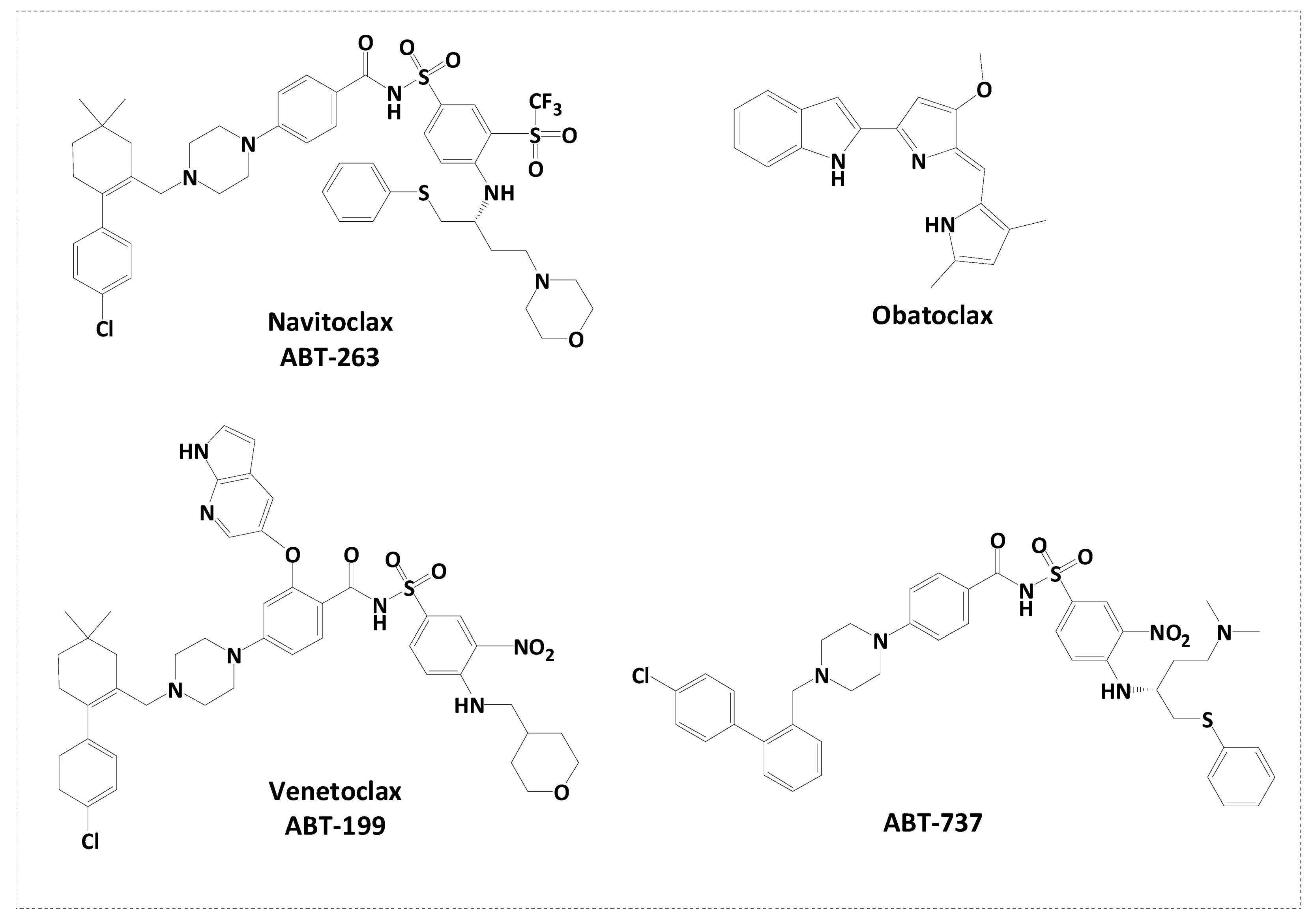
1. Introduction
2. Results
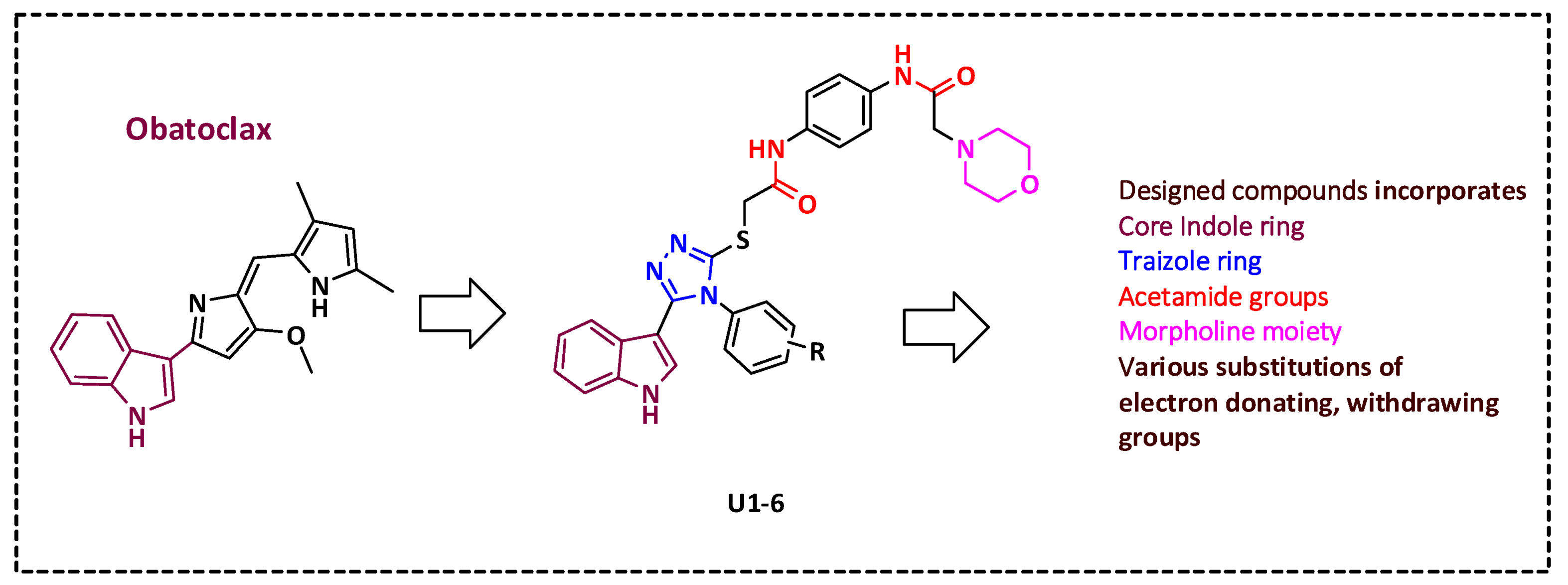
2.1. Rational Design
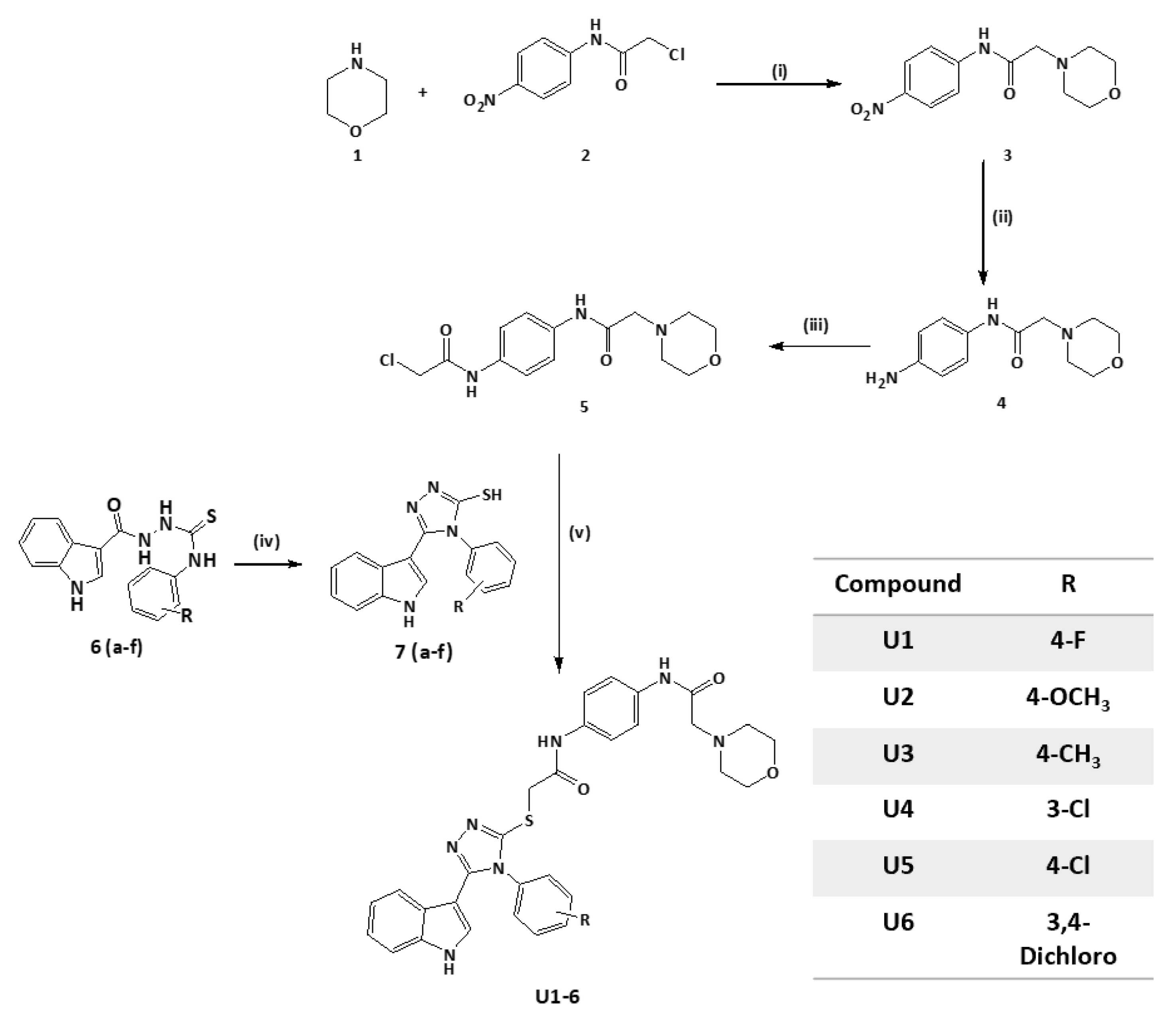
2.2. Synthesis of Title Compounds U1–6
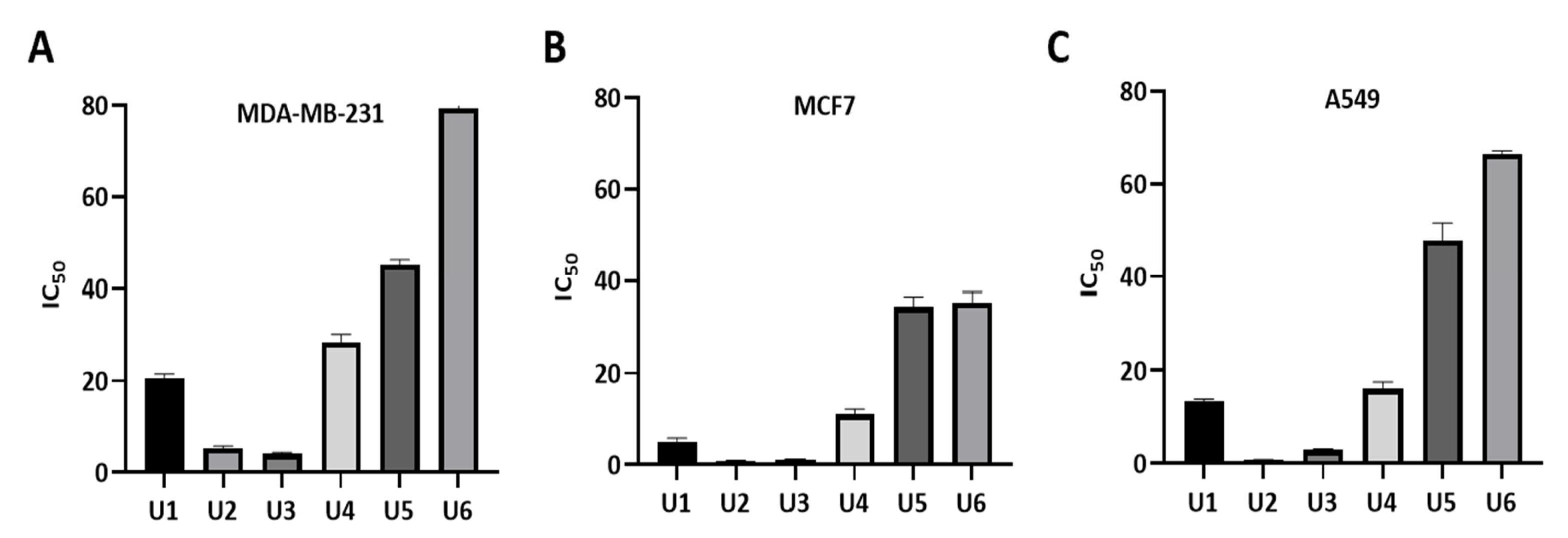
2.3. Compounds U2 and U3 Showed Potent Inhibitory Activity towards Bcl-2-Expressing Cancer Cells
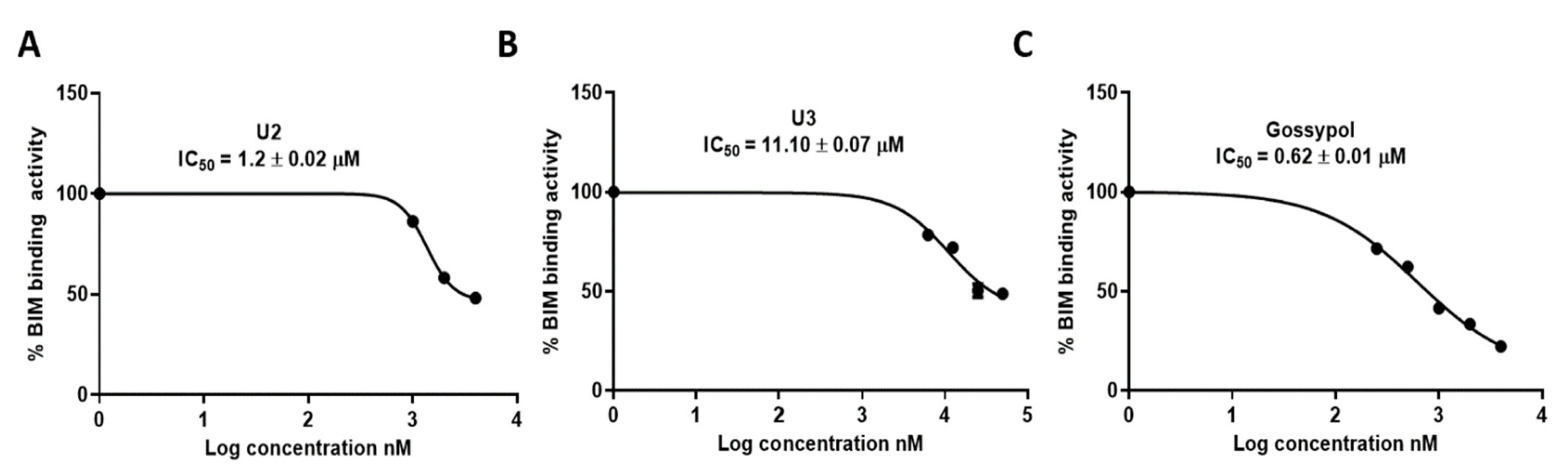
2.4. ELISA Indicated the Superior Activity of Compound U2 against Bcl-2 Protein
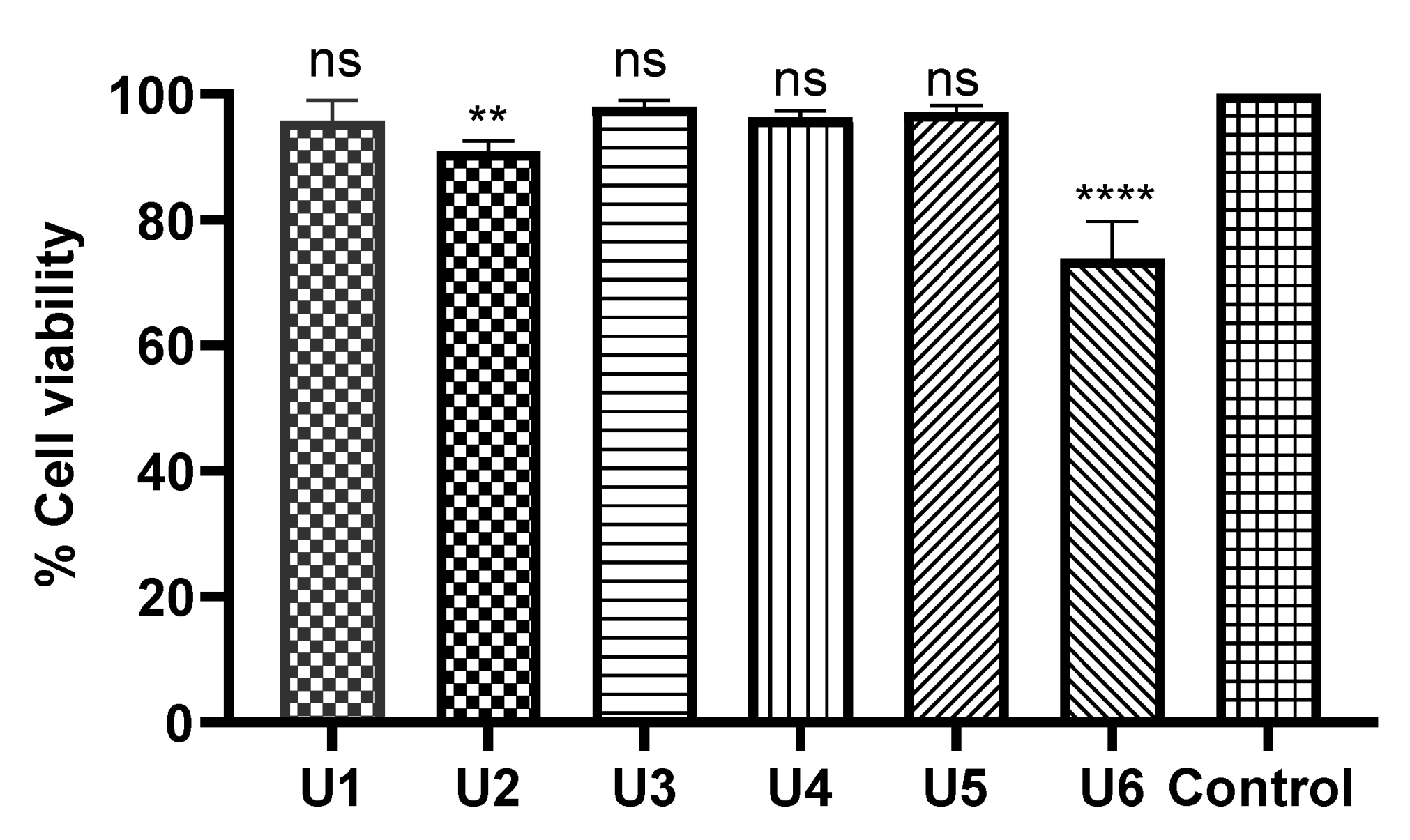
2.5. Compounds U1–6 Showed an Excellent Safety Profile in Human Normal Cells
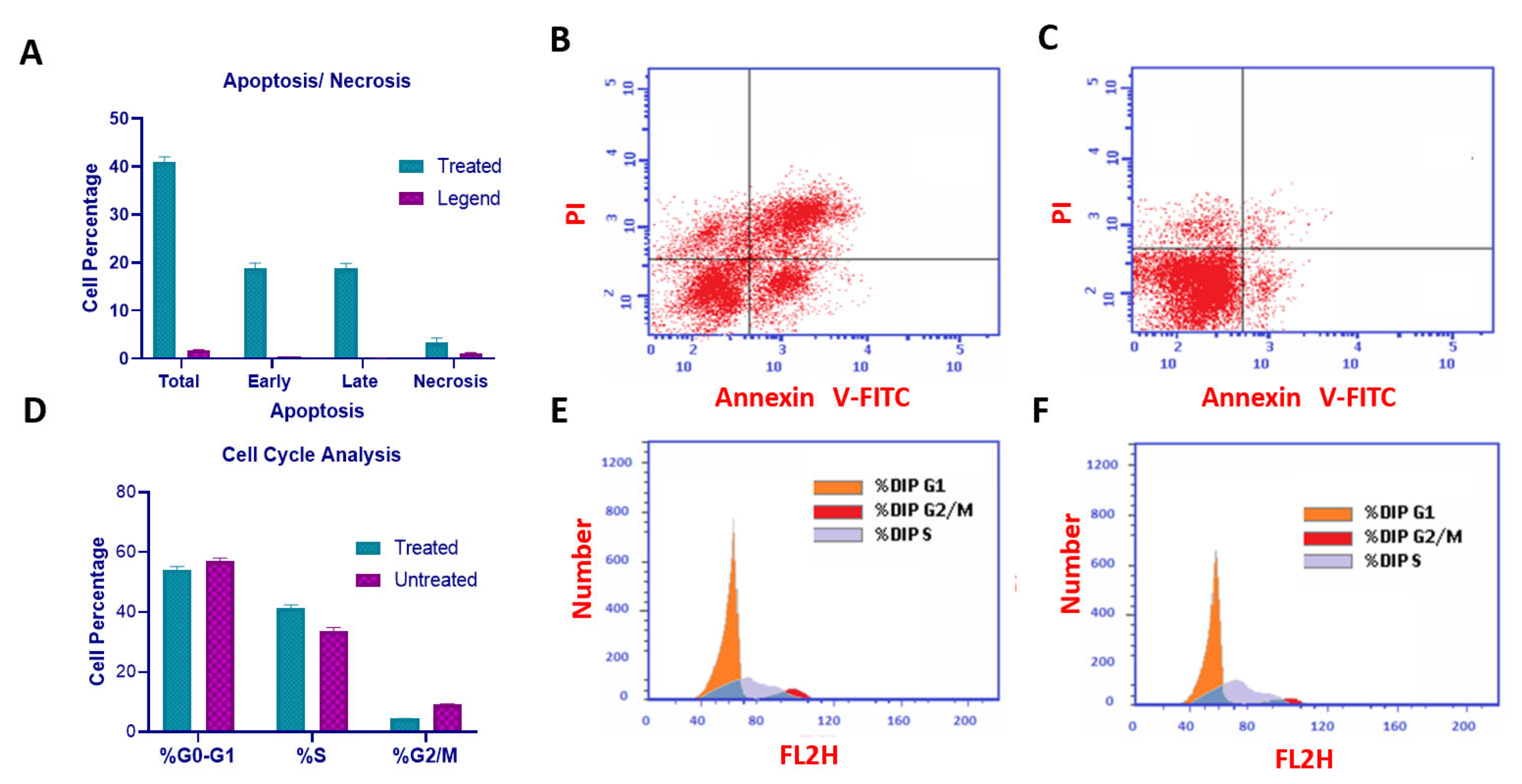
2.6. Compound U2 Induced Apoptosis and Cell Cycle Arrest at G1/S Phase
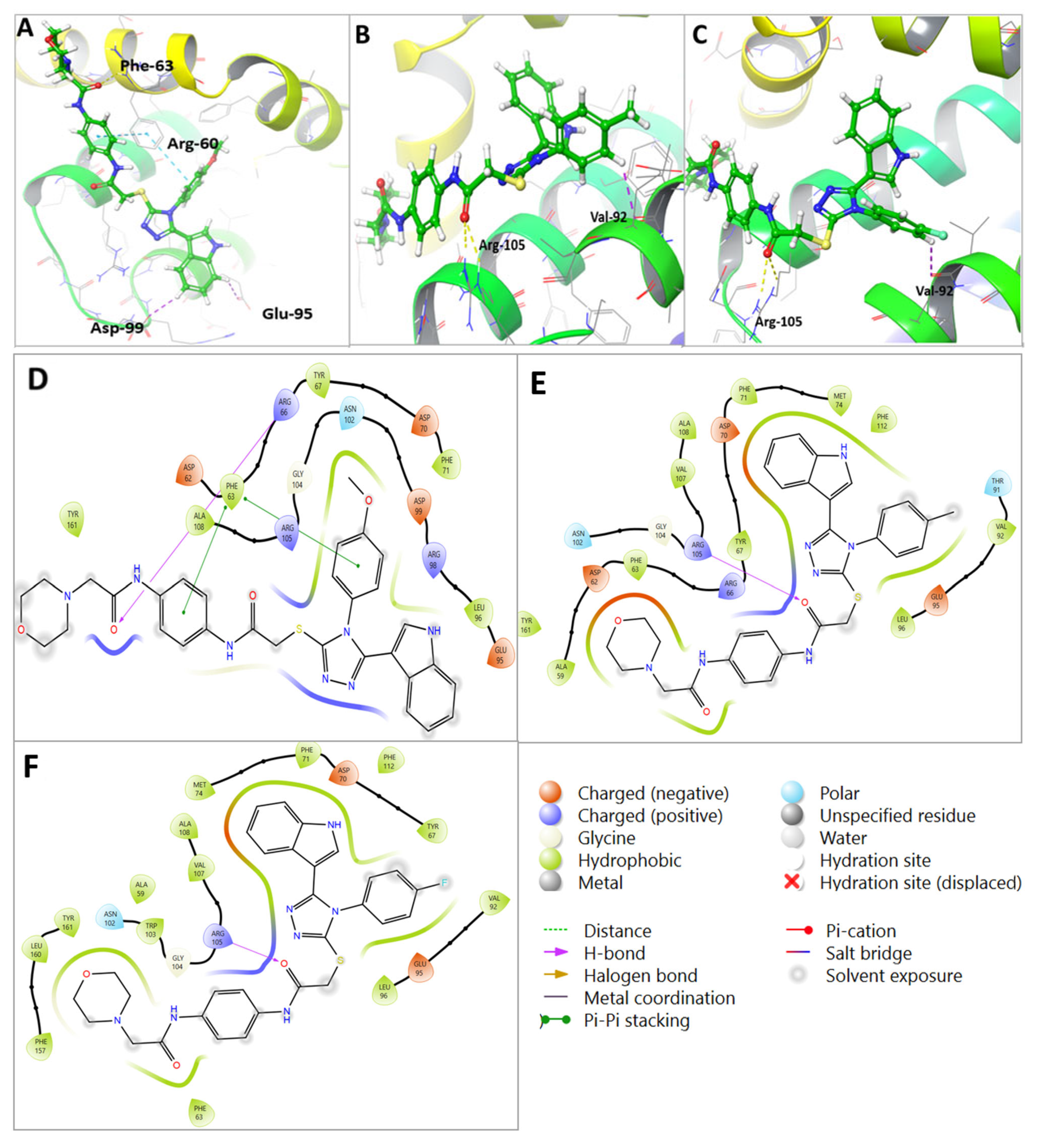
2.7. Molecular Docking Revealed Efficacy and Selectivity of U2 Compound against Bcl-2
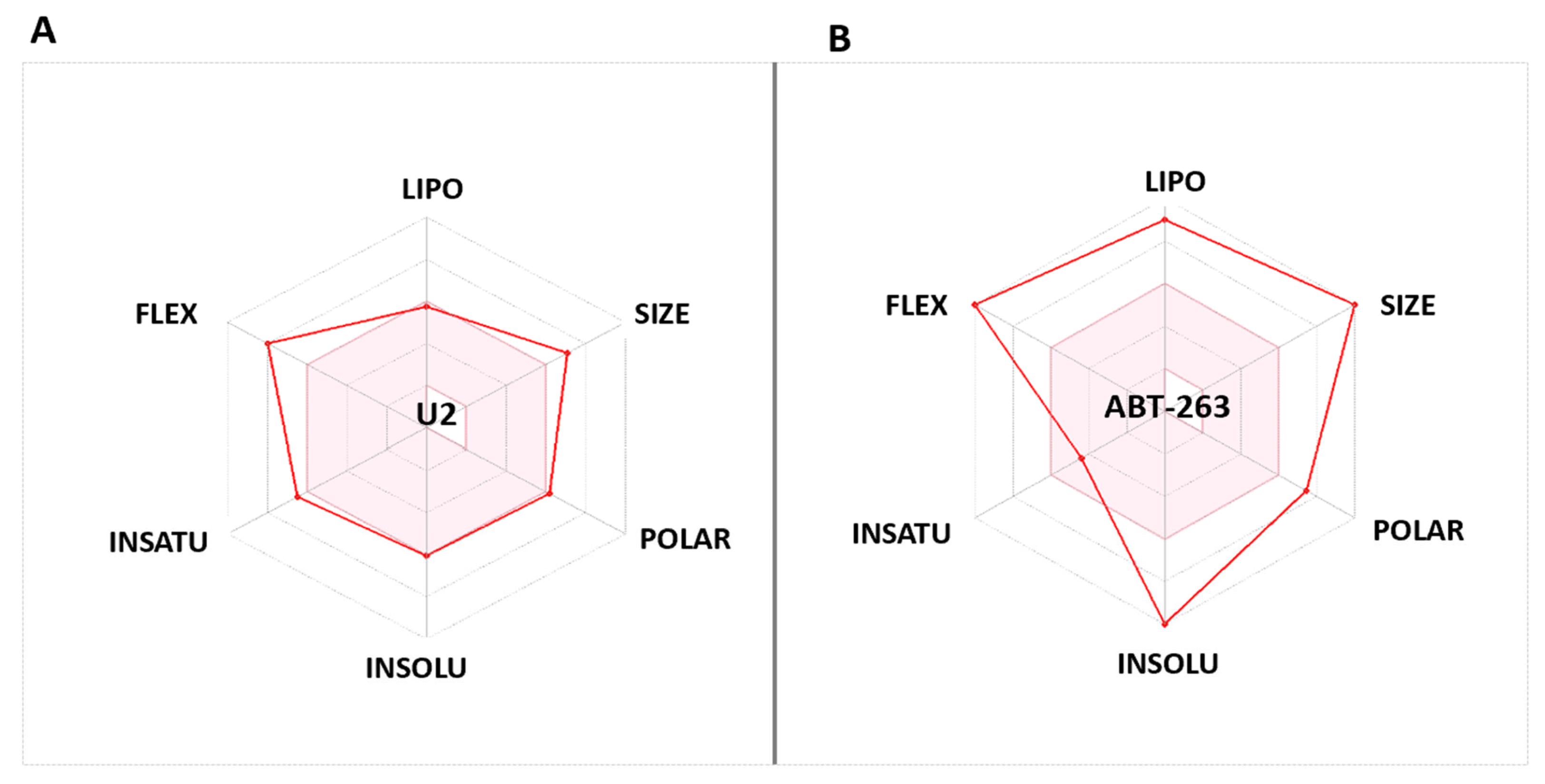
2.8. Compound U2 Showed Improved Properties Compared with Small-Molecule Inhibitor ABT263
3. Discussion
4. Experimental Section
4.1. Chemistry
4.1.1. Synthesis of 2-Chloro-N-(4-nitrophenyl)acetamide (2)
4.1.2. Synthesis of 2-Morpholin-4-yl-N-(4-nitrophenyl)acetamide (3)
4.1.3. Synthesis of N-(4-Amino-phenyl)-2-morpholin-4-yl-acetamide (4)
4.1.4. N-[4-(2-chloroacetylamino)phenyl]-2-morpholin-4-yl-acetamide (5)
4.1.5. General Procedure for Triazole Thiol (7a–f) Preparation
4-(4-Fluorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (7a)
4-(Methoxyphenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (7b)
5-(1H-Indol-3-yl)-4-methylphenyl-4H-1,2,4-triazole-3-thiol (7c)
4-(3-Chlorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (7d)
4-(4-Chlorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (7e)
4-(3,4-Dichlorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazole-3-thiol (7f)
4.1.6. General Procedure of S Alkylation of Triazole Thiol (U1–6)
2-(4-(4-Fluorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazol-3-ylthio)-N-(4-(2 morpholinoacetamido)phenyl)acetamide (U1)
2-(5-(1H-Indol-3-yl)-4-(4-methoxyphenyl)-4H-1,2,4-triazol-3-ylthio)-N-(4-(2-morpholinoacetamido)phenyl)acetamide (U2)
2-(5-(1H-Indol-3-yl)-4-methylphenyl-4H-1,2,4-triazol-3-ylthio)-N-(4-(2-morpholinoacetamido)phenyl)acetamide (U3)
2-(4-(3-Chlorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazol-3-ylthio)-N-(4-(2-morpholinoacetamido)phenyl) acetamide (U4)
2-(4-(4-Chlorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazol-3-ylthio)-N-(4-(2-morpholinoacetamido)phenyl)acetamide (U5)
2-[4-(3,4-Dichlorophenyl)-5-(1H-indol-3-yl)-4H-1,2,4-triazol-3-ylthio-N-[4-(2-morpholinoacetamido)phenyl acetamide (U6)
4.2. Biology
4.2.1. Cell Culture and Maintenance
4.2.2. Cytotoxicity Assay
4.2.3. Cell Cycle Assay
