Abstract
Cognitive dysfunction is an important non-motor symptom in amyotrophic lateral sclerosis (ALS) that has a negative impact on survival and caregiver burden. It shows a wide spectrum ranging from subjective cognitive decline to frontotemporal dementia (FTD) and covers various cognitive domains, mainly executive/attention, language and verbal memory deficits. The frequency of cognitive impairment across the different ALS phenotypes ranges from 30% to 75%, with up to 45% fulfilling the criteria of FTD. Significant genetic, clinical, and pathological heterogeneity reflects deficits in various cognitive domains. Modern neuroimaging studies revealed frontotemporal degeneration and widespread involvement of limbic and white matter systems, with hypometabolism of the relevant areas. Morphological substrates are frontotemporal and hippocampal atrophy with synaptic loss, associated with TDP-43 and other co-pathologies, including tau deposition. Widespread functional disruptions of motor and extramotor networks, as well as of frontoparietal, frontostriatal and other connectivities, are markers for cognitive deficits in ALS. Cognitive reserve may moderate the effect of brain damage but is not protective against cognitive decline. The natural history of cognitive dysfunction in ALS and its relationship to FTD are not fully understood, although there is an overlap between the ALS variants and ALS-related frontotemporal syndromes, suggesting a differential vulnerability of motor and non-motor networks. An assessment of risks or the early detection of brain connectivity signatures before structural changes may be helpful in investigating the pathophysiological mechanisms of cognitive impairment in ALS, which might even serve as novel targets for effective disease-modifying therapies.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal multi-system neurodegenerative disease with no effective treatment or cure. It is characterized primarily by motor neuron degeneration but may be accompanied by cognitive dysfunction. The involvement of respiratory muscles is a key feature and usually leads to death due to respiratory failure. The mean survival time is 2–5 years after disease onset [1]. Classification of the disease is based on the site of origin (spinal or bulbar onset), the severity of involvement, and the pattern of heritability. ALS has an incidence between 1.5 and 2.5 per 100,000/year and a point prevalence of around 5 per 100,000 population, with local variations [2,3,4,5]. ALS, which has an estimated heritability between 40 and 50% [6], occurs sporadically and familial, with an autosomal-dominant inheritance (5–10%) [7]. Four genes are associated with up to 70% of familial ALS (fALS), namely C9orf72 (chromosome 9 open reading frame 72), TARDBP (transactive response DNA binding protein), SOD1 (superoxide dismutase 1), and FUS (fused-in sarcoma protein) [8], but there is a long and growing list of mutations found in sporadic ALS (sALS) and/or fALS. The frequency of C9orf72 mutation is low in Japan, unlike in Europe and the USA, while SOD1 and FUS are more common [9]. ALS heritability is enriched in binding sites of RNA-binding proteins, including TDP-43 and FUS [10]. Genetic polymorphisms may exert a polygenic contribution to the risk of cortical disease and cognitive dysfunction in ALS [11]. Neuropsychiatric disorders in parents and the families of ALS patients are associated with cognitive (and behavioral) changes, such as poorer overall cognition and visuospatial or language disorders, overlapping with ALS or other motor neuron diseases [12].
At the onset, signs and symptoms of ALS are rather subtle, with the progressive involvement of the lower and upper motor neurons. The site of symptoms is important because of distinguishing between the limb, bulbar and respiratory onset of different ALS subtypes. Clinical diagnosis is usually made according to the revised El Escorial criteria [13], further modifications of which are aimed at increasing diagnostic sensitivity [14]. The three major ALS variants are sALS, fALS (about 20%), and the rare Western Pacific form. There is an overlap between ALS and frontotemporal lobe degeneration (FTLD), with the latter presenting with cognitive impairment (CI) progressing to dementia. The ALS-FTLD phenotype with no to severe CI is accompanied by ALS pathology and its clinical presentation. The most consistent ALS and ALS-FTLD pathology is a disturbance in transactive response DNA-binding protein 43 kDA (TDP-43) metabolism, observed in up to 97% of all cases [15,16], with phosphorylated TDP-43 pathology causing a disruption of synaptic structures [17,18]. Alterations in tau protein metabolism have also been observed in ALS-FTLD [19]. During the last 10 years, radiological, humoral, neurophysiological and genetic biomarkers have been reported in ALS and some of them correlate to the CI of ALS patients. Early detection and careful monitoring of ALSci even at early disease stages are crucial for patient and caregiver and may not only have prognostic implications but may also be important for future clinical trials [20]. The present review is restricted to the neuroimaging and pathological bases of CI in ALS and its pathogenesis, without a specific discussion of FTLD.
2. Cognitive Abnormalities in ALS
Cognitive (and behavioral) impairment has been known since the 19th century thanks to Biblioteca di Area Medica “Adolfo Ferrate” Sistema Bibliotecario di Ateneo, University of Pavia, Italy [21]. CI in ALS is more frequent than in patients with other neuromuscular diseases [22], and in the last decade, it has become clear that cognitive (and behavioral) changes in ALS are more frequent than previously recognized [23]. The incidence of CI in ALS varies considerably between 30% [22,24,25,26,27,28,29,30,31] and 75% [19,32,33], whilst the rate of behavioral dysfunction occurs in up to 50% of ALS patients [32]. The published data about the severity of CI in ALS vary considerably: mild cognitive impairment (MCI) was observed between 32% and 70% [33,34], and between 6% and 40–45% showed severe CI [33,34], while others reported frank dementia in 15% of ALS patients [19] with full symptoms of FTLD, predominantly the behavioral variant [35]. Cognitive functions were more impaired in patients with an age of onset over 65 years [33]. Moreover, 14% of ALS patients had evidence of CI without executive dysfunction, and no cognitive abnormality was detected in 24% to 46.9% [25,29,35].
In a recent study from China, the ALSci group (49.5% of a total of 1015 ALS patients) tended to harbor more ALS-plus (extramotor) symptoms than the cognitively normal ALS group [36]. In non-demented ALS patients, no cognitive/behavioral correlate for hippocampal dysfunction was found [37].
The prevalence and pattern of CI in fALS is similar to that in sALS. For patients with fALS, the site of symptoms does not correlate with CI, but age does [38]. The cognitive spectrum in ALS is heterogenous and consists of deficits in executive function (EF), attention, verbal fluency, naming, language, social cognition, visuospatial abilities, verbal memory and other cognitive domains [24,30,39,40,41,42,43]. Foremost, deficits are observed in the domains of attention, executive function and language function [35,44]. Memory deficits are distinctly different from those in amnestic MCI, which may be due to the fact that they are not exclusively caused by coexisting executive dysfunction in ALS. This indicates qualitative differences in the type of memory impairment between ALS and MCI and supports that temporal dysfunction is related with distinct cognitive impairments in ALS [45].
There is emerging evidence that presymptomatic disease is not uniformly clinically silent with mild motor, cognitive and/or behavioral impairment, representing a prodromal stage of ALS [20]. In contrast to motor functioning that declines concomitantly with disease progression, cognitive deficits appear in early disease, are essentially present at the initial visit and usually do not substantially decline on follow-up [46]. Cognitive deterioration in non-demented patients with ALS is a relatively slow process; selected CI in the form of verbal fluency deficits appears relatively early in the course of the disease, although language function may become vulnerable as the disease progresses [47]. Despite vast evidence for CI at disease onset in different domains, evidence for the evolution of these deficits, however, is rather limited, suggesting that ALS patients present with CI early in the course of disease [48]. Language impairment is characteristic of ALS in early disease stages and can develop independently of executive dysfunction, reflecting selective patterns of frontotemporal involvement [49]. Occurring in about 23% of non-demented ALS patients, it may also be significantly driven by executive dysfunction, but independent of other motor and non-motor features [50]. The spectrum of language impairment in ALS includes spontaneous speech, word fluency and action naming, whereas object naming, semantic memory, sentence comprehension and repetition are comparable to healthy controls. Spontaneous speech is reduced across the ALS spectrum even in those with intact core language abilities. Around 36% of ALS patients produced significantly fewer words per minute, suggesting that spontaneous speech is reduced across the ALS spectrum [26]. The frequency of language abnormalities in ALS was 8.5% for spontaneous speech, 25.7% for verbal comprehension, 8.8% for repetition, 14.7% for naming, 17.6% for reading, and 51.4% for writing, with dysfunction on the left angular gyrus probably being associated with writing errors observed in ALS [51]. A recent study reported a prevalence of language impairment of 22.7%, which is significantly driven by executive dysfunction and not associated with other motor or non-motor features [50].
While executive dysfunctions have been described first, alterations in verbal fluency, pragmatic and social cognition have also been observed [52]. The dysfunction of social cognition is well recognized as one of the CIs of ALS [42]. This is a new domain with a relatively large effect size, highlighting the overlap between ALS and FTLD [39]. ALS-FTLD patients have a worse prognosis and shorter survival rates than patients with pure ALS [53].
CI in both fALS and sALS may present before, at, or after the onset of motor neuron disease [25,54]. It usually develops without bias to movement impairment [39,55,56], whereas others have stated that cognitive deficits (and behavioral impairment) accompany the intensifying physical activity and are more frequent with a more severe disease stage [57]. In ALS, MCI is associated with exaggerated gait variability and an increased risk of falls [58]. A high level of CI arrives within 18 months of symptom onset [59], while others reported the worsening of CI after 6 months [25]. The decline in cognitive function was faster in patients who were cognitively impaired at baseline, while normal cognition at baseline was associated with a tendency to remain cognitively intact, with slowed motor and cognitive progression [60]. Weight loss, very common in ALS, is associated with executive dysfunction and cognitive decline, as well as poor survival [61], whereas there is no significant difference in the survival curves of pure ALS and ALS with cognitive and behavioral changes [62]. It was suggested that baseline executive status might predict survival in ALS [63].
Patients with bulbar onset and those with bilateral spinal onset were generally characterized by lower cognitive performance in most neuropsychological tests when compared to patients with lateralized onset, which was in keeping with the hemispheric lateralization of language and visuospatial abilities [64]. Cognitive deficits are more pronounced in ALS with predominant upper motor neuron (PUMN) involvement [19,65]. Some authors stated that cognitive changes are more frequent with more severe disease stages [57], whereas, according to others, cognitive performance remains stable [48]. ALS patients with dysarthria performed significantly worse than non-dysarthric patients, while cognitive functioning was not associated with the site of onset and had only a moderate association with dysarthria [66]. Others also stated that abnormalities in cognitive domains have only limited relevance for the patient’s everyday life, in comparison to the impact of behavioral alterations [67].
C9orf72 repeat expansions seem to cause a recognizable phenotype characterized by earlier disease onset, more severe CI, more rapid progression and shorter survival [68,69,70,71,72,73]; neuropsychological changes of these patients are associated with subcortical gray matter (GM) atrophy [74]. Likewise, those with dysarthria, family history of ALS, PUMN phenotype and bulbar onset have a higher risk for ALSci [75]. By contrast, patients with the atypical ALS type 8, caused by a mutation of the vesicle-trafficking protein VAPB [76], demonstrated similar deficits in most cognitive domains as patients with sALS [77], while the landscape of CI in SOD1 ALS is under discussion [78].
3. Neuroimaging Findings in ALSci
Early MRI and SPECT studies showed frontal atrophy, suggesting a continuum of cognitive disability in ALS patients corresponding to a pathological process in the frontal lobe ranging from normality to significant impairment [79]. Later studies suggested that cognitive dysfunction in ALS reflects functional and morphological deficits outside the primary motor system that are specific to the nature and evolution of the basic disease [46] (Table 1). While structural neuroimaging has not been able to establish a specific hypothesis of extramotor cortical atrophy beyond the combination of various frontal, temporal and limbic areas, functional neuroimaging supports the neuropsychological findings of frontotemporal dysfunction but also implies the involvement of somatosensory areas [80]. A significant decline in the cortical thickness of frontal, temporal and parietal regions is observed during disease progression, whereas the reduced cortical thickness of the precentral gyrus at the beginning of the disease remains stable [81,82]. Multimodal neuroimaging confirmed motor and non-motor GM and WM abnormalities in non-demented cognitively impaired ALS patients, as well as early extramotor pathologies in those without CI [83]. Cortical thinning in the frontoparietal region suggesting a disease-specific pattern of neurodegeneration was present in all ALS patients, irrespective of cognitive (and behavioral) status, whereas the thinning of inferior frontal, temporal, cingulate and insular cortices was typical for cognitive and behavior deficits, with language impairment mainly related to the left temporal pole and insular involvement [84] (Table 1). Menke et al. [85] assessed brain changes in patients with ALS over 2 years and reported widespread changes in both WM and GM in the cingulate gyrus, thalamic nuclei, caudate nuclei, pallidum, hippocampi, parahippocampal gyri, and insula (Table 1). These results indicate that over time, cerebral changes extend into extramotor areas, but it remains difficult to draw a link between these changes and clinical cognitive implications [86]. Cognitive changes are highly dependent upon cortical atrophy; patients with ALSci have significant atrophy across motor and somatosensory as well as adjacent frontal and parietal areas [87]. Subcortical GM structures are often involved before CI becomes evident [88]. The degeneration of basal ganglia in ALS is particularly seen in patients with CI (and behavioral involvement); thalamic atrophy is associated with poorer EF, and reduced volumes of globus pallidus, caudate nucleus, putamen and thalamus are associated with poorer cognitive scores [89]. A significant reduction in amygdala volume may also play an important role in cognitive dysfunction in ALS [90]. Parts of the hypothalamus, containing the paraventricular nucleus, show essential volume loss in ALS and ALS + FTD [91].

Table 1.
Major neuroimaging findings in ALSci.
While ALS-motor patients showed decreased GM volume in frontal and cerebellar areas and increased GM volume in the right supplementary motor cortex, ALSci patients showed diffused GM volume reduction in the primary motor cortex bilaterally, and frontotemporal, bilateral cerebellum, basal ganglia and bilateral superior longitudinal fasciculi white matter (WM) were seen [97]. The cortical thickness of the left entorhinal cortex, left inferior temporal gyrus, left medial orbitofrontal lobe and left insular lobe were significantly reduced (p < 0.05). This correlated with neuropsychological scores [31]. There was also decreased fractional anisotropy (FA) and increased radial diffusivity in the corticospinal tract bilaterally, corpus callosum, and extramotor tracts in ALS-motor patients, and decreased FA and increased axial diffusivity in motor and several WM tracts in ALSci patients [111].
C9orf72 ALS mutation carriers exhibited extensive cortical and subcortical damage with disease-specific patterns of thalamo-cortico-striatal atrophy. Cortical thinning was pronounced in posterior areas and extended to frontal regions. Bilateral atrophy of the mediodorsal and pulvinar nuclei emphasized a focal rather than global thalamus atrophy, indicating a central role of the thalamus in the pathogenic mechanisms of C9orf72-mediated disease [112]. C9orf72-repeat-negative ALS patients showed a volume reduction in the right nucleus accumbens, left caudate, and hippocampus [103].
Verbal fluency was associated with temporal lobe dysfunction, with increasing frontal involvement in ALS patients with greater CI [113]. Dissociated motor and cognitive components of speech deficits were related to cortical thickness in the primary motor cortex and perisylvian regions, and impaired speech duration was linked to the bilateral frontal operculum and left anterior insula [94]. Multifocal deficits in sentence expression that are independent of their motor disorder were related to GM atrophy in the left inferior frontal and anterior temporal regions and to reduced FA in superior longitudinal and inferior frontal-occipital fasciculi [93]. ALSci patients demonstrated cortical thinning in the bilateral precentral gyrus, right precuneus, and right frontal and temporal lobes; considerable frontoparietal atrophy, including the right insula and motor cortices, was associated with the involvement of the bilateral pallidum and putamen, with “pure” ALS displaying greater parietal atrophy [89]. These and other data support a more integral framework for the clinical relationship of frontal functioning as a correlate of cognitive deficits and executive dysfunction [114]. They furthermore support the concept that ALS patients with CI have a more widespread neurodegenerative process compared with a pure motor disease [107].
Resting-state functional MRI studies showed significant differences in the fractional amplitude of low-frequency fluctuation (fALFF)—(The amplitude of low-frequency fluctuation (ALFF) measures low-frequency oscillations of the blood-oxygen-level-dependent (BOLD) signal, characterizing local spontaneous activity during the resting state. ALFF is a commonly used measure for resting-state functional magnetic resonance imaging (rs-fMRI) in numerous basic and clinical neuroscience studies. (Verbatim from [115] in the left frontal lobe, right parietal lobe, and left cingulate gyrus, and there was regional homogeneity (ReHo) in these areas compared to those with and without CI. These changes were mainly located in the left prefrontal lobe and anterior cingulate cortex, indicating that hypoactivities are detected in extramotor areas in patients with CI in ALS [104] (Table 1). ALSci patients showed increased ReHo in bilateral inferior parietal lobules and the precuneus and inferior cerebellar areas, as well as in the right inferior parietal and right inferior cerebellar area relative to the ALS non-CI subgroup, while both ALS groups showed decreased ReHo in bilateral sensorimotor cortices. The altered regional functional coherence might indicate the underlying deficits in ALS with and without CI, supporting that ALS is a multi-system disease and providing evidence that alterations of ReHo in the right inferior cerebellar area might be a marker of ALSci [105].
In addition to a volume reduction in the basal ganglia [85,96,97], the atrophy of the amygdala [90] and the cholinergic nucleus accumbens was described in both ALS cases with and without CI [101,102,103]; the clinical implication of the latter is under discussion [102].
Recent MRI clustering revealed three ALS subtypes with specific neurodegeneration and clinical patterns. All subgroups displayed the involvement of the precentral gyrus and are featured, respectively, by (1) pure motor involvement, (2) orbitofrontal and temporal affection (frontotemporal cluster /FT/), and (3) involvement of posterior cingulate cortex, temporal operculum, parietal WM and cerebellum (cingulate-parieto-temporal cluster /CPT/). These subgroups showed distinct clinical profiles, among which FT and CPT revealed higher rates of CI— the FT had significantly higher frequencies of CI in language, EF and memory domains. Longitudinally, this clustering of subgroups remained stable: 90.4% of the patients remained stable at follow-up visits, clustered in the same subgroup as at baseline [100].
In conclusion, the cortical thickness of ALS patients is correlated with neuropsychological scores, which may reflect not only the cortical structure but also the regional homogeneity corresponding to the cognitive assessment and may provide help for the early diagnosis of cognitive changes in ALS patients [31]. The new clustering of ALS into three main patterns of neurodegeneration, each associated with distinct clinical manifestation and cognitive profiles, which remained stable during the disease process, designates a process in classifying this heterogeneous disorder based on characteristic morphological features.
4. Brain Perfusion and Metabolism Studies
PET studies showed reduced regional cerebral blood flow (rCBF) in the anterior cingulate cortex in both ALS with and without CI; ALS patients with impaired verbal fluency showed significantly reduced rCBF in the bilateral prefrontal cortex, rostral anterior cingulate, right parahippocampal gyrus and anterior thalamic nucleus. rCBF at rest in the right parahippocampal gyrus was significantly correlated with verbal fluency score, as was the activation of the anterior thalamic nucleus complex, indicating functional abnormalities in regions along a limbo-thalamo-cortical pathway [106]. 18F-FDG-PET showed moderately reduced frontal and prefrontal metabolism in ALSci, which increased with the severity of CI [107]. A combined MRI and PET study in patients with ALS found GM atrophy, predominantly in the temporal poles, and hypometabolism in the left superior medial cortex [95]. Hypermetabolism was also found in parts of the temporal lobes and the cerebellum. A series of negative correlations between cognitive performance and regional cerebral metabolism in functionally relevant areas suggest that hypermetabolism is more likely to reflect deleterious processes such as neuroinflammation rather than compensatory neuronal activity [86].
Structural neuroimaging revealed different patterns of GM and WM involvement in the hypo- and hypermetabolic brain regions in patients with ALSci; cortical thickness and surface area showed differential involvement in the hypo- and hypermetabolic regions [116].
In the ALS group, the metabolism in the medial frontal cluster positively correlated with that of frontotemporal regions, bilateral caudate nucleus, and right insula, and negatively with that of corticospinal tracts, cerebellum, and pons. The negative correlation between the medial frontal cluster and the cerebellum found only in ALS might reflect cerebellar compensation. In addition, there is a negative correlation between education and brain metabolism in the right anterior cingulate and bilateral medial frontal gyrus [117].
Brain metabolism was positively correlated with that of prefrontal, orbitofrontal and anterior cingulate cortices; it was lower in association with the APOE ε/2 allele, indicating its role as a risk factor for CI in ALS [110].
Magnetic resonance spectroscopy in ALS patients exhibited higher hippocampal total N-acetylaspartate (tNAA), tNAA/total creatine (tCr) and total choline (tChol) bilaterally, despite the absence of volumetric and perforant pathway zone diffusivity differences between ALS patients with and without CI. Furthermore, superior memory performance was associated with higher hippocampal tNAA/tCr bilaterally [118]. Other studies showed reduced N-acetylaspartate rates in the motor cortex at baseline, while NAA ratios were reduced in the prefrontal cortex in cognitively impaired ALS patients [119]. The anterior and superior tuberal subregions of the hypothalamus containing the paraventricular nucleus (housing oxytocin-producing neurons) display considerable volume loss in ALS and ALS-FTD, while the inferior tuberal subregions with the paraventricular nucleus (containing neuroendocrine neurons) were relatively preserved, supporting discrete neuropeptide expression abnormalities that may potentially underlie the pathogenesis of certain cognitive and behavioral symptoms in this disease spectrum [91].
5. Brain Network Studies
Brain network analyses have contributed valuable insights into cerebral pathology in vivo. Previous studies have reported conflicting data of increased, reduced or unaltered resting state networks (RSNs) and functional connectivity (FC) in ALS and did not report the contributions of upper neuron changes to RSN FC [120,121]. Impaired cognitive flexibility was related to GM atrophy in inferior frontal and insular regions, and to reduced FA in WM projections in the inferior fronto-occipital and uncinate fasciculi and corpus callosum [98]. Later studies suggested that executive dysfunction in ALS is associated with frontal and global network dysconnectivity, underlain by diminished WM integrity, which indicates domain-specific or general CI, depending on the degree of global network destruction [122]. Widespread disease-associated disruptions pointed to extensive dysfunctions in both motor and cognitive networks [123], with particular dysregulation and asymmetry of the hemodynamic-based frontal functional network [124].
In addition to multiple GM volume reductions in precentral gyri, posterior cingulate cortex, basal ganglia, thalamus and hippocampus, ALSci patients revealed the involvement of the superior longitudinal fasciculi and corpus callosum, reduced FC in the sensorimotor cortex—RSN and frontal pole in the bilateral thalamic-visual cortex network, and increased FC in the left primary motor cortex and left frontoparietal network [85]. There was a diffuse GM volume reduction in the primary motor cortex bilaterally, and the frontotemporal areas, cerebellum and basal ganglia were associated with decreased FA and increased radial diffusivity in the corticospinal tract bilaterally and in several WM tracts [83]. Diffusion tensor tractography detected a unique contribution of microstructural changes in hippocampal and frontotemporal WM tracts in ALS patients with impaired episodic memory [96]. ALSci patients demonstrated an “ALS with only motor deficits (ALS-cn)”-like pattern of structural damage, diverging from ALS-cn, with similar motor impairment for the presence of enhanced functional connectivity within sensorimotor areas, supporting the hypothesis that ALSci might be considered as a phenotypic variant of ALS, rather than a consequence of disease worsening [125]. Reduced GM in the left hippocampus, entorhinal cortex and posterior cingulate, as well as increased FA and decreased mean diffusivity in the left cingulum bundle (hippocampal part) was accompanied by decreased FC in the bilateral hippocampus, anterior and posterior parahippocampal and posterior cingulate gyrus, indicating widespread connectivity abnormalities in the Papez network [99]. While non-demented ALS patients without episodic memory deficits had no changes in the GM of the Papez circuit, those with delayed recall showed marked atrophy of the GM of the Papez circuit, indicating that it is differentially affected in ALS with and without cognitive changes [126]. Other studies revealed significant functional and structural connectivity changes across regions comprising the Papez circuit, and more extended areas including the cerebellum, frontal, temporal and parietal areas, supporting the theory of a multi-system pathology in ALS that spreads from cortical to subcortical structures [127].
Multimodal neuroimaging studies identified a significant volume reduction in the thalamus and putamen of non-fluent-variant primary progressive aphasia (nfvPPA) patients, while patients with semantic variant primary progressive aphasia (svPPA) only exhibited changes in the left hippocampus. The bulk of striatal and thalamic pathology in nfvPPA patients was identified in foci projecting to motor areas. Subcortical density alterations in svPPA patients were limited to motor areas. Striatal and thalamic changes indicated selected network-defined vulnerability patterns mirroring cortical pathology. Multimodal cortico-basal imaging confirmed that subcortical GM profiles of PPA phenotypes support the concept of network-wise degeneration, preferential vulnerability and disease propagation along specific connectivity patterns [128].
ALS individuals displayed a higher intra-network resting state functional connectivity in the sensorimotor, default mode, bilateral frontoparietal and orbitofrontal RSNs, underpinning both motor and cognitive impairment [129]. They showed an increased functional network, predominantly encompassing the connections between the default mode network (DMN) and the frontoparietal network (FPN). The increased structural connection involved interregional connections between the limbic network and the DMN, and the salient/ventral attention network and FPN, while the decreased structural connections mainly involved those between the limbic and the subcortical network. These FC changes are related to cognitive performance and predict the progression of disease [130]. The dysfunction of circuits connecting basal ganglia with the frontal lobe, in particular, sensorimotor and limbic circuits, contributes to cognition and motivation disorders [129,131] (Figure 1). A reduction in empathic skills, intention and emotional attribution are correlated with FA along the forceps minor, genu of corpus callosum, right uncinate and inferior fronto-occipital fasciculi; the involvement of frontal commissural fiber tracts and associative fronto-limbic pathways is the microstructural hallmark of the impairment of the high-order processing of socio-emotional stimuli in ALS [132].
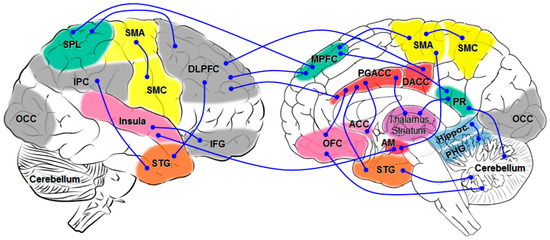
Figure 1.
Schematic overview of major network connectivity in ALSci changes. AM amygdala; DACC dorsal anterior cingulate gyrus; DLPFC dorsolateral prefrontal cortex; IFG inferior frontal gyrus; IPC inferior parietal cortex; MPFC medial prefrontal cortex; OCC occipital; OFC orbital prefrontal cortex; PGACC perigenual anterior cingulate cortex; PHG parahippocampal gyrus; PR precuneus; SMA supplementary motor cortex; SMC sensorimotor cortex; SPL superior parietal lobule; STG superior temporal gyrus.
CI in ALS, most commonly EF and language impairment, are associated with broad network dysfunctions in frontostriatal and frontotemporal systems [133], while altered decision-making showed a strong correlation with decreased connectivity in medial cingulate gyrus and the prefrontal area [134]. The letter fluency fMRI task revealed significantly impaired activation in the middle and inferior frontal gyri and anterior cingulate gyrus, in addition to regions of the temporal and parietal lobes; confrontation naming was associated with impaired activation in less extensive prefrontal regions, including the inferior frontal gyrus and regions in the temporal, parietal and occipital lobes. These findings further illustrate the heterogeneity of cognitive and cerebral changes in ALS [135]. Other studies showed that different executive deficits, like letter fluency, processing speed or dual-task performance, are related to distinct (inferolateral) prefrontal dysfunction [136]. Resting-state fMRI revealed increasing functional breakdown within the frontostriatal and frontoparietal networks related to EF frontal dysfunction [131,137]. Disease progression was associated with a frontal functional network disruption that was primarily observed in the right prefrontal cortex, suggesting that dysregulation, centralization and asymmetry of the hemodynamic-based frontal functional network are potential neurotopological markers of ALS pathogenesis [124]. Similar patterns of hypoconnectivity in the bilateral motor and frontotemporal cortices emerged when comparing ALSci and ALS-FTLD patients with those not cognitively impaired [138]. Diffusion tensor MR imaging that is sensitive to microstructural changes in WM tracts showed that attention and EF impairment correlated with changes in the corpus callosum and association WM tracts bilaterally, including cingulum, inferior longitudinal, inferior fronto-occipital, and uncinate fasciculi. Verbal learning scores were associated with fornix microstructural changes, and visuospatial abilities correlated with left uncinate FA, underlining the role of WM network abnormalities in the development of CI in patients with ALS [139].
In ALS, the inferior frontal gyri show significantly lower baseline activity compared to healthy controls; these activities correlate with motor decline and cognitive inhibition, indicating a symptom-preceding and -associated motor and cognitive cortical network decline [140]. Resting-state fMRI showed that FC within the DMN, as well as between the DMN, the sensorimotor network (SMN), the frontoparietal network and the salience network (SN), are predictive of both global cognition and motor function in ALS [141]. Moreover, the mental fatigue score is directly related to FC in the right and left insula (within the SN) and inversely related to that in the left middle temporal gyrus (within the DMN), thus correlating with CI due to alterations of FCs in extramotor networks [142]. Decreased FC was observed for the SMN and DMN in the left medial frontal and left postcentral gyrus, for DMN in the right medial frontal gyrus and left precuneus, for FPN in the right and left middle frontal and left inferior frontal gyrus, and for subcortical network in the right and left anterior insular cortex and anterior cingulate cortex, whereas the DMN showed increased FC in the middle temporal cortex [142] (Figure 2). Furthermore, some alterations of fronto-temporo-parietal-cerebellar circuits could be related to the pseudobulbar effect in ALS. In particular, abnormal FC between the cerebellum and posterior cingulate and the left middle frontal cortex indicates a crucial role of the cerebellum in regulating emotional expression in ALS [142].