4.2.4. Apoptosis Assay
4.2.5. Bim Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Computational Modelling
4.3.1. Protein and Ligand Preparation
4.3.2. Grid Generation and Molecular Docking
4.3.3. Pharmacokinetics Predication
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. Role of Bcl-2 family proteins in photodynamic therapy mediated cell survival and regulation. Molecules 2020, 25, 5308. [Google Scholar] [CrossRef]
- Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; Jones, A.T.; Westwell, A.D. New quinoline-based heterocycles as anticancer agents targeting bcl-2. Molecules 2019, 24, 1274. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; El-Sadek, M.; Lashin, E.; Jones, A.T.; Westwell, A.D. Design, synthesis and evaluation of new bioactive oxadiazole derivatives as anticancer agents targeting bcl-2. Int. J. Mol. Sci. 2020, 21, 8980. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.A.; Huang, C.-y.; Ivessa, A.; Singh, S.; Romanienko, P.J.; Nakamura, M. Bcl-x short-isoform is essential for maintaining homeostasis of multiple tissues. Iscience 2023, 26, 106409. [Google Scholar] [CrossRef]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef]
- Longley, D.; Johnston, P. Molecular mechanisms of drug resistance. J. Pathol. A J. Pathol. Soc. Gt. Br. Irel. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P. Broad Targeting of Resistance to Apoptosis in Cancer; Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015; pp. S78–S103. [Google Scholar]
- Thomas, S.; Quinn, B.A.; Das, S.K.; Dash, R.; Emdad, L.; Dasgupta, S.; Wang, X.-Y.; Dent, P.; Reed, J.C.; Pellecchia, M. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. [Google Scholar] [CrossRef]
- Ploumaki, I.; Triantafyllou, E.; Koumprentziotis, I.-A.; Karampinos, K.; Drougkas, K.; Karavolias, I.; Trontzas, I.; Kotteas, E.A. Bcl-2 pathway inhibition in solid tumors: A review of clinical trials. Clin. Transl. Oncol. 2023, 25, 1554–1578. [Google Scholar] [CrossRef]
- Oh, S.; Ni, D.; Pirooz, S.; Lee, J.; Lee, D.; Zhao, Z.; Lee, S.; Lee, H.; Ku, B.; Kowalik, T. Downregulation of autophagy by Bcl-2 promotes MCF7 breast cancer cell growth independent of its inhibition of apoptosis. Cell Death Differ. 2011, 18, 452–464. [Google Scholar] [CrossRef]
- Akar, U.; Chaves-Reyez, A.; Barria, M.; Tari, A.; Sanguino, A.; Kondo, Y.; Kondo, S.; Arun, B.; Lopez-Berestein, G.; Ozpolat, B. Silencing of Bcl-2 expression by small interfering RNA induces autophagic cell death in MCF-7 breast cancer cells. Autophagy 2008, 4, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.G.; Halleran, D.R.; Nevaldine, B.; Shapiro, A.; Hutchison, R.E.; Hahn, P.J. Microarray determination of Bcl-2 family protein inhibition sensitivity in breast cancer cells. Exp. Biol. Med. 2013, 238, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Geng, J.-X.; Hu, X.-Y. Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac. J. Cancer Prev. 2013, 14, 4599–4602. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zheng, Y.; Jiang, P.; Liu, R.; Liu, X.; Chu, Y. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int. J. Biol. Sci. 2011, 7, 805. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.; Vicari, H.P.; Carlos, J.A.E.G.; da Silva, J.C.L.; Figueiredo-Pontes, L.L.D.; Rego, E.M.; Machado-Neto, J.A. Obatoclax reduces cell viability of acute myeloid leukemia cell lines independently of their sensitivity to venetoclax. Hematol. Transfus. Cell Ther. 2022, 44, 124–127. [Google Scholar] [CrossRef]
- Daniel, P.T.; Schulze-Osthoff, K.; Belka, C.; Güner, D. Guardians of cell death: The Bcl-2 family proteins. Essays Biochem. 2003, 39, 73–88. [Google Scholar]
- Warren, C.F.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Bajwa, N.; Liao, C.; Nikolovska-Coleska, Z. Inhibitors of the anti-apoptotic Bcl-2 proteins: A patent review. Expert Opin. Ther. Pat. 2012, 22, 37–55. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Mullard, A. Pioneering apoptosis-targeted cancer drug poised for FDA approval: AbbVie’s BCL-2 inhibitor venetoclax—The leading small-molecule protein-protein interaction inhibitor—Could soon become the first marketed drug to directly target the ability of cancer cells to evade apoptosis. Nat. Rev. Drug Discov. 2016, 15, 147–150. [Google Scholar]
- King, A.C.; Peterson, T.J.; Horvat, T.Z.; Rodriguez, M.; Tang, L.A. Venetoclax: A first-in-class oral BCL-2 inhibitor for the management of lymphoid malignancies. Ann. Pharmacother. 2017, 51, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Korycka-Wolowiec, A.; Wolowiec, D.; Kubiak-Mlonka, A.; Robak, T. Venetoclax in the treatment of chronic lymphocytic leukemia. Expert Opin. Drug Metab. Toxicol. 2019, 15, 353–366. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Mason, K.D.; Vandenberg, C.J.; Scott, C.L.; Wei, A.H.; Cory, S.; Huang, D.C.; Roberts, A.W. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc. Natl. Acad. Sci. USA 2008, 105, 17961–17966. [Google Scholar] [CrossRef]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, S.; Abraham, V.; Huang, X.; Liu, B.; Mitten, M.J.; Nimmer, P.; Lin, X.; Smith, M.; Shen, Y. The Bcl-2/Bcl-XL/Bcl-w Inhibitor, Navitoclax, Enhances the Activity of Chemotherapeutic Agents In Vitro and In VivoNavitoclax Enhances the Activity of Chemotherapeutic Agents. Mol. Cancer Ther. 2011, 10, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Camidge, D.R.; de Oliveira, M.R.; Bonomi, P.; Gandara, D.; Khaira, D.; Hann, C.L.; McKeegan, E.M.; Litvinovich, E.; Hemken, P.M. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 2011, 29, 909. [Google Scholar] [CrossRef]