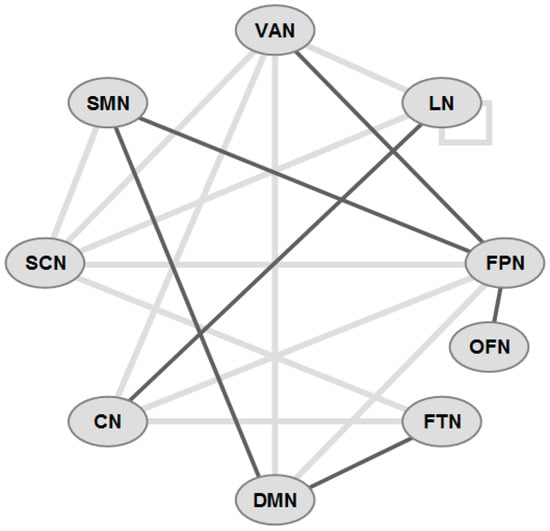
Figure 2.
Schematic overview of functional connectivity (FC) in some major networks in ALS-CI (incomplete). Gray lines: decreased FC, black lines: increased FC. Network abbreviations: VAN ventral attention; LN limbic; FPN frontoparietal network; OFN orbitofrontal; FTN frontotemporal; DMN default mode; CN cerebellar; SCN subcortical; SMN sensorimotor.
In conclusion, brain network analyses revealed involvement of multiple FC dysfunctions related to CI in ALS, particularly affecting fronto-striatal, frontotemporal, and frontoparietal systems, the Papez circuit, fronto-temporo-parieto-cerebellar circuits, and DMN, SMN and SN, with the affection of different networks contributing to specific cognitive and motivation disorders. A schematic overview of the major disrupted architectures is shown in Figure 2 (see also Supplementary Table S1).
6. Neurophysiological Studies in ALSci
The evaluation of event-related potentials (ERP) in ALS patients with CI detected changes in the neural substrate of cognitive changes [143], confirming earlier studies of ERPs showing lower P3 (novelty P300) amplitudes, indicating a subclinical impairment of cognitive functions, which may be related to dysfunction of the frontal network [144]. The determination of cognitive profiles in non-demented patients with early-stage sALS by using ERPs revealed significantly prolonged latencies, indicating subclinical cognitive deficits in non-demented sALS patients [145]. A loss in performance in cognitively impaired ALS patients was accompanied by a decrease in the P300 event-related potential and a decrease in the task-relevant EEG band power [146]. In a similar way, reduced amplitudes and delayed latencies in attenuated error-related potentials may indicate ALS-associated impairment of executive functions, potentially due to disturbances in neural networks that involve the anterior cingulate cortex [147]. Auditory ERPs and delayed latencies were associated with attention impairment in ALS, supporting the hypothesis that ALS involves extramotor cognitive functions including auditory attentional processing [148].
Recent studies have shown that resting-state EEG can quantitatively capture abnormal patterns of cognitive network disruptions in ALS. These changes have been identified across multiple frequency bands by measuring neuronal activity (spectral power) and connectivity on source-located brain oscillations from high-density EEG. Based on data-driven methods (spectral clustering and similarity network fusion), a clustering analysis was undertaken to identify disease subphenotypes and to determine whether different patterns of network disruption may be predictive of disease outcome. According to these results, ALS patients can be subgrouped into phenotypes with distinct neurophysiological profiles. They are characterized by varying degrees of disruption in (1) the somatomotor (alpha-band synchrony) network, (2) the frontotemporal (beta-band neural activity and gamma-l-band synchrony), (3) frontoparietal (gamma-l-band co-modulation) network, which reliably correlate with distinct clinical profiles and different patterns of network disturbance. These data demonstrate that neuroelectric analysis can distinguish various disease subtypes based on different patterns of neurophysiological network disturbances, which may reflect the underlying disease neuropathobiology. Advanced network profiling in ALS may enable new therapeutic strategies that are based on neurobiology and designed to modulate network disruption [149]. In a similar way, the source localization of evoked potentials can reliably discriminate patterns of functional network impairment in ALS subgroups with dysfunctions of attention. The discriminating ability of the detected cognitive changes in specific brain regions (in particular, the left inferior frontal, dorsolateral prefrontal, superior temporal, and posterior parietal cortices) is comparable to those of fMRI and thus provides a novel non-invasive quantitative biomarker of network disruption in ALSci patients [150].
In conclusion, neurophysiological methods not only revealed early cognitive and executive dysfunctions in ALS but demonstrated different patterns of neural network disruptions, thus providing novel non-invasive quantitative biomarkers of network disorders related to cognitive disorders in ALS.
7. Neuropathological Findings
ALS is histologically characterized by large variety of cytoplasmic inclusions in both neuronal and glial cells positive for transactivation response DNA-binding protein 43 kDa (TDP-43); this is a key feature for up to 97% of ALS cases [15,16], whereas others show FUS and/or tau reactivity (see [151,152]). TDP-43 and FUS as the defining pathological hallmarks in ALS, coupled with ALS-causing mutations of both genes, indicate that their dysfunction damages the motor system and cognition [153]. In sALS, a model of the staging of TDP-43 has been proposed: the involvement of the agranular motor cortex and alpha-motor neurons of the brainstem and spinal cord (stage 1), in the prefrontal neocortex (middle frontal gyrus), reticular formation and the precerebellar nuclei (stage 2), in further areas of the prefrontal neocortex (gyrus rectus, orbitofrontal gyri), postcentral sensory cortex and basal ganglia (stage 3), and in anteromedial temporal lobe including the hippocampus (stage 4). Based on this staging, a corticofugal model for a “prion-like” spreading of TDP-43 pathology was hypothesized suggesting that it starts from the primary motor cortex and spreads from there via axonal projections in a spatiotemporal manner to lower motor neurons and to subcortical structures [154,155,156,157]. TDP-43 is self-templating and -propagating across the brain, due to the presence of a glycine-rich C-terminal domain that is prone to aggregation and misfolding; this induces the formation of TDP-43 oligomers and invariably reduces cell viability [158,159]. Moreover, TDP-43 can transport intracellularly in an anterograde and retrograde fashion, supporting the region-to-region spread of TDP-43 pathology [160].
From the clinical standpoint, ALS patients affected by extramotor deficits including cognitive disturbances seem to correspond to the pathological involvement of the relevant cerebral structures [161]. Loss of TDP-43 from the nucleus and the subsequent aggregation in the cytoplasm, occurring in susceptible regions may be associated with neuronal loss, although this may also occur without TDP-43 accumulation. Therefore, it remains controversial whether the stepwise appearance of TDP-43-positive inclusions is based on direct cell-to-cell propagation [152]. In C9orf72 repeat mutations, the most common genetic impairment causal to ALS, early cortical network dysfunction is due to impaired synaptic plasticity, attributable to impaired pre-synaptic vesicle dynamics, which directly impact cortical function [162].
Under the assumption of a single common progression pattern, a recent staging of TDP-43 proteinopathies using SuStaIn classification was proposed. The SuStaIn stage can be thought of as a proxy for progression along a pathological trajectory [163,164]. SuStaIn estimated that TDP-43 deposition in ALS began in the spinal cord, before progressing to the medulla and motor cortex (stages 2–5). Subsequently, in stages 6–13, there was TDP-43 deposition in the caudate/putamen, thalamus, globus pallidus, midbrain, substantia nigra, pons and anterior cingulate cortex. In stages 14–15, it progressed to the middle frontal and angular gyri, and by stage 21, TDP-43 was found in all regions except the cerebellum, including the medial temporal lobe [165]. In addition, ALS subtypes were assessed for differences in demographic, pathological and genetic variables. Two confounding factors significantly discriminated between two subtypes: ALS subtype 1 (subcortical predominant) has the tendency to have less overall brain pathology (e.g., lower SuStaIn stage) compared with ALS stage 2 (corticolimbic predominant), with the onset of motor cortex pathology. SuStaIn inferred the spinal cord as the first susceptible region for both ALS subtypes [165]. Studies to identify molecular targets in cognitively affected and unaffected brain regions in ALS patients, using sensitive mRNA sequencing, identified 50 significantly dysregulated genes that are distinct between these brain regions. Using in situ hybridization and macromolecular complex regulation, notably, NLRP3 inflammasome modulation was found as a potential pathological correlate of cognitive resilience in ALS [166].
Several studies showed that TDP-43 pathologic burden in ALS is associated with CI, though no association with disease duration or rate of progression was seen [62,167]. However, TDP-43-positive neuronal and glial inclusions were more numerous in ALSci than in classical ALS [168], and a close relationship between cognition and the extent of TDP-43 pathology was seen in non-primary motor areas [169]. ALS patients with impaired EFs showed TPD-43 pathology as well as significant neuronal loss and significant microglial activation in the middle frontal and superior or middle temporal gyrus [101]. TDP-43 burden in the limbic system (amygdala, dentate nucleus, CA1 sector of the hippocampus, subiculum, and entorhinal cortex) was greater in older than in younger ALS patients, indicating that the amygdala and hippocampus are vulnerable to TDP-43 pathology in older ALS patients [170]. Bulbar ALS cases showed severe neuronal loss and TDP-43 pathology across most speech regions, inducing a link between the severity of bulbar ALS and speech network damage, whereas spinal onset ALS cases showed no neuronal loss but mild TDP-43 pathology in focal regions, and cases without ante mortem bulbar ALS demonstrated an absence of pathology [171].
Other pathology studies showed that all ALSci patients had TDP-43 pathology in extramotor brain regions, associated with disorders of executive, language and fluency domains, although there was a subgroup with no cognitive dysfunction, despite having substantial TDP-43 pathology [27]. Postmortem studies in a large cohort of ALS cases, 38.5% of which had cognitive decline, revealed TDP-43 pathology and hippocampal sclerosis. Mutation carriers presented a higher burden of TDP-43 pathology than sporadic cases. Moreover, 89% presented some degree of concomitant pathologies, which were associated with older age at death, indicating that other findings can influence cognitive status, particularly in older age groups [172], although no cognitive burden from vascular risk factors was found in patients with ALS [173].
8. Cognitive Reserve in ALS
Long-time life experiences, such as education, occupational attainment, leisure activities, and bilingualism, have been considered proxies of cognitive reserve (CR). In neurodegenerative diseases, CR is considered a modulator of more favorable cognitive trajectory and motor functions. Cross-sectional studies and longitudinal assessment in cohorts of ALS patients measured CR by combining education, occupational attainment, amount of leisure activation, physical activity, etc. Jobs requiring greater reasoning abilities, social skills and humanities knowledge were related to preserved cognitive functioning consistent with CR [174]. It was hypothesized that higher CR would correlate with better performance on cognitive test batteries. The analyses provided moderate to strong evidence that higher CR was associated with a mild increase in performance with regard to verbal fluency functions, working memory, verbal learning and recognition, and visuoconstructive ability. These results indicated that CR moderates the effects of brain morphology on cognition in ALS. Executive functions presented a dissociation: while verbally assessed functions benefit from CR, non-verbally assessed functions did not [175]. Thus, CR was a significant predictor of baseline neuropsychological performance, with high-CR individuals performing better than those with medium or low CR. Better cognitive performance in high-CR individuals was maintained longitudinally for social cognition, EF or confrontational naming. High-CR patients displayed little cognitive decline over the course of follow-up, which suggests that CR plays a role in the presentation of CI at diagnosis but is not protective against cognitive decline [176]. However, according to others, there is evidence that CR protected letter fluency from further decline [177]. Another study documented the association between CR and cognitive performance in all patients and the predictive role of CR in modulating the extent of cognitive decline and functional bulbar impairment, suggesting that the concept of reserve applied to ALS should encompass cognitive but also motor domains [56].
Usually, there is an association between the pathological burden of TDP-43 misfolding and CI in ALS, demonstrating high specificity, but correspondingly low sensitivity characterizes a subset of individuals with no evidence of cognitive deficits despite the high burden of TDP-43 pathology—called “mismatch” cases. These cases have demonstrated predominantly the expression of clusterin, a topologically dynamic chaperone protein with the ability to participate in both intra- and extracellular proteostasis. Mismatch cases showed a differential spatial expression of clusterin, with a predominantly neuronal pattern, compared to cases with a cognitive manifestation of their TDP-43 pathology, which demonstrated a predominantly glial distribution. These data indicate that in individuals with TDP-43 pathology, a predominantly neuronal expression of clusterin in extramotor brain regions may indicate a cell protective mechanism, delaying the clinical consequences of TDP-43 pathology such as cognitive dysfunction [178].
Another key molecular target underlying cognitive resilience in ALS is NLRP3 inflammasome modulation, which might be a potential therapeutically targetable correlate of cognitive resilience in ALS [166]. However, future research is needed to examine the interaction between CR and other objective correlates of CI in ALS.
9. Experimental Models of ALSci
Experimental animal models are intended to provide information about the mechanisms and the brain regions that are involved in the alterations occurring in CI in ALS.
Dominant missense mutations in TDP-43 cause up to 95% of ALS, and the cytoplasmic accumulation of TDP-43 is a pathological hallmark in ALS. Investigations of the transgenic (tg) mouse model expressing the disease-causing human TDP-43 M337V mutant have shown robust motor and cognitive deficits in hemizygous mice by 8 months of age. After 12 months, cortical neurons were significantly affected by cytoplasmic TDP-43 mislocation, mitochondrial dysfunction, and neuronal loss. Compared with age-matched non-tg mice, TDP-43 M337V mice showed a similar expression of TDP-43 but higher levels in mitochondria, while an inhibitory peptide abolished cytoplasmic TDP-43 accumulation, restored mitochondrial function, prevented neuronal loss and alleviated both motor and cognitive deficits [179]. A tg mouse model overexpressing human wild-type TDP-43 protein showed a rapid development in cognitive/social deficits involving working and recognition memory in the absence of overt motor abnormalities [180]. In a mouse line expressing an ALS-linked mutation in TDP-43 (Q331K), motor and cognitive deficits were observed at 3 months of age, persisting through 12 months of age. Among the cognitive modalities, the hippocampus-mediated spatial learning and memory were normal, whereas the frontal-mediated working memory and cognitive flexibility were impaired. Q331K TDP-43 was largely retained at the nucleus without apparent aggregates, which was comparable with the physiological level of human TDP-43 protein in cerebral cortex and hippocampus. These data suggest that the motor and frontal cortex are more vulnerable to disease-linked mutation in TDP-43 [181]. A recently developed C9orf72 BAC tg mouse model showed the widespread loss of H3K9me3 in astrocytes and neurons in the spinal cord, motor cortex, and hippocampus, associated with hippocampal-dependent CI [182].
SOD1 mice, a model for fALS, showed a reduction in dendritic length and branch nodes of basal dendrites in the pre/infralimbic medial prefrontal cortex neurons, indicating abnormal prefrontal connectivity and function before the onset of motor disturbances [183], while another SOD1 mouse model showed CI due to disordered cortico-striatal and hippocampal synaptic plasticity, dendritic branching and glutamate receptor function [184]. A mouse model expressing wild-type human FUS (found in sALS carrying FUS mutations) developed hippocampus-mediated cognitive deficits, accompanied by an age-dependent reduction in spine density and long-term potentiation in their hippocampi. However, there were no definite FUS aggregates or cytosolic FUS accumulation. It was found that FUS directly binds to Sema5a (a gene involved in axon guidance and spine dynamics) mRNA and regulates Sema5a expression in a FUS-dependent manner, suggesting that FUS-driven Sema5a deregulation may be related to cognitive deficits in FUS tg mice [185]. Another mouse model expressing an ALS-linked human FUS mutation (R514G-FUS) developed cognitive deficits accompanied by a reduction in spine density and long-term potentiation within the hippocampus, accompanied by a disruption in protein homeostasis and mitochondrial functions, a widespread reduction in cortical volumes, and enhanced FC between the hippocampus, basal ganglia and neocortex. These findings suggest that disease-linked mutation in FUS may lead to changes in proteostasis and mitochondrial dysfunction that affect brain structure and connectivity, resulting in cognitive deficits [186].
In conclusion, various tg animal models of ALS have provided insight into the heterogeneity of brain lesions associated with cognitive dysfunction, into and the role of gene mutations in the development of cognitive disorders in ALS.
10. Putative Pathogenic Mechanisms
ALS is a multi-system neurodegenerative disorder with both clinical and biological heterogeneity for which the dysregulation of several pathogenic mechanisms related to TDP-43 pathology has been postulated, including disorders of protein homeostasis, RNA processing, nuclear and axonal transport, energy metabolism, dysfunctional immune regulation, oxidative stress, excitotoxicity, endosomal and vesicular transport impairment, autophagy, neuroinflammation involving glial cells and circulating immune cells, and mitochondrial dysfunction [16,23,151,187,188,189,190,191]. On the other hand, evidence for the primary dysfunction of mitochondrial oxidative phosphorylation was detected and provided in vivo evidence for bioenergetic dysfunction in ALS brains as a central pathogenic factor [190]. Disease-linked mutations in FUS may lead to changes in proteostasis and mitochondrial dysfunction that in turn affect brain structures and connectivity, leading to cognitive deficits [186]. The molecular background of protein aggregation in the pathogenesis of ALS was reviewed recently [192]. In addition to oxidative stress [193], there is recent evidence that the endoplasmic reticulum stress pathway is an essential pathological mechanism in ALS [194].
Electron microscopy analyses of ALS patient brain samples revealed prominent mitochondrial impairment, confirming that increased TDP-43 expression induced mitochondrial dysfunction and elevated the production of reactive oxygen species. TDP-43 expression depressed mitochondrial complex I activity, reduced mitochondrial ATP synthesis, and activated the mitochondrial unfolded protein response, though it down-regulated mitochondrial protein, increased mitochondrial TDP-43 levels, and exacerbated mitochondrial damage as well as neurodegeneration. These data demonstrated that TDP-43-induced mitochondrial impairment is a critical pathogenic factor in ALS and other TPD-43 proteinopathies [195].
There is growing evidence for a relationship between TDP-43 and glial pathology, indicating pathogenic glial cell-autonomous dysfunction and dysregulated glial immune responses to neuronal TDP-43 [196]. Serum levels of glial fibrillary acidic protein provide neurochemical evidence of astrocyte involvement in ALS pathophysiology, particularly in the development of CI [197]. However, the relationship between TDP-43 and neurodegeneration is not absolute, and other pathophysiological processes, such as neuroinflammation (with the prominent role of microglia, cortical hyperexcitability, and synaptic dysfunction), play a central role in ALS pathophysiology [189]. There are links between the C9orf72 repeat expansion and neuroinflammatory signatures that exist across genetic and sporadic ALS cohorts [198]. Cortical hyperexcitability, probably associated with TDP-43 accumulation, was more prominent in cognitively impaired ALS [199].
Increasing evidence suggests that synaptic dysfunction is a central and possibly triggering factor in ALSci [17,18,200,201,202]. Cognitively impaired ALS cases showed a relation between TDP-43 pathology and synapse loss in the frontal cortex, which was not due to cortical atrophy nor associated with other dementia-related neuropathologies, indicating that synapse loss in the prefrontal cortex represents a neurobiological substrate for cognitive decline in ALS [200]. The impaired clearance of TDP-43 and synaptic alterations may induce neuronal dysfunction related to TDP-43 pathology [203]. In addition to TDP-43 mislocation or aggregation as a hallmark of ALS, there is evidence for alterations in the metabolism of the microtubule-associated protein tau, which is characterized by pathological phosphorylation at residue threonine 175, and the formation of cytoplasmic inclusions. Immunohistochemical studies using polyclonal phospho-tau antibodies and antibodies directed against PHF tau detected tau-positive intraneuronal and neuritic aggregates throughout the amygdala, the entorhinal, anterior cingulate and superior frontal cortices, and the substantia nigra, as well as immunoreactive tufted astrocytes in the same regions in ALSci brains. The extent of pathological tau inclusions showed no correlation with that of TDP-43 pathology, and nuclear TDP-43 immunoreactivity was absent in neurons with tau pathology. These findings indicate that the ALSci brain is characterized by the co-occurrence of TDP-43 and tau pathology [204]. Other studies corroborated this hypothesis—for example, increased TDP-43 accumulation and lethality were observed in an in vivo model co-expressing both proteins [205]. The same group also demonstrated that low levels of TDP-43 already promote neurotoxicity, leading to selective neurodegeneration [206]. These data indicate that TDP-43 may promote tau aggregation [207,208]. The interaction between both proteins suggests that they share pathomechanistic events that exert a synergistic role in ALSci. A potential role of TDP-43 acting synergistically with pathological tau metabolism has been proposed [209] and demonstrated in a rodent model [210].
On the molecular level, TDP-43 and FUS participate in the biogenesis and metabolism of coding RNAs and in the transport and translation of mRNA as part of cytoplasmic mRNA-ribonuclein, many of which are involved in synaptic transmission and plasticity, indicating that synaptic dysfunction could be an early process contributing to motor and cognitive deficits in ALS [153]. In vivo studies of synaptic density as a possible biomarker with 18F-SynVesT-1 PET also showed reduced synaptic density in the bilateral superior temporal gyrus, hippocampus-insula, anterior cingulate, and left inferior frontal gyrus in ALSci patients compared to healthy controls, which could be a potentially useful biomarker for ALS and for estimating cognitive decline [211].
Synaptic proteomics experiments found more than 30 ALS-associated proteins in synaptoneurosomes, including TDP-43, FUS, SOD1 and C9orf72. Stratifying the ALS cohort by cognitive status revealed almost 500 specific alterations in cognitively impaired ALS synaptic preparations, thus generating an unbiased map of the human ALS synaptic proteome, which highlighted the influence of cognitive decline and C9orf72-repeat expansion on synaptic composition [212].
In conclusion, a complex multi-step process of events associated with TDP-43 and other mechanisms is engaged in the development of motor and cognitive disorders in ALS. Among these, besides other alterations, mitochondrial and synaptic impairment and proteasomic dysfunction represent the main mechanisms underlying ALS etiopathogenesis. Functional and structural events probably represent important mechanisms underlying an adaptive capability, causing the partial and transient resiliency of the CNS affected by neurodegeneration, while on the other hand, the failure of synaptic functions and plasticity are part of an early pathogenic process in ALS responsible for cognitive dysfunction [213].
11. Conclusions and Outlook
ALS is a multi-system neurodegenerative disorder with clinical and biological heterogeneity that is accompanied by a wide spectrum of cognitive decline, covering various domains. The pathogenesis of ALS and the accompanying cognitive dysfunctions include heterogeneous mechanisms, dominated by the mislocation and aggregation of TDP-43, which is associated with a wide variety of mechanisms, including disorders of proteostasis, mitochondrial dysfunction, oxidative stress, neuroinflammation and immune dysregulation. This leads to the neurodegeneration of essential cerebral systems, with synaptic dysfunction as a central and triggering factor of CI in ALS and related FTLD. Modern neuroimaging techniques revealed the widespread degeneration of fronto-limbic systems with the microdestruction of GM and WM systems, hypometabolism in frontotemporal regions, and the disruption of frontoparietal, frontostriatal and other networks as essential markers of cognitive deficits. Neuropathology confirmed the central role of TDP-43 burden, related neurodegeneration and synapse loss in frontal and other cognition-relevant cerebral regions. ALS is characterized by the dysfunction of specific neuronal networks and alterations of synaptic connectivity, contributing to neuronal degeneration, which leads to the impairment of motor and cognitive functions [214]. The role of cognitive reserve has become the focus of modern neuroscience and of the discussion of the diagnostic and prognostic value of modern biomarkers in ALS and their potential to reveal insights into the pathophysiology of CI in ALS. Emerging imaging biomarkers of extramotor neurodegeneration that enable the monitoring of disease progression are particularly promising. In addition, a growing arsenal of biofluid biomarkers linked to neurodegeneration and neuroinflammation as well as to synapse dysfunction and neuronal demise are important for improving the diagnostic accuracy and identification of patients with a faster progression of both motor and cognitive dysfunctions.
Neurofilaments, indicating ongoing neuronal or axonal injury, are one of the most promising biomarkers of ALS, although their role in the pathophysiology of ALS is unclear [215]. A significant elevation of the neurofilament light chain (NfL) in cerebrospinal fluid (CSF) and serum was found in ALS patients, compared to healthy controls. Increased values were found particularly in ALS patients with known mutations but have also been applied to sALS patients and may shorten the diagnostic delay by up to 3 months [20]. ALS is characterized by increased CSF and serum levels of NfL, a marker of neuroaxonal degeneration [216,217] that may be associated with CI [218]. On the other hand, the finding of moderate serum NfL elevation in patients with a long ALS duration underlined its value as a progression marker [219]. Sensitivity and specificity values of NfL in CSF have been reviewed recently [220]. Since inflammatory processes involving astroglia and peripheral immune cells are also characteristic in the pathophysiology of ALS, proinflammatory biomarkers may provide information on the disease stage, progression rate and pathophysiological mechanisms [220].
The development and validation of biomarkers that detect the pathogenic aggregates of TDP-43 and related pathological proteins in vivo are notably expected to further elucidate the pathophysiological mechanisms underlying the basic disorder and the ensuing cognitive decline. Novel biomarkers tracking the different aspects of ALS pathophysiology and the mechanisms of related CI are notably expected to further elucidate the different aspects of ALS pathogenesis and to pave the way to precision medicine approaches in the ALSci continuum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241914647/s1 [221,222].
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Acknowledgments
The author thanks E. Mitter-Ferstl for secretarial and editorial work.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| ALSci | amyotrophic lateral sclerosis with cognitive impairment |
| CI | cognitive impairment |
| CR | cognitive reserve |
| CSF | cerebrospinal fluid |
| DMN | default mode network |
| EF | executive function |
| ERP | event-related potentials |
| FA | fractional anisotropy |
| fALS | familial ALS |
| FTLD | frontotemporal lobe degeneration |
| GM | gray matter |
| MCI | mild cognitive impairment |
| rCBF | regional cerebral blood flow |
| ReHo | regional homogeneity |
| RSN | resting state network |
| sALS | sporadic ALS |
| SMN | sensorimotor network |
| SN | salience network |
| tg | transgenic |
References
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Berry, J.D.; Blanchard, M.; Bonar, K.; Drane, E.; Murton, M.; Ploug, U.; Ricchetti-Masterson, K.; Savic, N.; Worthington, E.; Heiman-Patterson, T. Epidemiology and economic burden of amyotrophic lateral sclerosis in the United States: A literature review. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 436–448. [Google Scholar] [CrossRef]
- Logroscino, G.; Piccininni, M. Amyotrophic lateral sclerosis descriptive epidemiology: The origin of geographic difference. Neuroepidemiology 2019, 52, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.; Boumédiene, F.; Logroscino, G.; Couratier, P.; Babron, M.C.; Leutenegger, A.L.; Copetti, M.; Preux, P.M.; Beghi, E. Variation in worldwide incidence of amyotrophic lateral sclerosis: A meta-analysis. Int. J. Epidemiol. 2017, 46, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2020, 267, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Hop, P.J.; Zwamborn, R.A.J.; Hannon, E.; Shireby, G.L.; Nabais, M.F.; Walker, E.M.; van Rheenen, W.; van Vugt, J.; Dekker, A.M.; Westeneng, H.J.; et al. Genome-wide study of DNA methylation shows alterations in metabolic, inflammatory, and cholesterol pathways in ALS. Sci. Transl. Med. 2022, 14, eabj0264. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lin, F.; Ye, C.H.; Huang, H.; Li, X.; Yao, X.; Xu, Y.; Wang, C. Rare, low-frequency and common coding variants of ARHGEF28 gene and their association with sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 2020, 87, 138.e131–138.e136. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.D.; Khan, Y.S. Motor Neuron Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560774/ (accessed on 10 May 2023).