- Tolcher, A.W.; LoRusso, P.; Arzt, J.; Busman, T.A.; Lian, G.; Rudersdorf, N.S.; Vanderwal, C.A.; Kirschbrown, W.; Holen, K.D.; Rosen, L.S. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2015, 76, 1025–1032. [Google Scholar] [CrossRef]
- Bose, P.; Gandhi, V.; Konopleva, M. Pathways and mechanisms of venetoclax resistance. Leuk. Lymphoma 2017, 58, 2026–2039. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Claxton, D.F.; Crump, M.; Faderl, S.; Kipps, T.; Keating, M.J.; Viallet, J.; Cheson, B.D. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan–Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood J. Am. Soc. Hematol. 2009, 113, 299–305. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the target-based design of anticancer agents: A versatile scaffold with diverse mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Dhuguru, J.; Skouta, R. Role of indole scaffolds as pharmacophores in the development of anti-lung cancer agents. Molecules 2020, 25, 1615. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.; Hayallah, A.M.; Kovacic, S.; Abdelhamid, D.; Abdel-Aziz, M. Recent progress in biologically active indole hybrids: A mini review. Pharmacol. Rep. 2022, 74, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Structural optimization of indole based compounds for highly promising anti-cancer activities: Structure activity relationship studies and identification of lead molecules. Eur. J. Med. Chem. 2014, 74, 440–450. [Google Scholar]
- Jia, H.-W.; Yang, H.-L.; Xiong, Z.-L.; Deng, M.-H.; Wang, T.; Liu, Y.; Cheng, M. Design, synthesis and antitumor activity evaluation of novel indole acrylamide derivatives as IMPDH inhibitors. Bioorganic Chem. 2022, 129, 106213. [Google Scholar] [CrossRef]
- Mehra, A.; Sharma, V.; Verma, A.; Venugopal, S.; Mittal, A.; Singh, G.; Kaur, B. Indole derived anticancer agents. ChemistrySelect 2022, 7, e202202361. [Google Scholar] [CrossRef]
- Schimmer, A.D.; O’Brien, S.; Kantarjian, H.; Brandwein, J.; Cheson, B.D.; Minden, M.D.; Yee, K.; Ravandi, F.; Giles, F.; Schuh, A. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008, 14, 8295–8301. [Google Scholar] [CrossRef]
- Nagy, M.I.; Darwish, K.M.; Kishk, S.M.; Tantawy, M.A.; Nasr, A.M.; Qushawy, M.; Swidan, S.A.; Mostafa, S.M.; Salama, I. Design, synthesis, anticancer activity, and solid lipid nanoparticle formulation of indole-and benzimidazole-based compounds as pro-apoptotic agents targeting bcl-2 protein. Pharmaceuticals 2021, 14, 113. [Google Scholar] [CrossRef]