- Suzuki, N.; Nishiyama, A.; Warita, H.; Aoki, M. Genetics of amyotrophic lateral sclerosis: Seeking therapeutic targets in the era of gene therapy. J. Hum. Genet. 2023, 68, 131–152. [Google Scholar] [CrossRef]
- Megat, S.; Mora, N.; Sanogo, J.; Roman, O.; Catanese, A.; Alami, N.O.; Freischmidt, A.; Mingaj, X.; De Calbiac, H.; Muratet, F.; et al. Integrative genetic analysis illuminates ALS heritability and identifies risk genes. Nat. Commun. 2023, 14, 342. [Google Scholar] [CrossRef]
- Placek, K.; Benatar, M.; Wuu, J.; Rampersaud, E.; Hennessy, L.; Van Deerlin, V.M.; Grossman, M.; Irwin, D.J.; Elman, L.; McCluskey, L.; et al. Machine learning suggests polygenic risk for cognitive dysfunction in amyotrophic lateral sclerosis. EMBO Mol. Med. 2021, 13, e12595. [Google Scholar] [CrossRef]
- McHutchison, C.A.; Leighton, D.J.; McIntosh, A.; Cleary, E.; Warner, J.; Porteous, M.; Chandran, S.; Pal, S.; Abrahams, S. Relationship between neuropsychiatric disorders and cognitive and behavioural change in MND. J. Neurol. Neurosurg. Psychiatry 2020, 91, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron. Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Carvalho, M.D.; Swash, M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotroph. Lateral Scler. 2009, 10, 53–57. [Google Scholar] [CrossRef]
- Luan, W.; Wright, A.L.; Brown-Wright, H.; Le, S.; San Gil, R.; Madrid San Martin, L.; Ling, K.; Jafar-Nejad, P.; Rigo, F.; Walker, A.K. Early activation of cellular stress and death pathways caused by cytoplasmic TDP-43 in the rNLS8 mouse model of ALS and FTD. Mol. Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Gurfinkel, Y.; Polain, N.; Lamont, W.; Lyn Rea, S. Molecular mechanisms underlying TDP-43 pathology in cellular and animal models of ALS and FTLD. Int. J. Mol. Sci. 2021, 22, 4705. [Google Scholar] [CrossRef] [PubMed]
- Aousji, O.; Feldengut, S.; Antonucci, S.; Schön, M.; Boeckers, T.M.; Matschke, J.; Mawrin, C.; Ludolph, A.C.; Del Tredici, K.; Roselli, F.; et al. Patterns of synaptic loss in human amyotrophic lateral sclerosis spinal cord: A clinicopathological study. Acta Neuropathol. Commun. 2023, 11, 120. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Bonthron, C.; Waddington, J.; Smith, W.V.; Lopez, M.F.; Burley, S.; Valli, J.; Zhu, F.; Komiyama, N.H.; Smith, C.; et al. Selective vulnerability of tripartite synapses in amyotrophic lateral sclerosis. Acta Neuropathol. 2022, 143, 471–486. [Google Scholar] [CrossRef]
- Rusina, R.; Vandenberghe, R.; Bruffaerts, R. Cognitive and behavioral manifestations in ALS: Beyond motor system involvement. Diagnostics 2021, 11, 624. [Google Scholar] [CrossRef]
- Benatar, M.; Turner, M.R.; Wuu, J. Presymptomatic amyotrophic lateral sclerosis: From characterization to prevention. Curr. Opin. Neurol. 2023, 36, 360–364. [Google Scholar] [CrossRef]
- Corcia, P.; Couratier, P. Cognitive impairment in ALS has been known since the nineteenth century. Neurol. Sci. 2023, 44, 735–736. [Google Scholar] [CrossRef]
- Xu, Z.; Alruwaili, A.R.S.; Henderson, R.D.; McCombe, P.A. Screening for cognitive and behavioural impairment in amyotrophic lateral sclerosis: Frequency of abnormality and effect on survival. J. Neurol. Sci. 2017, 376, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Leiva, D.; Dols-Icardo, O.; Sirisi, S.; Cortés-Vicente, E.; Turon-Sans, J.; de Luna, N.; Blesa, R.; Belbin, O.; Montal, V.; Alcolea, D.; et al. Pathophysiological underpinnings of extra-motor neurodegeneration in amyotrophic lateral sclerosis: New insights from biomarker studies. Front. Neurol. 2022, 12, 750543. [Google Scholar] [CrossRef]
- Beeldman, E.; Raaphorst, J.; Klein Twennaar, M.; Govaarts, R.; Pijnenburg, Y.A.L.; de Haan, R.J.; de Visser, M.; Schmand, B.A. The cognitive profile of behavioural variant FTD and its similarities with ALS: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 995–1002. [Google Scholar] [CrossRef]
- Bersano, E.; Sarnelli, M.F.; Solara, V.; Iazzolino, B.; Peotta, L.; De Marchi, F.; Facchin, A.; Moglia, C.; Canosa, A.; Calvo, A.; et al. Decline of cognitive and behavioral functions in amyotrophic lateral sclerosis: A longitudinal study. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ceslis, A.; Argall, R.; Henderson, R.D.; McCombe, P.A.; Robinson, G.A. The spectrum of language impairments in amyotrophic lateral sclerosis. Cortex 2020, 132, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.M.; McDade, K.; Bak, T.H.; Pal, S.; Chandran, S.; Smith, C.; Abrahams, S. Executive, language and fluency dysfunction are markers of localised TDP-43 cerebral pathology in non-demented ALS. J. Neurol. Neurosurg. Psychiatry 2020, 91, 149–157. [Google Scholar] [CrossRef] [PubMed]
- McMillan, C.T.; Wuu, J.; Rascovsky, K.; Cosentino, S.; Grossman, M.; Elman, L.; Quinn, C.; Rosario, L.; Stark, J.H.; Granit, V.; et al. Defining cognitive impairment in amyotrophic lateral sclerosis: An evaluation of empirical approaches. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 517–526. [Google Scholar] [CrossRef]
- Rabkin, J.; Goetz, R.; Murphy, J.M.; Factor-Litvak, P.; Mitsumoto, H. Cognitive impairment, behavioral impairment, depression, and wish to die in an ALS cohort. Neurology 2016, 87, 1320–1328. [Google Scholar] [CrossRef]
- Rippon, G.A.; Scarmeas, N.; Gordon, P.H.; Murphy, P.L.; Albert, S.M.; Mitsumoto, H.; Marder, K.; Rowland, L.P.; Stern, Y. An observational study of cognitive impairment in amyotrophic lateral sclerosis. Arch. Neurol. 2006, 63, 345–352. [Google Scholar] [CrossRef]
- Ye, S.; Jin, P.P.; Zhang, N.; Wu, H.B.; Shi, L.; Zhao, Q.; Yang, K.; Yuan, H.S.; Fan, D.S. Cortical thickness and cognitive impairment in patients with amyotrophic lateral sclerosis. Beijing Da Xue Xue Bao Yi Xue Ban 2022, 54, 1158–1162. [Google Scholar]
- Huynh, W.; Ahmed, R.; Mahoney, C.J.; Nguyen, C.; Tu, S.; Caga, J.; Loh, P.; Lin, C.S.; Kiernan, M.C. The impact of cognitive and behavioral impairment in amyotrophic lateral sclerosis. Expert Rev. Neurother. 2020, 20, 281–293. [Google Scholar] [CrossRef]
- Watanabe, Y.; Raaphorst, J.; Izumi, Y.; Yoshino, H.; Ito, S.; Adachi, T.; Takigawa, H.; Masuda, M.; Atsuta, N.; Adachi, Y.; et al. Cognitive and behavioral status in Japanese ALS patients: A multicenter study. J. Neurol. 2020, 267, 1321–1330. [Google Scholar] [CrossRef]
- Ringholz, G.M.; Appel, S.H.; Bradshaw, M.; Cooke, N.A.; Mosnik, D.M.; Schulz, P.E. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005, 65, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Phukan, J.; Elamin, M.; Bede, P.; Jordan, N.; Gallagher, L.; Byrne, S.; Lynch, C.; Pender, N.; Hardiman, O. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. J. Neurol. Neurosurg. Psychiatry 2012, 83, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhao, Q.; Liu, P.; Yuan, Y.; Liu, Z.; Hu, Y.; Li, W.; Hou, X.; Tang, X.; Jiao, B.; et al. ALS-plus related clinical and genetic study from China. Neurol. Sci. 2023, 44, 3557–3566. [Google Scholar] [CrossRef]
- Maier, P.M.; Iggena, D.; Meyer, T.; Finke, C.; Ploner, C.J. Memory-guided navigation in amyotrophic lateral sclerosis. J. Neurol. 2023, 270, 4031–4040. [Google Scholar] [CrossRef]
- Wheaton, M.W.; Salamone, A.R.; Mosnik, D.M.; McDonald, R.O.; Appel, S.H.; Schmolck, H.I.; Ringholz, G.M.; Schulz, P.E. Cognitive impairment in familial ALS. Neurology 2007, 69, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Beeldman, E.; Raaphorst, J.; Klein Twennaar, M.; de Visser, M.; Schmand, B.A.; de Haan, R.J. The cognitive profile of ALS: A systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry 2016, 87, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Nakamura, T.; Suzuki, M.; Ueda, M.; Hirayama, M.; Katsuno, M. Impaired pain processing and its association with attention disturbance in patients with amyotrophic lateral sclerosis. Neurol. Sci. 2021, 42, 3327–3335. [Google Scholar] [CrossRef]
- Oh, S.I.; Park, A.; Kim, H.J.; Oh, K.W.; Choi, H.; Kwon, M.J.; Ki, C.S.; Kim, H.T.; Kim, S.H. Spectrum of cognitive impairment in Korean ALS patients without known genetic mutations. PLoS ONE 2014, 9, e87163. [Google Scholar] [CrossRef]
- Panopoulou, N.; Christidi, F.; Kourtesis, P.; Ferentinos, P.; Karampetsou, P.; Tsirtsiridis, G.; Theodosiou, T.; Xirou, S.; Zouvelou, V.; Evdokimidis, I.; et al. The association of theory of mind with language and visuospatial abilities in amyotrophic lateral sclerosis: A pilot study. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Sampson, J.B. Cognitive profile of C9orf72 in frontotemporal dementia and amyotrophic lateral sclerosis. Curr. Neurol. Neurosci. Rep. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Paulus, K.S.; Magnano, I.; Piras, M.R.; Solinas, M.A.; Solinas, G.; Sau, G.F.; Aiello, I. Visual and auditory event-related potentials in sporadic amyotrophic lateral sclerosis. Clin. Neurophysiol. 2002, 113, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Machts, J.; Bittner, V.; Kasper, E.; Schuster, C.; Prudlo, J.; Abdulla, S.; Kollewe, K.; Petri, S.; Dengler, R.; Heinze, H.J.; et al. Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: A comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neurosci. 2014, 15, 83. [Google Scholar] [CrossRef]
- Schreiber, H.; Gaigalat, T.; Wiedemuth-Catrinescu, U.; Graf, M.; Uttner, I.; Muche, R.; Ludolph, A.C. Cognitive function in bulbar- and spinal-onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J. Neurol. 2005, 252, 772–781. [Google Scholar] [CrossRef]
- Abrahams, S.; Leigh, P.N.; Goldstein, L.H. Cognitive change in ALS: A prospective study. Neurology 2005, 64, 1222–1226. [Google Scholar] [CrossRef]
- Finsel, J.; Uttner, I.; Vázquez Medrano, C.R.; Ludolph, A.C.; Lulé, D. Cognition in the course of ALS—A meta-analysis. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 2–13. [Google Scholar] [CrossRef]
- Pinto-Grau, M.; Donohoe, B.; O’Connor, S.; Murphy, L.; Costello, E.; Heverin, M.; Vajda, A.; Hardiman, O.; Pender, N. Patterns of language impairment in early amyotrophic lateral sclerosis. Neurol. Clin. Pract. 2021, 11, e634–e644. [Google Scholar] [CrossRef]
- Solca, F.; Aiello, E.N.; Torre, S.; Carelli, L.; Ferrucci, R.; Verde, F.; Ticozzi, N.; Silani, V.; Monti, A.; Poletti, B. Prevalence and determinants of language impairment in non-demented amyotrophic lateral sclerosis patients. Eur. J. Neurol. 2023, 30, 606–611. [Google Scholar] [CrossRef]
- Sakurai, T.; Hirano, S.; Abe, M.; Uji, Y.; Shimizu, K.; Suzuki, M.; Nakano, Y.; Ishikawa, A.; Kojima, K.; Shibuya, K.; et al. Dysfunction of the left angular gyrus may be associated with writing errors in ALS. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 267–275. [Google Scholar] [CrossRef]
- De Marchi, F.; Carrarini, C.; De Martino, A.; Diamanti, L.; Fasano, A.; Lupica, A.; Russo, M.; Salemme, S.; Spinelli, E.G.; Bombaci, A. Cognitive dysfunction in amyotrophic lateral sclerosis: Can we predict it? Neurol. Sci. 2021, 42, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Saxon, J.A.; Thompson, J.C.; Harris, J.M.; Richardson, A.M.; Langheinrich, T.; Rollinson, S.; Pickering-Brown, S.; Chaouch, A.; Ealing, J.; Hamdalla, H.; et al. Cognition and behaviour in frontotemporal dementia with and without amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.; Lippa, C.F.; Swearer, J.M. Cognition and amyotrophic lateral sclerosis (ALS). Am. J. Alzheimers Dis. Other Demen. 2007, 22, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Consonni, M.; Dalla Bella, E.; Bersano, E.; Lauria, G. Cognitive and behavioural impairment in amyotrophic lateral sclerosis: A landmark of the disease? A mini review of longitudinal studies. Neurosci. Lett 2021, 754, 135898. [Google Scholar] [CrossRef] [PubMed]
- Consonni, M.; Dalla Bella, E.; Bersano, E.; Telesca, A.; Lauria, G. Cognitive reserve is associated with altered clinical expression in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 237–247. [Google Scholar] [CrossRef]
- Crockford, C.; Newton, J.; Lonergan, K.; Chiwera, T.; Booth, T.; Chandran, S.; Colville, S.; Heverin, M.; Mays, I.; Pal, S.; et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 2018, 91, e1370–e1380. [Google Scholar] [CrossRef]
- Dubbioso, R.; Spisto, M.; Hausdorff, J.M.; Aceto, G.; Iuzzolino, V.V.; Senerchia, G.; De Marco, S.; Marcuccio, L.; Femiano, C.; Iodice, R.; et al. Cognitive impairment is associated with gait variability and fall risk in amyotrophic lateral sclerosis. Eur. J. Neurol. 2023, 30, 3056–3067. [Google Scholar] [CrossRef]
- Murphy, J.; Factor-Litvak, P.; Goetz, R.; Lomen-Hoerth, C.; Nagy, P.L.; Hupf, J.; Singleton, J.; Woolley, S.; Andrews, H.; Heitzman, D.; et al. Cognitive-behavioral screening reveals prevalent impairment in a large multicenter ALS cohort. Neurology 2016, 86, 813–820. [Google Scholar] [CrossRef]
- Elamin, M.; Bede, P.; Byrne, S.; Jordan, N.; Gallagher, L.; Wynne, B.; O’Brien, C.; Phukan, J.; Lynch, C.; Pender, N.; et al. Cognitive changes predict functional decline in ALS: A population-based longitudinal study. Neurology 2013, 80, 1590–1597. [Google Scholar] [CrossRef]
- Wei, Q.Q.; Ou, R.; Cao, B.; Chen, Y.; Hou, Y.; Zhang, L.; Wu, F.; Shang, H. Early weight instability is associated with cognitive decline and poor survival in amyotrophic lateral sclerosis. Brain Res. Bull. 2021, 171, 10–15. [Google Scholar] [CrossRef]
- Ye, S.; Jin, P.; Chen, L.; Zhang, N.; Fan, D. Prognosis of amyotrophic lateral sclerosis with cognitive and behavioural changes based on a sixty-month longitudinal follow-up. PLoS ONE 2021, 16, e0253279. [Google Scholar] [CrossRef]
- Stojkovic, T.; Stefanova, E.; Pekmezovic, T.; Peric, S.; Stevic, Z. Executive dysfunction and survival in patients with amyotrophic lateral sclerosis: Preliminary report from a Serbian centre for motor neuron disease. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 543–547. [Google Scholar] [CrossRef]
- Manera, U.; Peotta, L.; Iazzolino, B.; Canosa, A.; Vasta, R.; Palumbo, F.; Torrieri, M.C.; Solero, L.; Daviddi, M.; Grassano, M.; et al. The characteristics of cognitive impairment in als patients depend on the lateralization of motor damage. Brain Sci. 2020, 10, 650. [Google Scholar] [CrossRef]
- Aiello, E.N.; Pain, D.; Radici, A.; Aktipi, K.M.; Sideri, R.; Appollonio, I.; Mora, G. Cognition and motor phenotypes in ALS: A retrospective study. Neurol. Sci. 2022, 43, 5397–5402. [Google Scholar] [CrossRef] [PubMed]
- Sterling, L.E.; Jawaid, A.; Salamone, A.R.; Murthy, S.B.; Mosnik, D.M.; McDowell, E.; Wheaton, M.; Strutt, A.M.; Simpson, E.; Appel, S.; et al. Association between dysarthria and cognitive impairment in ALS: A prospective study. Amyotroph. Lateral Scler. 2010, 11, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Schrempf, T.; Finsel, J.; Uttner, I.; Ludolph, A.C.; Lulé, D. Neuropsychological deficits have only limited impact on psychological well-being in amyotrophic lateral sclerosis. J. Neurol. 2022, 269, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Elamin, M.; Bede, P.; Shatunov, A.; Walsh, C.; Corr, B.; Heverin, M.; Jordan, N.; Kenna, K.; Lynch, C.; et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: A population-based cohort study. Lancet Neurol. 2012, 11, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; Vasta, R.; Brunetti, M.; Barberis, M.; Corrado, L.; D’Alfonso, S.; Bersano, E.; et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019, 93, e984–e994. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Poletti, B.; Maranzano, A.; Peverelli, S.; Solca, F.; Colombrita, C.; Torre, S.; Tiloca, C.; Verde, F.; Bonetti, R.; et al. Motor, cognitive and behavioural profiles of C9orf72 expansion-related amyotrophic lateral sclerosis. J. Neurol. 2023, 270, 898–908. [Google Scholar] [CrossRef]
- Iazzolino, B.; Peotta, L.; Zucchetti, J.P.; Canosa, A.; Manera, U.; Vasta, R.; Grassano, M.; Palumbo, F.; Brunetti, M.; Barberis, M.; et al. Differential neuropsychological profile of patients with amyotrophic lateral sclerosis with and without C9orf72 mutation. Neurology 2021, 96, e141–e152. [Google Scholar] [CrossRef]
- Irwin, D.J.; McMillan, C.T.; Brettschneider, J.; Libon, D.J.; Powers, J.; Rascovsky, K.; Toledo, J.B.; Boller, A.; Bekisz, J.; Chandrasekaran, K.; et al. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Urso, D.; Tortelli, R. The challenge of amyotrophic lateral sclerosis descriptive epidemiology: To estimate low incidence rates across complex phenotypes in different geographic areas. Curr. Opin. Neurol. 2022, 35, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Machts, J.; Loewe, K.; Kaufmann, J.; Jakubiczka, S.; Abdulla, S.; Petri, S.; Dengler, R.; Heinze, H.J.; Vielhaber, S.; Schoenfeld, M.A.; et al. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology 2015, 85, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hou, Y.; Li, C.; Cao, B.; Cheng, Y.; Wei, Q.; Zhang, L.; Shang, H. Risk factors for cognitive impairment in amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 688–693. [Google Scholar] [CrossRef]
- Nishimura, A.L.; Mitne-Neto, M.; Silva, H.C.; Richieri-Costa, A.; Middleton, S.; Cascio, D.; Kok, F.; Oliveira, J.R.; Gillingwater, T.; Webb, J.; et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004, 75, 822–831. [Google Scholar] [CrossRef] [PubMed]
- de Alcântara, C.; Cruzeiro, M.M.; França, M.C., Jr.; Alencar, M.A.; Jaeger, A.; de Araújo, C.M.; da Gama, N.A.S.; Camargos, S.T.; de Souza, L.C. A comparative study of cognitive and behavioral profiles between sporadic and type 8 amyotrophic lateral sclerosis. Muscle Nerve 2023, 68, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Zucchi, E.; Simonini, C.; Gianferrari, G.; Zamboni, G.; Pinti, M.; Mandrioli, J. The landscape of cognitive impairment in superoxide dismutase 1–amyotrophic lateral sclerosis. Neural Regen. Res. 2023, 18, 1427–1433. [Google Scholar] [CrossRef]
- Abe, K.; Fujimura, H.; Toyooka, K.; Sakoda, S.; Yorifuji, S.; Yanagihara, T. Cognitive function in amyotrophic lateral sclerosis. J. Neurol. Sci. 1997, 148, 95–100. [Google Scholar] [CrossRef]
- Tsermentseli, S.; Leigh, P.N.; Goldstein, L.H. The anatomy of cognitive impairment in amyotrophic lateral sclerosis: More than frontal lobe dysfunction. Cortex 2012, 48, 166–182. [Google Scholar] [CrossRef]
- Schuster, C.; Kasper, E.; Machts, J.; Bittner, D.; Kaufmann, J.; Benecke, R.; Teipel, S.; Vielhaber, S.; Prudlo, J. Longitudinal course of cortical thickness decline in amyotrophic lateral sclerosis. J. Neurol. 2014, 261, 1871–1880. [Google Scholar] [CrossRef]
- Verstraete, E.; Veldink, J.H.; Hendrikse, J.; Schelhaas, H.J.; van den Heuvel, M.P.; van den Berg, L.H. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Karavasilis, E.; Riederer, F.; Zalonis, I.; Ferentinos, P.; Velonakis, G.; Xirou, S.; Rentzos, M.; Argiropoulos, G.; Zouvelou, V.; et al. Gray matter and white matter changes in non-demented amyotrophic lateral sclerosis patients with or without cognitive impairment: A combined voxel-based morphometry and tract-based spatial statistics whole-brain analysis. Brain Imaging Behav. 2018, 12, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Consonni, M.; Contarino, V.E.; Catricalà, E.; Dalla Bella, E.; Pensato, V.; Gellera, C.; Lauria, G.; Cappa, S.F. Cortical markers of cognitive syndromes in amyotrophic lateral sclerosis. Neuroimage Clin. 2018, 19, 675–682. [Google Scholar] [CrossRef]
- Menke, R.A.L.; Proudfoot, M.; Talbot, K.; Turner, M.R. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. Neuroimage Clin. 2017, 17, 953–961. [Google Scholar] [CrossRef]
- Benbrika, S.; Desgranges, B.; Eustache, F.; Viader, F. Cognitive, emotional and psychological manifestations in amyotrophic lateral sclerosis at baseline and overtime: A review. Front. Neurosci. 2019, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Lillo, P.; Yew, B.; Hsieh, S.; Savage, S.; Hodges, J.R.; Kiernan, M.C.; Hornberger, M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 2013, 80, 1117–1123. [Google Scholar] [CrossRef]
- Tae, W.S.; Sung, J.H.; Baek, S.H.; Lee, C.N.; Kim, B.J. Shape Analysis of the Subcortical Nuclei in Amyotrophic Lateral Sclerosis without Cognitive Impairment. J. Clin. Neurol. 2020, 16, 592–598. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Bocchetta, M.; Todd, E.G.; Tse, N.Y.; Devenney, E.M.; Tu, S.; Caga, J.; Hodges, J.R.; Halliday, G.M.; Irish, M.; et al. Tackling clinical heterogeneity across the amyotrophic lateral sclerosis-frontotemporal dementia spectrum using a transdiagnostic approach. Brain Commun. 2021, 3, fcab257. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Ren, Q.; Zhang, D.; Shao, K.; Lin, P.; Yuan, Y.; Dai, T.; Zhang, Y.; Li, L.; et al. Amygdala abnormalities across disease stages in patients with sporadic amyotrophic lateral sclerosis. Hum. Brain Mapp. 2022, 43, 5421–5431. [Google Scholar] [CrossRef]
- Tse, N.Y.; Bocchetta, M.; Todd, E.G.; Devenney, E.M.; Tu, S.; Caga, J.; Hodges, J.R.; Halliday, G.M.; Irish, M.; Kiernan, M.C.; et al. Distinct hypothalamic involvement in the amyotrophic lateral sclerosis-frontotemporal dementia spectrum. Neuroimage Clin. 2023, 37, 103281. [Google Scholar] [CrossRef]
- Schuster, C.; Kasper, E.; Dyrba, M.; Machts, J.; Bittner, D.; Kaufmann, J.; Mitchell, A.J.; Benecke, R.; Teipel, S.; Vielhaber, S.; et al. Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.; Olm, C.; McMillan, C.T.; Boller, A.; Irwin, D.J.; McCluskey, L.; Elman, L.; Grossman, M. Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nevler, N.; Ash, S.; McMillan, C.; Elman, L.; McCluskey, L.; Irwin, D.J.; Cho, S.; Liberman, M.; Grossman, M. Automated analysis of natural speech in amyotrophic lateral sclerosis spectrum disorders. Neurology 2020, 95, e1629–e1639. [Google Scholar] [CrossRef]
- Buhour, M.S.; Doidy, F.; Mondou, A.; Pélerin, A.; Carluer, L.; Eustache, F.; Viader, F.; Desgranges, B. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res. 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Karavasilis, E.; Zalonis, I.; Ferentinos, P.; Giavri, Z.; Wilde, E.A.; Xirou, S.; Rentzos, M.; Zouvelou, V.; Velonakis, G.; et al. Memory-related white matter tract integrity in amyotrophic lateral sclerosis: An advanced neuroimaging and neuropsychological study. Neurobiol. Aging 2017, 49, 69–78. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, S.I.; de Leon, M.; Wang, X.; Oh, K.W.; Park, J.S.; Deshpande, A.; Buj, M.; Kim, S.H. Structural explanation of poor prognosis of amyotrophic lateral sclerosis in the non-demented state. Eur. J. Neurol. 2017, 24, 122–129. [Google Scholar] [CrossRef]
- Evans, J.; Olm, C.; McCluskey, L.; Elman, L.; Boller, A.; Moran, E.; Rascovsky, K.; Bisbing, T.; McMillan, C.T.; Grossman, M. Impaired cognitive flexibility in amyotrophic lateral sclerosis. Cogn. Behav. Neurol. 2015, 28, 17–26. [Google Scholar] [CrossRef]
- Bueno, A.P.A.; Pinaya, W.H.L.; Moura, L.M.; Bertoux, M.; Radakovic, R.; Kiernan, M.C.; Teixeira, A.L.; de Souza, L.C.; Hornberger, M.; Sato, J.R. Structural and functional Papez circuit integrity in amyotrophic lateral sclerosis. Brain Imaging Behav. 2018, 12, 1622–1630. [Google Scholar] [CrossRef]
- Tan, H.H.G.; Westeneng, H.J.; Nitert, A.D.; van Veenhuijzen, K.; Meier, J.M.; van der Burgh, H.K.; van Zandvoort, M.J.E.; van Es, M.A.; Veldink, J.H.; van den Berg, L.H. MRI clustering reveals three ALS subtypes with unique neurodegeneration patterns. Ann. Neurol. 2022, 92, 1030–1045. [Google Scholar] [CrossRef]
- Brettschneider, J.; Libon, D.J.; Toledo, J.B.; Xie, S.X.; McCluskey, L.; Elman, L.; Geser, F.; Lee, V.M.; Grossman, M.; Trojanowski, J.Q. Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol. 2012, 123, 395–407. [Google Scholar] [CrossRef]
- Mavridis, I.N.; Pyrgelis, E.S. Nucleus accumbens changes in amyotrophic lateral sclerosis. Am. J. Neurodegener. Dis. 2023, 12, 85–88. [Google Scholar] [PubMed]
- Bede, P.; Elamin, M.; Byrne, S.; McLaughlin, R.L.; Kenna, K.; Vajda, A.; Pender, N.; Bradley, D.G.; Hardiman, O. Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology 2013, 81, 2107–2115. [Google Scholar] [CrossRef]
- Shen, D.C.; Hou, B.; Cui, B.; Li, X.L.; Peng, P.; Tai, H.F.; Zhang, K.; Liu, S.W.; Fu, H.H.; Liu, M.S.; et al. Resting-state functional MRI studies of amyotrophic lateral sclerosis patients with various levels of cognitive impairment. Zhonghua Yi Xue Za Zhi 2018, 98, 2002–2006. [Google Scholar] [PubMed]
- Hu, T.; Hou, Y.; Wei, Q.; Yang, J.; Luo, C.; Chen, Y.; Gong, Q.; Shang, H. Patterns of brain regional functional coherence in cognitive impaired ALS. Int. J. Neurosci. 2020, 130, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kew, J.J.; Goldstein, L.H.; Leigh, P.N.; Abrahams, S.; Cosgrave, N.; Passingham, R.E.; Frackowiak, R.S.; Brooks, D.J. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain 1993, 116, 1399–1423. [Google Scholar] [CrossRef]
- Canosa, A.; Moglia, C.; Manera, U.; Vasta, R.; Torrieri, M.C.; Arena, V.; D’Ovidio, F.; Palumbo, F.; Zucchetti, J.P.; Iazzolino, B.; et al. Metabolic brain changes across different levels of cognitive impairment in ALS: A 18F-FDG-PET study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 357–363. [Google Scholar] [CrossRef]
- Carluer, L.; Mondou, A.; Buhour, M.S.; Laisney, M.; Pélerin, A.; Eustache, F.; Viader, F.; Desgranges, B. Neural substrate of cognitive theory of mind impairment in amyotrophic lateral sclerosis. Cortex 2015, 65, 19–30. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, D.; Hou, B.; Sun, X.; Yang, X.; Gao, J.; Liu, M.; Feng, F.; Cui, L. Brain structural and perfusion changes in amyotrophic lateral sclerosis-frontotemporal dementia patients with cognitive and motor onset: A preliminary study. Brain Imaging Behav. 2022, 16, 2164–2174. [Google Scholar] [CrossRef]
- Canosa, A.; Pagani, M.; Brunetti, M.; Barberis, M.; Iazzolino, B.; Ilardi, A.; Cammarosano, S.; Manera, U.; Moglia, C.; Calvo, A.; et al. Correlation between apolipoprotein E genotype and brain metabolism in amyotrophic lateral sclerosis. Eur. J. Neurol. 2019, 26, 306–312. [Google Scholar] [CrossRef]
- Saito, Y. Diffusion magnetic resonance imaging-based surrogate marker in amyotrophic lateral sclerosis. Explor. Neuroprot. Ther. 2023, 3, 186–206. [Google Scholar] [CrossRef]
- Nigri, A.; Umberto, M.; Stanziano, M.; Ferraro, S.; Fedeli, D.; Medina Carrion, J.P.; Palermo, S.; Lequio, L.; Denegri, F.; Agosta, F.; et al. C9orf72 ALS mutation carriers show extensive cortical and subcortical damage compared to matched wild-type ALS patients. Neuroimage Clin. 2023, 38, 103400. [Google Scholar] [CrossRef] [PubMed]
- Lepow, L.; Van Sweringen, J.; Strutt, A.M.; Jawaid, A.; MacAdam, C.; Harati, Y.; Schulz, P.E.; York, M.K. Frontal and temporal lobe involvement on verbal fluency measures in amyotrophic lateral sclerosis. J. Clin. Exp. Neuropsychol. 2010, 32, 913–922. [Google Scholar] [CrossRef]
- Yunusova, Y.; Ansari, J.; Ramirez, J.; Shellikeri, S.; Stanisz, G.J.; Black, S.E.; Gillingham, S.M.; Kiss, A.; Stuss, D.T.; Zinman, L. Frontal anatomical correlates of cognitive and speech motor deficits in amyotrophic lateral sclerosis. Behav. Neurol. 2019, 2019, 9518309. [Google Scholar] [CrossRef]