- Ziedan, N.I.; Hamdy, R.; Cavaliere, A.; Kourti, M.; Prencipe, F.; Brancale, A.; Jones, A.T.; Westwell, A.D. Virtual screening, SAR, and discovery of 5-(indole-3-yl)-2-[(2-nitrophenyl) amino][1,3,4]-oxadiazole as a novel Bcl-2 inhibitor. Chem. Biol. Drug Des. 2017, 90, 147–155. [Google Scholar] [CrossRef]
- Hajra, S.; Patra, A.R.; Basu, A.; Saha, P.; Bhattacharya, S. Indole-3-Carbinol (I3C) enhances the sensitivity of murine breast adenocarcinoma cells to doxorubicin (DOX) through inhibition of NF-κβ, blocking angiogenesis and regulation of mitochondrial apoptotic pathway. Chem.-Biol. Interact. 2018, 290, 19–36. [Google Scholar] [CrossRef]
- Singh, A.A.; Jo, S.H.; Kiddane, A.T.; Niyonizigiye, I.; Kim, G.D. Indole-3-carbinol induces apoptosis in AGS cancer cells via mitochondrial pathway. Chem. Biol. Drug Des. 2023, 101, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.R.; Pandya, J.G.; Vaidya, F.U.; Pathak, C.; Bhatt, B.S.; Patel, M.N. Biological activities of pyrazoline-indole based Re (I) carbonyls: DNA interaction, antibacterial, anticancer, ROS production, lipid peroxidation, in vivo and in vitro cytotoxicity studies. Chem.-Biol. Interact. 2020, 330, 109231. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelian, B.; Benkendorff, K.; Johnston, M.R.; Abbott, C.A. Purified brominated indole derivatives from Dicathais orbita induce apoptosis and cell cycle arrest in colorectal cancer cell lines. Mar. Drugs 2013, 11, 3802–3822. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, Z.; He, Y.; Xiao, Y.; Xia, C. Single and dual target inhibitors based on Bcl-2: Promising anti-tumor agents for cancer therapy. Eur. J. Med. Chem. 2020, 201, 112446. [Google Scholar] [CrossRef]
- Bang, S.; Baek, J.Y.; Kim, G.J.; Kim, J.; Kim, S.; Deyrup, S.T.; Choi, H.; Kang, K.S.; Shim, S.H. Azaphilones from an endophytic Penicillium sp. prevent neuronal cell death via inhibition of MAPKs and reduction of Bax/Bcl-2 ratio. J. Nat. Prod. 2021, 84, 2226–2237. [Google Scholar] [CrossRef]
- Kamath, P.R.; Sunil, D.; Joseph, M.M.; Salam, A.A.A.; Sreelekha, T. Indole-coumarin-thiadiazole hybrids: An appraisal of their MCF-7 cell growth inhibition, apoptotic, antimetastatic and computational Bcl-2 binding potential. Eur. J. Med. Chem. 2017, 136, 442–451. [Google Scholar] [CrossRef]
- Hamdy, R.; Ziedan, N.; Ali, S.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 3-(benzylthio)-5-(1H-indol-3-yl)-1,2,4-triazol-4-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2391–2394. [Google Scholar] [CrossRef]
- Hamdy, R.; Ziedan, N.I.; Ali, S.; Bordoni, C.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 5-(1H-indol-3-yl)-N-aryl-1 3,4-oxadiazol-2-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 1037–1040. [Google Scholar] [CrossRef]