- Mao, D.; Ding, Z.; Jia, W.; Liao, W.; Li, X.; Huang, H.; Yuan, J.; Zang, Y.F.; Zhang, H. Low-frequency fluctuations of the resting brain: High magnitude does not equal high reliability. PLoS ONE 2015, 10, e0128117. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Pioro, E.P. Degeneration of gray and white matter differs between hypometabolic and hypermetabolic brain regions in a patient with ALS-FTD: A longitudinal MRI-PET multimodal study. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Canosa, A.; Palumbo, F.; Iazzolino, B.; Peotta, L.; Di Pede, F.; Manera, U.; Vasta, R.; Grassano, M.; Solero, L.; Arena, V.; et al. The interplay among education, brain metabolism, and cognitive impairment suggests a role of cognitive reserve in Amyotrophic Lateral Sclerosis. Neurobiol. Aging 2021, 98, 205–213. [Google Scholar] [CrossRef]
- Christidi, F.; Argyropoulos, G.D.; Karavasilis, E.; Velonakis, G.; Zouvelou, V.; Kourtesis, P.; Pantoleon, V.; Tan, E.L.; Daponte, A.; Aristeidou, S.; et al. Hippocampal metabolic alterations in amyotrophic lateral sclerosis: A magnetic resonance spectroscopy study. Life 2023, 13, 571. [Google Scholar] [CrossRef]
- Ta, D.; Ishaque, A.; Srivastava, O.; Hanstock, C.; Seres, P.; Eurich, D.T.; Luk, C.; Briemberg, H.; Frayne, R.; Genge, A.L.; et al. Progressive neurochemical abnormalities in cognitive and motor subgroups of amyotrophic lateral sclerosis: A prospective multicenter study. Neurology 2021, 97, e803–e813. [Google Scholar] [CrossRef]
- Chenji, S.; Jha, S.; Lee, D.; Brown, M.; Seres, P.; Mah, D.; Kalra, S. Investigating default mode and sensorimotor network connectivity in amyotrophic lateral sclerosis. PLoS ONE 2016, 11, e0157443. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Wang, Y.; Hu, J.; Wang, J.; Zhang, J.; Jiang, T. Disrupted effective connectivity of the sensorimotor network in amyotrophic lateral sclerosis. J. Neurol. 2016, 263, 508–516. [Google Scholar] [CrossRef]
- Dimond, D.; Ishaque, A.; Chenji, S.; Mah, D.; Chen, Z.; Seres, P.; Beaulieu, C.; Kalra, S. White matter structural network abnormalities underlie executive dysfunction in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2017, 38, 1249–1268. [Google Scholar] [CrossRef] [PubMed]
- Dukic, S.; McMackin, R.; Buxo, T.; Fasano, A.; Chipika, R.; Pinto-Grau, M.; Costello, E.; Schuster, C.; Hammond, M.; Heverin, M.; et al. Patterned functional network disruption in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2019, 40, 4827–4842. [Google Scholar] [CrossRef] [PubMed]
- Borgheai, S.B.; McLinden, J.; Mankodiya, K.; Shahriari, Y. Frontal functional network disruption associated with amyotrophic lateral sclerosis: An fNIRS-based minimum spanning tree analysis. Front. Neurosci. 2020, 14, 613990. [Google Scholar] [CrossRef] [PubMed]
- Cividini, C.; Basaia, S.; Spinelli, E.G.; Canu, E.; Castelnovo, V.; Riva, N.; Cecchetti, G.; Caso, F.; Magnani, G.; Falini, A.; et al. Amyotrophic lateral sclerosis-frontotemporal dementia: Shared and divergent neural correlates across the clinical spectrum. Neurology 2021, 98, e402–e415. [Google Scholar] [CrossRef]
- Bueno, A.P.A.; de Souza, L.C.; Pinaya, W.H.L.; Teixeira, A.L.; de Prado, L.G.R.; Caramelli, P.; Hornberger, M.; Sato, J.R. Papez circuit gray matter and episodic memory in amyotrophic lateral sclerosis and behavioural variant frontotemporal dementia. Brain Imaging Behav. 2021, 15, 996–1006. [Google Scholar] [CrossRef]
- Trojsi, F.; Di Nardo, F.; Caiazzo, G.; Siciliano, M.; D’Alvano, G.; Ferrantino, T.; Passaniti, C.; Ricciardi, D.; Esposito, S.; Lavorgna, L.; et al. Hippocampal connectivity in amyotrophic lateral sclerosis (ALS): More than Papez circuit impairment. Brain Imaging Behav. 2021, 15, 2126–2138. [Google Scholar] [CrossRef]
- Bede, P.; Omer, T.; Finegan, E.; Chipika, R.H.; Iyer, P.M.; Doherty, M.A.; Vajda, A.; Pender, N.; McLaughlin, R.L.; Hutchinson, S.; et al. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: A multimodal neuroimaging study. Brain Imaging Behav. 2018, 12, 1696–1707. [Google Scholar] [CrossRef]
- Bharti, K.; Graham, S.J.; Benatar, M.; Briemberg, H.; Chenji, S.; Dupré, N.; Dionne, A.; Frayne, R.; Genge, A.; Korngut, L.; et al. Functional alterations in large-scale resting-state networks of amyotrophic lateral sclerosis: A multi-site study across Canada and the United States. PLoS ONE 2022, 17, e0269154. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Z.; Ke, Z.; Xu, Y.; Bai, F.; Liu, Z. Aberrant multimodal connectivity pattern involved in default mode network and limbic network in amyotrophic lateral sclerosis. Brain Sci. 2023, 13, 803. [Google Scholar] [CrossRef]
- Castelnovo, V.; Canu, E.; De Mattei, F.; Filippi, M.; Agosta, F. Basal ganglia alterations in amyotrophic lateral sclerosis. Front. Neurosci. 2023, 17, 1133758. [Google Scholar] [CrossRef]
- Crespi, C.; Cerami, C.; Dodich, A.; Canessa, N.; Iannaccone, S.; Corbo, M.; Lunetta, C.; Falini, A.; Cappa, S.F. Microstructural correlates of emotional attribution impairment in non-demented patients with amyotrophic lateral sclerosis. PLoS ONE 2016, 11, e0161034. [Google Scholar] [CrossRef] [PubMed]
- Pender, N.; Pinto-Grau, M.; Hardiman, O. Cognitive and behavioural impairment in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2020, 33, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Masuda, M.; Watanabe, H.; Ogura, A.; Ohdake, R.; Tanaka, Y.; Kato, T.; Kawabata, K.; Riku, Y.; Hara, K.; et al. The neural network basis of altered decision-making in patients with amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 2115–2126. [Google Scholar] [CrossRef]
- Abrahams, S.; Goldstein, L.H.; Simmons, A.; Brammer, M.; Williams, S.C.; Giampietro, V.; Leigh, P.N. Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain 2004, 127, 1507–1517. [Google Scholar] [CrossRef]
- Pettit, L.D.; Bastin, M.E.; Smith, C.; Bak, T.H.; Gillingwater, T.H.; Abrahams, S. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain 2013, 136, 3290–3304. [Google Scholar] [CrossRef]
- Castelnovo, V.; Canu, E.; Calderaro, D.; Riva, N.; Poletti, B.; Basaia, S.; Solca, F.; Silani, V.; Filippi, M.; Agosta, F. Progression of brain functional connectivity and frontal cognitive dysfunction in ALS. Neuroimage Clin. 2020, 28, 102509. [Google Scholar] [CrossRef]
- Temp, A.G.M.; Dyrba, M.; Büttner, C.; Kasper, E.; Machts, J.; Kaufmann, J.; Vielhaber, S.; Teipel, S.; Prudlo, J. Cognitive profiles of amyotrophic lateral sclerosis differ in resting-state functional connectivity: An fMRI study. Front. Neurosci. 2021, 15, 682100. [Google Scholar] [CrossRef] [PubMed]
- Sarro, L.; Agosta, F.; Canu, E.; Riva, N.; Prelle, A.; Copetti, M.; Riccitelli, G.; Comi, G.; Filippi, M. Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: A diffusion tensor tractography study. AJNR Am. J. Neuroradiol. 2011, 32, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- McMackin, R.; Dukic, S.; Costello, E.; Pinto-Grau, M.; McManus, L.; Broderick, M.; Chipika, R.; Iyer, P.M.; Heverin, M.; Bede, P.; et al. Cognitive network hyperactivation and motor cortex decline correlate with ALS prognosis. Neurobiol. Aging 2021, 104, 57–70. [Google Scholar] [CrossRef]
- Wei, L.; Baeken, C.; Liu, D.; Zhang, J.; Wu, G.R. Functional connectivity-based prediction of global cognition and motor function in riluzole-naive amyotrophic lateral sclerosis patients. Netw. Neurosci. 2022, 6, 161–174. [Google Scholar] [CrossRef]
- Trojsi, F.; Di Nardo, F.; D’Alvano, G.; Passaniti, C.; Sharbafshaaer, M.; Canale, F.; Russo, A.; Silvestro, M.; Lavorgna, L.; Cirillo, M.; et al. Cognitive, behavioral, and brain functional connectivity correlates of fatigue in amyotrophic lateral sclerosis. Brain Behav. 2023, 13, e2931. [Google Scholar] [CrossRef]
- Lange, F.; Lange, C.; Joop, M.; Seer, C.; Dengler, R.; Kopp, B.; Petri, S. Neural correlates of cognitive set shifting in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2016, 127, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Hanagasi, H.A.; Gurvit, I.H.; Ermutlu, N.; Kaptanoglu, G.; Karamursel, S.; Idrisoglu, H.A.; Emre, M.; Demiralp, T. Cognitive impairment in amyotrophic lateral sclerosis: Evidence from neuropsychological investigation and event-related potentials. Brain Res. Cogn. Brain Res. 2002, 14, 234–244. [Google Scholar] [CrossRef]
- Ogawa, T.; Tanaka, H.; Hirata, K. Cognitive deficits in amyotrophic lateral sclerosis evaluated by event-related potentials. Clin. Neurophysiol. 2009, 120, 659–664. [Google Scholar] [CrossRef]
- Geronimo, A.; Simmons, Z.; Schiff, S.J. Performance predictors of brain-computer interfaces in patients with amyotrophic lateral sclerosis. J. Neural Eng. 2016, 13, 026002. [Google Scholar] [CrossRef] [PubMed]
- Seer, C.; Joop, M.; Lange, F.; Lange, C.; Dengler, R.; Petri, S.; Kopp, B. Attenuated error-related potentials in amyotrophic lateral sclerosis with executive dysfunctions. Clin. Neurophysiol. 2017, 128, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Volpato, C.; Prats Sedano, M.A.; Silvoni, S.; Segato, N.; Cavinato, M.; Merico, A.; Piccione, F.; Palmieri, A.; Birbaumer, N. Selective attention impairment in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Dukic, S.; McMackin, R.; Costello, E.; Metzger, M.; Buxo, T.; Fasano, A.; Chipika, R.; Pinto-Grau, M.; Schuster, C.; Hammond, M.; et al. Resting-state EEG reveals four subphenotypes of amyotrophic lateral sclerosis. Brain 2022, 145, 621–631. [Google Scholar] [CrossRef]
- McMackin, R.; Dukic, S.; Broderick, M.; Iyer, P.M.; Pinto-Grau, M.; Mohr, K.; Chipika, R.; Coffey, A.; Buxo, T.; Schuster, C.; et al. Dysfunction of attention switching networks in amyotrophic lateral sclerosis. Neuroimage Clin. 2019, 22, 101707. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Cairns, N.J. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration. In Neuropathology of Neurodegenerative Diseases: A Practical Guide; Kovacs, G.G., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 209–248. [Google Scholar]
- Takeda, T.; Kitagawa, K.; Arai, K. Phenotypic variability and its pathological basis in amyotrophic lateral sclerosis. Neuropathology 2020, 40, 40–56. [Google Scholar] [CrossRef]
- Ling, S.C. Synaptic paths to neurodegeneration: The emerging role of TDP-43 and FUS in synaptic functions. Neural. Plast. 2018, 2018, 8413496. [Google Scholar] [CrossRef]
- Braak, H.; Neumann, M.; Ludolph, A.C.; Del Tredici, K. Does sporadic amyotrophic lateral sclerosis spread via axonal connectivities? Neurol. Int. Open 2017, 01, E136–E141. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Tan, R.; Halliday, G.M.; Kril, J.J. Spread of pathology in amyotrophic lateral sclerosis: Assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol. Commun. 2015, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.M.; van der Burgh, H.K.; Nitert, A.D.; Bede, P.; de Lange, S.C.; Hardiman, O.; van den Berg, L.H.; van den Heuvel, M.P. Connectome-based propagation model in amyotrophic lateral sclerosis. Ann. Neurol. 2020, 87, 725–738. [Google Scholar] [CrossRef]
- Riemenschneider, H.; Guo, Q.; Bader, J.; Frottin, F.; Farny, D.; Kleinberger, G.; Haass, C.; Mann, M.; Hartl, F.U.; Baumeister, W.; et al. Gel-like inclusions of C-terminal fragments of TDP-43 sequester stalled proteasomes in neurons. EMBO Rep. 2022, 23, e53890. [Google Scholar] [CrossRef]
- Smethurst, P.; Newcombe, J.; Troakes, C.; Simone, R.; Chen, Y.R.; Patani, R.; Sidle, K. In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol. Dis. 2016, 96, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Feiler, M.S.; Strobel, B.; Freischmidt, A.; Helferich, A.M.; Kappel, J.; Brewer, B.M.; Li, D.; Thal, D.R.; Walther, P.; Ludolph, A.C.; et al. TDP-43 is intercellularly transmitted across axon terminals. J. Cell Biol. 2015, 211, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Del Tredici, K.; Braak, H.; Ludolph, A. The multisystem degeneration amyotrophic lateral sclerosis—Neuropathological staging and clinical translation. Arch. Ital. Biol. 2017, 155, 118–130. [Google Scholar] [CrossRef]
- Perkins, E.M.; Burr, K.; Banerjee, P.; Mehta, A.R.; Dando, O.; Selvaraj, B.T.; Suminaite, D.; Nanda, J.; Henstridge, C.M.; Gillingwater, T.H.; et al. Altered network properties in C9ORF72 repeat expansion cortical neurons are due to synaptic dysfunction. Mol. NeuroDegener. 2021, 16, 13. [Google Scholar] [CrossRef]
- Aksman, L.M.; Wijeratne, P.A.; Oxtoby, N.P.; Eshaghi, A.; Shand, C.; Altmann, A.; Alexander, D.C.; Young, A.L. pySuStaIn: A Python implementation of the Subtype and Stage Inference algorithm. SoftwareX 2021, 16, 100811. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Vogel, J.W.; Aksman, L.M.; Wijeratne, P.A.; Eshaghi, A.; Oxtoby, N.P.; Williams, S.C.R.; Alexander, D.C. Ordinal SuStaIn: Subtype and Stage Inference for clinical scores, visual ratings, and other ordinal data. Front. Artif. Intell. 2021, 4, 613261. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Vogel, J.W.; Robinson, J.L.; McMillan, C.T.; Ossenkoppele, R.; Wolk, D.A.; Irwin, D.J.; Elman, L.; Grossman, M.; Lee, V.M.Y.; et al. Data-driven neuropathological staging and subtyping of TDP-43 proteinopathies. Brain 2023, 146, 2975–2988. [Google Scholar] [CrossRef]
- Banerjee, P.; Elliott, E.; Rifai, O.M.; O’Shaughnessy, J.; McDade, K.; Abrahams, S.; Chandran, S.; Smith, C.; Gregory, J.M. NLRP3 inflammasome as a key molecular target underlying cognitive resilience in amyotrophic lateral sclerosis. J. Pathol. 2022, 256, 262–268. [Google Scholar] [CrossRef]
- Cykowski, M.D.; Powell, S.Z.; Peterson, L.E.; Appel, J.W.; Rivera, A.L.; Takei, H.; Chang, E.; Appel, S.H. Clinical significance of TDP-43 neuropathology in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2017, 76, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, C.F.; Mori, F.; Tanji, K.; Kakita, A.; Takahashi, H.; Wakabayashi, K. TDP-43–immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008, 115, 115–122. [Google Scholar] [CrossRef]
- Prudlo, J.; König, J.; Schuster, C.; Kasper, E.; Büttner, A.; Teipel, S.; Neumann, M. TDP-43 pathology and cognition in ALS: A prospective clinicopathologic correlation study. Neurology 2016, 87, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Koga, S.; Sekiya, H.; Oskarsson, B.; Boylan, K.; Petrucelli, L.; Josephs, K.A.; Dickson, D.W. Old age amyotrophic lateral sclerosis and limbic TDP-43 pathology. Brain Pathol 2022, 32, e13100. [Google Scholar] [CrossRef]
- Shellikeri, S.; Keith, J.; Black, S.E.; Zinman, L.; Yunusova, Y. Neuropathology of speech network distinguishes bulbar from nonbulbar amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2020, 79, 284–295. [Google Scholar] [CrossRef]
- Borrego-Écija, S.; Turon-Sans, J.; Ximelis, T.; Aldecoa, I.; Molina-Porcel, L.; Povedano, M.; Rubio, M.A.; Gámez, J.; Cano, A.; Paré-Curell, M.; et al. Cognitive decline in amyotrophic lateral sclerosis: Neuropathological substrate and genetic determinants. Brain Pathol. 2021, 31, e12942. [Google Scholar] [CrossRef]
- Yang, T.; Wei, Q.; Li, C.; Cao, B.; Ou, R.; Hou, Y.; Zhang, L.; Chen, Y.; Shang, H. Association between vascular risk factors and cognitive impairment in amyotrophic lateral sclerosis: A case-control study. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 185–194. [Google Scholar] [CrossRef]
- Rhodes, E.; Alfa, S.; Jin, H.; Massimo, L.; Elman, L.; Amado, D.; Baer, M.; Quinn, C.; McMillan, C.T. Cognitive reserve in ALS: The role of occupational skills and requirements. medRxiv 2023. [Google Scholar] [CrossRef]
- Temp, A.G.M.; Prudlo, J.; Vielhaber, S.; Machts, J.; Hermann, A.; Teipel, S.J.; Kasper, E. Cognitive reserve and regional brain volume in amyotrophic lateral sclerosis. Cortex 2021, 139, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.; Rooney, J.; Pinto-Grau, M.; Burke, T.; Elamin, M.; Bede, P.; McMackin, R.; Dukic, S.; Vajda, A.; Heverin, M.; et al. Cognitive reserve in amyotrophic lateral sclerosis (ALS): A population-based longitudinal study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 460–465. [Google Scholar] [CrossRef]
- Temp, A.G.M.; Kasper, E.; Machts, J.; Vielhaber, S.; Teipel, S.; Hermann, A.; Prudlo, J. Cognitive reserve protects ALS-typical cognitive domains: A longitudinal study. Ann. Clin. Transl. Neurol. 2022, 9, 1212–1223. [Google Scholar] [CrossRef]
- Gregory, J.M.; Elliott, E.; McDade, K.; Bak, T.; Pal, S.; Chandran, S.; Abrahams, S.; Smith, C. Neuronal clusterin expression is associated with cognitive protection in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2020, 46, 255–263. [Google Scholar] [CrossRef]
- Wang, W.; Arakawa, H.; Wang, L.; Okolo, O.; Siedlak, S.L.; Jiang, Y.; Gao, J.; Xie, F.; Petersen, R.B.; Wang, X. Motor-coordinative and cognitive dysfunction caused by mutant TDP-43 could be reversed by inhibiting its mitochondrial localization. Mol. Ther. 2017, 25, 127–139. [Google Scholar] [CrossRef]
- Alfieri, J.A.; Silva, P.R.; Igaz, L.M. Early cognitive/social deficits and late motor phenotype in conditional wild-type TDP-43 transgenic mice. Front. Aging Neurosci. 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Ho, W.Y.; Yen, Y.C.; Sanford, E.; Ling, S.C. The vulnerability of motor and frontal cortex-dependent behaviors in mice expressing ALS-linked mutation in TDP-43. Neurobiol. Aging 2020, 92, 43–60. [Google Scholar] [CrossRef]
- Jury, N.; Abarzua, S.; Diaz, I.; Guerra, M.V.; Ampuero, E.; Cubillos, P.; Martinez, P.; Herrera-Soto, A.; Arredondo, C.; Rojas, F.; et al. Widespread loss of the silencing epigenetic mark H3K9me3 in astrocytes and neurons along with hippocampal-dependent cognitive impairment in C9orf72 BAC transgenic mice. Clin. Epigenetics 2020, 12, 32. [Google Scholar] [CrossRef]
- Sgobio, C.; Trabalza, A.; Spalloni, A.; Zona, C.; Carunchio, I.; Longone, P.; Ammassari-Teule, M. Abnormal medial prefrontal cortex connectivity and defective fear extinction in the presymptomatic G93A SOD1 mouse model of ALS. Genes Brain Behav. 2008, 7, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Spalloni, A.; Longone, P. Cognitive impairment in amyotrophic lateral sclerosis, clues from the SOD1 mouse. Neurosci. Biobehav. Rev. 2016, 60, 12–25. [Google Scholar] [CrossRef]
- Ho, W.Y.; Chang, J.C.; Tyan, S.H.; Yen, Y.C.; Lim, K.; Tan, B.S.Y.; Ong, J.; Tucker-Kellogg, G.; Wong, P.; Koo, E.; et al. FUS-mediated dysregulation of Sema5a, an autism-related gene, in FUS mice with hippocampus-dependent cognitive deficits. Hum. Mol. Genet. 2019, 28, 3777–3791. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Agrawal, I.; Tyan, S.H.; Sanford, E.; Chang, W.T.; Lim, K.; Ong, J.; Tan, B.S.Y.; Moe, A.A.K.; Yu, R.; et al. Dysfunction in nonsense-mediated decay, protein homeostasis, mitochondrial function, and brain connectivity in ALS-FUS mice with cognitive deficits. Acta Neuropathol. Commun. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, L.; Yan, T.; Perry, G.; Wang, X. TDP-43 proteinopathy and mitochondrial abnormalities in neurodegeneration. Mol. Cell Neurosci. 2019, 100, 103396. [Google Scholar] [CrossRef]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and cellular mechanisms affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef]
- Rifai, O.M.; Longden, J.; O’Shaughnessy, J.; Sewell, M.; Pate, J.; McDade, K.; Daniels, M.J.; Abrahams, S.; Chandran, S.; McColl, B.W.; et al. Random forest modelling demonstrates microglial and protein misfolding features to be key phenotypic markers in C9orf72–ALS. J. Pathol. 2022, 258, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Sassani, M.; Alix, J.J.; McDermott, C.J.; Baster, K.; Hoggard, N.; Wild, J.M.; Mortiboys, H.J.; Shaw, P.J.; Wilkinson, I.D.; Jenkins, T.M. Magnetic resonance spectroscopy reveals mitochondrial dysfunction in amyotrophic lateral sclerosis. Brain 2020, 143, 3603–3618. [Google Scholar] [CrossRef]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Duranti, E.; Villa, C. Molecular investigations of protein aggregation in the pathogenesis of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2022, 24, 704. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Santella, R.M.; Liu, X.; Bogdanov, M.; Zipprich, J.; Wu, H.C.; Mahata, J.; Kilty, M.; Bednarz, K.; Bell, D.; et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph. Lateral Scler. 2008, 9, 177–183. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Kwon, Y.; Lee, S.; Kim, H.J. Potential roles of the endoplasmic reticulum stress pathway in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2023, 15, 1047897. [Google Scholar] [CrossRef]
- Wang, P.; Deng, J.; Dong, J.; Liu, J.; Bigio, E.H.; Mesulam, M.; Wang, T.; Sun, L.; Wang, L.; Lee, A.Y.; et al. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 2019, 15, e1007947. [Google Scholar] [CrossRef]
- Prater, K.E.; Latimer, C.S.; Jayadev, S. Glial TDP-43 and TDP-43 induced glial pathology, focus on neurodegenerative proteinopathy syndromes. Glia 2022, 70, 239–255. [Google Scholar] [CrossRef]
- Verde, F.; Milone, I.; Maranzano, A.; Colombo, E.; Torre, S.; Solca, F.; Doretti, A.; Gentile, F.; Manini, A.; Bonetti, R.; et al. Serum levels of glial fibrillary acidic protein in patients with amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2023, 10, 118–129. [Google Scholar] [CrossRef]
- Rifai, O.M.; O’Shaughnessy, J.; Dando, O.R.; Munro, A.F.; Sewell, M.D.E.; Abrahams, S.; Waldron, F.M.; Sibley, C.R.; Gregory, J.M. Distinct neuroinflammatory signatures exist across genetic and sporadic amyotrophic lateral sclerosis cohorts. Brain 2023, awad243. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, M.; Pavey, N.; van den Bos, M.; Menon, P.; Kiernan, M.C.; Vucic, S. Association of cortical hyperexcitability and cognitive impairment in patients with amyotrophic lateral sclerosis. Neurology 2021, 96, e2090–e2097. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Sideris, D.I.; Carroll, E.; Rotariu, S.; Salomonsson, S.; Tzioras, M.; McKenzie, C.A.; Smith, C.; von Arnim, C.A.F.; Ludolph, A.C.; et al. Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol. 2018, 135, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.G.; Slade, J.; Chinnery, R.M.; McKenzie, J.; Royston, C.; Roberts, G.W.; Shaw, P.J. Quantitative study of synaptophysin immunoreactivity of cerebral cortex and spinal cord in motor neuron disease. J. Neuropathol. Exp. Neurol. 1995, 54, 673–679. [Google Scholar] [CrossRef]
- Sasaki, S.; Maruyama, S. Synapse loss in anterior horn neurons in amyotrophic lateral sclerosis. Acta Neuropathol. 1994, 88, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Riku, Y.; Seilhean, D.; Duyckaerts, C.; Boluda, S.; Iguchi, Y.; Ishigaki, S.; Iwasaki, Y.; Yoshida, M.; Sobue, G.; Katsuno, M. Pathway from TDP-43-related pathology to neuronal dysfunction in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Int. J. Mol. Sci. 2021, 22, 3843. [Google Scholar] [CrossRef]
- Yang, W.; Strong, M.J. Widespread neuronal and glial hyperphosphorylated tau deposition in ALS with cognitive impairment. Amyotroph. Lateral Scler. 2012, 13, 178–193. [Google Scholar] [CrossRef]
- Latimer, C.S.; Burke, B.T.; Liachko, N.F.; Currey, H.N.; Kilgore, M.D.; Gibbons, L.E.; Henriksen, J.; Darvas, M.; Domoto-Reilly, K.; Jayadev, S.; et al. Resistance and resilience to Alzheimer’s disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol. Commun. 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.S.; Stair, J.G.; Hincks, J.C.; Currey, H.N.; Bird, T.D.; Keene, C.D.; Kraemer, B.C.; Liachko, N.F. TDP-43 promotes tau accumulation and selective neurotoxicity in bigenic Caenorhabditis elegans. Dis. Model Mech. 2022, 15, dmm049323. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.A.; Gan, K.A.; Dowell, J.A.; Cairns, N.J.; Gitcho, M.A. TDP-43 expression influences amyloidβ plaque deposition and tau aggregation. Neurobiol. Dis. 2017, 103, 154–162. [Google Scholar] [CrossRef]
- Montalbano, M.; McAllen, S.; Cascio, F.L.; Sengupta, U.; Garcia, S.; Bhatt, N.; Ellsworth, A.; Heidelman, E.A.; Johnson, O.D.; Doskocil, S.; et al. TDP-43 and tau oligomers in Alzheimer’s disease, amyotrophic lateral sclerosis, and frontotemporal dementia. Neurobiol. Dis. 2020, 146, 105130. [Google Scholar] [CrossRef]
- Moszczynski, A.J.; Hintermayer, M.A.; Strong, M.J. Phosphorylation of threonine 175 tau in the induction of tau pathology in amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD). A review. Front. Neurosci. 2018, 12, 259. [Google Scholar] [CrossRef]
- Strong, M.J.; Donison, N.S.; Volkening, K. Alterations in tau metabolism in ALS and ALS-FTSD. Front. Neurol. 2020, 11, 598907. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, P.; Li, W.; Liu, Z.; Zhou, M.; Li, J.; Yuan, Y.; Fang, L.; Wang, M.; Shen, L.; et al. Detection of changes in synaptic density in amyotrophic lateral sclerosis patients using 18F-SynVesT-1 positron emission tomography. Eur. J. Neurol. 2022, 29, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, Z.I.; Hindley, N.; Sanchez Avila, A.; Kline, R.A.; Eaton, S.L.; Lamont, D.J.; Smith, C.; Spires-Jones, T.L.; Wishart, T.M.; Henstridge, C.M. Synaptic proteomics reveal distinct molecular signatures of cognitive change and C9ORF72 repeat expansion in the human ALS cortex. Acta Neuropathol. Commun. 2022, 10, 156. [Google Scholar] [CrossRef]
- Gulino, R. Synaptic dysfunction and plasticity in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2023, 24, 4613. [Google Scholar] [CrossRef]
- Mora, S.; Allodi, I. Neural circuit and synaptic dysfunctions in ALS-FTD pathology. Front. Neural Circuits 2023, 17, 1208876. [Google Scholar] [CrossRef]
- Bacioglu, M.; Maia, L.F.; Preische, O.; Schelle, J.; Apel, A.; Kaeser, S.A.; Schweighauser, M.; Eninger, T.; Lambert, M.; Pilotto, A.; et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016, 91, 56–66. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Verde, F.; Milone, I.; Colombo, E.; Maranzano, A.; Solca, F.; Torre, S.; Doretti, A.; Gentile, F.; Manini, A.; Bonetti, R.; et al. Phenotypic correlates of serum neurofilament light chain levels in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2023, 15, 1132808. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Portelius, E.; Cullen, N.C.; Sandelius, Å.; Zetterberg, H.; Andreasson, U.; Höglund, K.; Irwin, D.J.; Grossman, M.; Weintraub, D.; et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019, 76, 318–325. [Google Scholar] [CrossRef]
- Meyer, T.; Salkic, E.; Grehl, T.; Weyen, U.; Kettemann, D.; Weydt, P.; Günther, R.; Lingor, P.; Koch, J.C.; Petri, S.; et al. Performance of serum neurofilament light chain in a wide spectrum of clinical courses of amyotrophic lateral sclerosis—A cross-sectional multicenter study. Eur. J. Neurol. 2023, 30, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, M.; Müschen, L.H.; Brakemeier, S.; Machetanz, G.; Naumann, M.; Castro-Gomez, S. Current state and future directions in the diagnosis of amyotrophic lateral sclerosis. Cells 2023, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Trojsi, F.; Di Nardo, F.; D’Alvano, G.; Caiazzo, G.; Passaniti, C.; Mangione, A.; Sharbafshaaer, M.; Russo, A.; Silvestro, M.; Siciliano, M.; et al. Resting state fMRI analysis of pseudobulbar affect in Amyotrophic Lateral Sclerosis (ALS): Motor dysfunction of emotional expression. Brain Imaging Behav. 2023, 17, 77–89. [Google Scholar] [CrossRef]
- Tedeschi, G.; Trojsi, F.; Tessitore, A.; Corbo, D.; Sagnelli, A.; Paccone, A.; D’Ambrosio, A.; Piccirillo, G.; Cirillo, M.; Cirillo, S.; et al. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol. Aging 2012, 33, 886–898. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).