- Dwivedi, A.R.; Kumar, V.; Prashar, V.; Verma, A.; Kumar, N.; Parkash, J.; Kumar, V. Morpholine substituted quinazoline derivatives as anticancer agents against MCF-7, A549 and SHSY-5Y cancer cell lines and mechanistic studies. RSC Med. Chem. 2022, 13, 599–609. [Google Scholar] [CrossRef]
- Ono, Y.; Ninomiya, M.; Kaneko, D.; Sonawane, A.D.; Udagawa, T.; Tanaka, K.; Nishina, A.; Koketsu, M. Design and synthesis of quinoxaline-1, 3, 4-oxadiazole hybrid derivatives as potent inhibitors of the anti-apoptotic Bcl-2 protein. Bioorg. Chem. 2020, 104, 104245. [Google Scholar] [CrossRef]
- Kulabaş, N.; Tatar, E.; Özakpınar, Ö.B.; Özsavcı, D.; Pannecouque, C.; de Clercq, E.; Küçükgüzel, İ. Synthesis and antiproliferative evaluation of novel 2-(4H-1, 2, 4-triazole-3-ylthio) acetamide derivatives as inducers of apoptosis in cancer cells. Eur. J. Med. Chem. 2016, 121, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Yu, L.; Yang, L. Mechanisms of venetoclax resistance and solutions. Front. Oncol. 2022, 12, 1005659. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.; Schmidt, C.; Bruch, P.-M.; Blank, M.F.; Rohde, C.; Waclawiczek, A.; Heid, D.; Renders, S.; Göllner, S.; Vierbaum, L. Venetoclax synergizes with gilteritinib in FLT3 wild-type high-risk acute myeloid leukemia by suppressing MCL-1. Blood J. Am. Soc. Hematol. 2022, 140, 2594–2610. [Google Scholar] [CrossRef]
- Timucin, A.C.; Basaga, H.; Kutuk, O. Selective targeting of antiapoptotic BCL-2 proteins in cancer. Med. Res. Rev. 2019, 39, 146–175. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Lartigue, L.; Perkins, G. Targeting Mcl-1 and other Bcl-2 family member proteins in cancer therapy. Pharmacol. Ther. 2019, 195, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Dai, N.; Tang, Z.; Fang, H. Small-molecule Mcl-1 inhibitors: Emerging anti-tumor agents. Eur. J. Med. Chem. 2018, 146, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wan, Y.; Liu, R.; Ma, L.; Li, M.; Fang, H. Design, synthesis and preliminary biological evaluation of indole-3-carboxylic acid-based skeleton of Bcl-2/Mcl-1 dual inhibitors. Bioorg. Med. Chem. 2017, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, T.; Zhou, Y.; Yang, X.; Fang, H. 1-Phenyl-1H-indole derivatives as a new class of Bcl-2/Mcl-1 dual inhibitors: Design, synthesis, and preliminary biological evaluation. Bioorg. Med. Chem. 2017, 25, 5548–5556. [Google Scholar] [CrossRef]
- Kamath, P.R.; Sunil, D.; Ajees, A.A.; Pai, K.; Das, S. Some new indole–coumarin hybrids; Synthesis, anticancer and Bcl-2 docking studies. Bioorganic Chem. 2015, 63, 101–109. [Google Scholar] [CrossRef]
- Nayak, S.; Gaonkar, S.L.; Musad, E.A.; Dawsar, A.M.A. 1, 3, 4-Oxadiazole-containing hybrids as potential anticancer agents: Recent developments, mechanism of action and structure-activity relationships. J. Saudi Chem. Soc. 2021, 25, 101284. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Jones, A.T.; El-Sadek, M.; Hamoda, A.M.; Shakartalla, S.B.; Al Shareef, Z.M.; Soliman, S.S.; Westwell, A.D. New bioactive fused triazolothiadiazoles as Bcl-2-targeted anticancer agents. Int. J. Mol. Sci. 2021, 22, 12272. [Google Scholar] [CrossRef] [PubMed]
- Ashimori, N.; Zeitlin, B.D.; Zhang, Z.; Warner, K.; Turkienicz, I.M.; Spalding, A.C.; Teknos, T.N.; Wang, S.; Nör, J.E. TW-37, a small-molecule inhibitor of Bcl-2, mediates S-phase cell cycle arrest and suppresses head and neck tumor angiogenesis. Mol. Cancer Ther. 2009, 8, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Gu, C.; Shi, L.; Xun, T.; Li, X.; Liu, S.; Yu, L. Obatoclax Induces G1/G0-Phase Arrest via p38/p21waf1/Cip1 Signaling Pathway in Human Esophageal Cancer Cells. J. Cell. Biochem. 2014, 115, 1624–1635. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar] [CrossRef]
- Opydo-Chanek, M.; Cichoń, I.; Rak, A.; Kołaczkowska, E.; Mazur, L. The pan-Bcl-2 inhibitor obatoclax promotes differentiation and apoptosis of acute myeloid leukemia cells. Investig. New Drugs 2020, 38, 1664–1676. [Google Scholar] [CrossRef]
- Wang, Y.-c.; Rao, P.N. Effect of gossypol on DNA synthesis and cell cycle progression of mammalian cells in vitro. Cancer Res. 1984, 44, 35–38. [Google Scholar]
- Chan, G.K.Y.; Kleinheinz, T.L.; Peterson, D.; Moffat, J.G. A simple high-content cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assays. PLoS ONE 2013, 8, e63583. [Google Scholar] [CrossRef]
- Vertrees, R.A.; Das, G.C.; Popov, V.L.; Coscio, A.M.; Goodwin, T.J.; Logrono, R.; Zwischenberger, J.B.; Boor, P.J. Synergistic interaction of hyperthermia and gemcitabine in lung cancer. Cancer Biol. Ther. 2005, 4, 1144–1153. [Google Scholar] [CrossRef][Green Version]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput.-Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Fayed, B.; Mostafa, A.; Shama, N.M.A.; Mahmoud, S.H.; Mehta, C.H.; Nayak, Y.; Soliman, S.S.M. Iterated virtual screening-assisted antiviral and enzyme inhibition assays reveal the discovery of novel promising anti-SARS-CoV-2 with dual activity. Int. J. Mol. Sci. 2021, 22, 9057. [Google Scholar] [CrossRef] [PubMed]
- Baltrukevich, H.; Bartos, P. RIG-I and RNA Complex MD Simulations in ff19SB+ OL3, ff14SB+ OL3, OPLS4 and AMOEBA Force Fields. Available online: https://erepo.uef.fi/handle/123456789/29290 (accessed on 25 July 2023).
- Giardina, S.F.; Werner, D.S.; Pingle, M.; Feinberg, P.B.; Foreman, K.W.; Bergstrom, D.E.; Arnold, L.D.; Barany, F. Novel, Self-Assembling Dimeric Inhibitors of Human β Tryptase. J. Med. Chem. 2020, 63, 3004–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-J.; Foloppe, N. Drug-like bioactive structures and conformational coverage with the LigPrep/ConfGen suite: Comparison to programs MOE and catalyst. J. Chem. Inf. Model. 2010, 50, 822–839. [Google Scholar] [CrossRef]
- Hamdy, R.; Hamoda, A.M.; Al-Khalifa, M.; Menon, V.; El-Awady, R.; Soliman, S.S. Efficient selective targeting of Candida CYP51 by oxadiazole derivatives designed from plant cuminaldehyde. RSC Med. Chem. 2022, 13, 1322–1340. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Weininger, D. SMILES. 3. DEPICT. Graphical depiction of chemical structures. J. Chem. Inf. Comput. Sci. 1990, 30, 237–243. [Google Scholar] [CrossRef]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S.; Hamoda, A.M.; Alkhayat, F.; Khademi, N.N.; Al Joud, S.M.A.; El-Keblawy, A.A.; Soliman, S.S. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2021, 21, 291–312. [Google Scholar] [CrossRef] [PubMed]
| Compound | R | IC50 * | ||
|---|---|---|---|---|
| MDA-MB-231 | MCF-7 | A549 | ||
| U1 | 4-F | 20.66 ± 0.85 | 5.05 ± 0.80 | 13.34 ± 0.58 |
| U2 | 4-OCH3 | 5.22 ± 0.55 | 0.83 ± 0.11 | 0.73 ± 0.07 |
| U3 | 4-CH3 | 4.07 ± 0.35 | 1.17 ± 0.10 | 2.98 ± 0.19 |
| U4 | 3-Cl | 28.38 ± 1.79 | 11.0 ± 1.2 | 16.11 ± 1.4 |
| U5 | 4-Cl | 45.1 ± 1.15 | 34.43 ± 2.20 | 47.77 ± 3.7 |
| U6 | 3,4-Dichloro | 79.4 ± 4.04 | 35.45 ± 2.14 | 66.5 ± 0.62 |
| Gossypol | 5.5 ± 0.35 | 4.43 ± 0.54 | 3.45 ± 0.40 | |
| Compound | IC50 * |
|---|---|
| U2 | 1.2 ± 0.02 |
| U3 | 11.10 ± 0.07 |
| Gossypol | 0.62 ± 0.01 |
| Compound | Moiety | Interaction | Amino Acid |
|---|---|---|---|
| U1 | Carbonyl group | 2 H-bond interaction | Arg-105 |
| Phenyl ring | Aromatic H-bond | Val-92 | |
| Phenyl ring | Hydrophobic interaction | Tyr-67 | |
| Phenyl ring | Hydrophobic interaction | Phe-63 | |
| Phenyl ring | Hydrophobic interaction | Glu-95 | |
| Sulfanyl group | Hydrophobic interaction | Leu-96 | |
| U2 | Carbonyl group | H-bond interaction | Arg-60 |
| Phenyl ring | Pi-pi staking | Phe-63 | |
| Phenyl ring | Aromatic H-bond | Asp-99 | |
| Phenyl ring | Pi-pi staking | Phe-63 | |
| Phenyl ring | Aromatic H-bond | Glu-95 | |
| Sulfanyl group | Hydrophobic interaction | Arg-105, Tyr-63, and Ala-108 | |
| Phenyl ring | Hydrophobic interaction | Ala-108 | |
| Phenyl ring | Hydrophobic interaction | Tyr-67 | |
| Phenyl ring | Hydrophobic interaction | Glu-95 | |
| U3 | Carbonyl group | 2H-bond interaction | Arg-105 |
| Phenyl ring | Aromatic H-bond | Val-92 | |
| CH3 | Hydrophobic interaction | Glu-95 | |
| Sulfanyl group | Hydrophobic interaction | Leu-96 | |
| Carbonyl group | Hydrophobic interaction | Arg-105 | |
| Phenyl ring | Hydrophobic interaction | Asp-70 | |
| Phenyl ring | Hydrophobic interaction | Tyr-67 and Glu-95 | |
| Phenyl ring | Hydrophobic interaction | Val-92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almehdi, A.M.; Soliman, S.S.M.; El-Shorbagi, A.-N.A.; Westwell, A.D.; Hamdy, R. Design, Synthesis, and Potent Anticancer Activity of Novel Indole-Based Bcl-2 Inhibitors. Int. J. Mol. Sci. 2023, 24, 14656. https://doi.org/10.3390/ijms241914656
Almehdi AM, Soliman SSM, El-Shorbagi A-NA, Westwell AD, Hamdy R. Design, Synthesis, and Potent Anticancer Activity of Novel Indole-Based Bcl-2 Inhibitors. International Journal of Molecular Sciences. 2023; 24(19):14656. https://doi.org/10.3390/ijms241914656
Chicago/Turabian StyleAlmehdi, Ahmed M., Sameh S. M. Soliman, Abdel-Nasser A. El-Shorbagi, Andrew D. Westwell, and Rania Hamdy. 2023. "Design, Synthesis, and Potent Anticancer Activity of Novel Indole-Based Bcl-2 Inhibitors" International Journal of Molecular Sciences 24, no. 19: 14656. https://doi.org/10.3390/ijms241914656
APA StyleAlmehdi, A. M., Soliman, S. S. M., El-Shorbagi, A.-N. A., Westwell, A. D., & Hamdy, R. (2023). Design, Synthesis, and Potent Anticancer Activity of Novel Indole-Based Bcl-2 Inhibitors. International Journal of Molecular Sciences, 24(19), 14656. https://doi.org/10.3390/ijms241914656





