Nicotine: From Discovery to Biological Effects
Abstract
1. Introduction
1.1. History of Tobacco
| Years | Note | References |
|---|---|---|
| Around 6000 BCE | It is widely acknowledged that tobacco originated and began to grow in the Americas. | [37] |
| 1492 | Cristoforo Colombo met Arawaks who offered them gifts, including their much valued dried leaves of tobacco. | [34] |
| 1493 | Ramon Pane, a Catholic monk who accompanied Columbus on his second expedition to the West Indies, provided detailed descriptions of the practice of using snuff, a form of smokeless tobacco created by grinding tobacco leaves into a fine powder and typically inhaled through the nose. Pane is commonly acknowledged as the individual who introduced tobacco to Europe during this time. | [41] |
| 1499 | Amerigo Vespucci noticed the curious habit of Native Americans chewing green leaves mixed with a white powder. | [42] |
| 1518 | Fernando Cortez brought tobacco to Spain, as requested by Ramon Pane. | [4] |
| 1530–1604 | The French Ambassador to Portugal, Jean Nicot de Villemain, introduced tobacco to Catherine de Medici, the queen consort and regent of France. Initially, Nicot sent snuff to help treat her son Francis II’s migraine headaches. Later, the queen referred to tobacco as “Herba Regina” or “Herba Medicea”. However, there is some confusion in sources: some assert that it cured Catherine’s own headaches by inducing sneezing. | [4] |
| 1565 | Adam Lonicer, Adam Lonitzer or Adamus Lonicerus, in homage to Nicot, named the botanical species Nicotiana and its product nicotine. | [4] |
| 1571 | Nicolas Monardes, a physician in Seville, wrote a book on the history of medicinal plants of the new world, De Hierba Panacea, which stated that tobacco could cure 36 different ills. It became a standard medical textbook across Europe. | [34] |
| 1588 | Thomas Harriot, also spelled Harriott, Hariot or Heriot, in Virginia, cited the health benefits of tobacco. | [34] |
| 1600 | Tobacco was probably introduced to England by Sir John Hawkins and his crew, although legend reports that it was first used by Sir Francis Drake. There is a legend that Sir Walter Raleigh convinced Queen Elizabeth I to smoke. | [42] |
| 29 October 1618 | The tradition that Raleigh smoked a pipe or two on the morning of his execution is well established. | [34] |
| mid-1660s | When the Great Plague struck London, people believed that smoking tobacco could protect them from infection. (The Great Plague was an outbreak of the bubonic plague that killed more than one-fifth of London’s population.) Eton, a school near the city, even made smoking a requirement in hopes of keeping the plague away. | [42] |
| 1638 | Virginia became the primary supplier of tobacco to Western Europe. | [34] |
| 1761 | British physician John Hill published Warnings against Excessive Use of Snuff, probably the first clinical study of the effects of tobacco. Hill warned snuff users that they were susceptible to nose cancer. | [4] |
| 1776 | Tobacco was used by the revolutionaries as collateral for the loans they were getting from France. | [4] |
| 1847 | Philip Morris was established in the UK. They were the first to start selling hand-rolled Turkish cigarettes. | [4] |
| 1854–1856 | With the return of the veterans from the Crimean War (1854–1856), where soldiers learned to roll tobacco from the Turks, the cigarette spreads throughout Europe. | [4] |
| 1881 | Invention of the cigarette-making machine by James Bonsack. He went into business with James ‘Buck’ Duke, and the American Tobacco Company was born. | [4] |
| 1914–1918 | The use of cigarettes exploded during the First World War (1914–1918). Everywhere cigarettes were called “soldier’s smoke”. | [4] |
| 1924 | Phillip Morris began marketing Marlboro as a women’s cigarette, dubbed “Mild as May!” To combat this publicity, the American Tobacco Company, manufacturer of the Lucky Strike brand, began marketing its cigarettes to women and gained 38% of the market. Lucky Strike urged customers to “Look for a Lucky instead of a sweet”. | [4] |
| 1925–1935 | The smoking rate among teenagers triples. | [4] |
| 1940 | Americans smoked 2558 cigarettes per person per year, 2.5 times their consumption in 1930; in addition, 7121 cases of lung cancer were reported. | [4] |
| 1941 | Ochsner and DeBakey reported a correlation between the increase in tobacco sales and the increasing prevalence of lung cancer, concluding that the latter was mainly due to tobacco. | [4] |
| 1939–1945 | During the Second World War (1939–1945), cigarette sales peaked. | [4] |
| 1952–today | Tobacco is classified as a cancer-causing agent due to the presence of various compounds that are considered carcinogenic to humans (at least 70 known to cause cancer, Tobacco-specific nitrosamines (TSNAs), and Polycyclic aromatic hydrocarbons (PAHs)) | [4] |
| 2023 |
| [43] |
1.2. Tobacco Plant
1.3. Nicotine as Secondary Metabolite
2. Nicotine
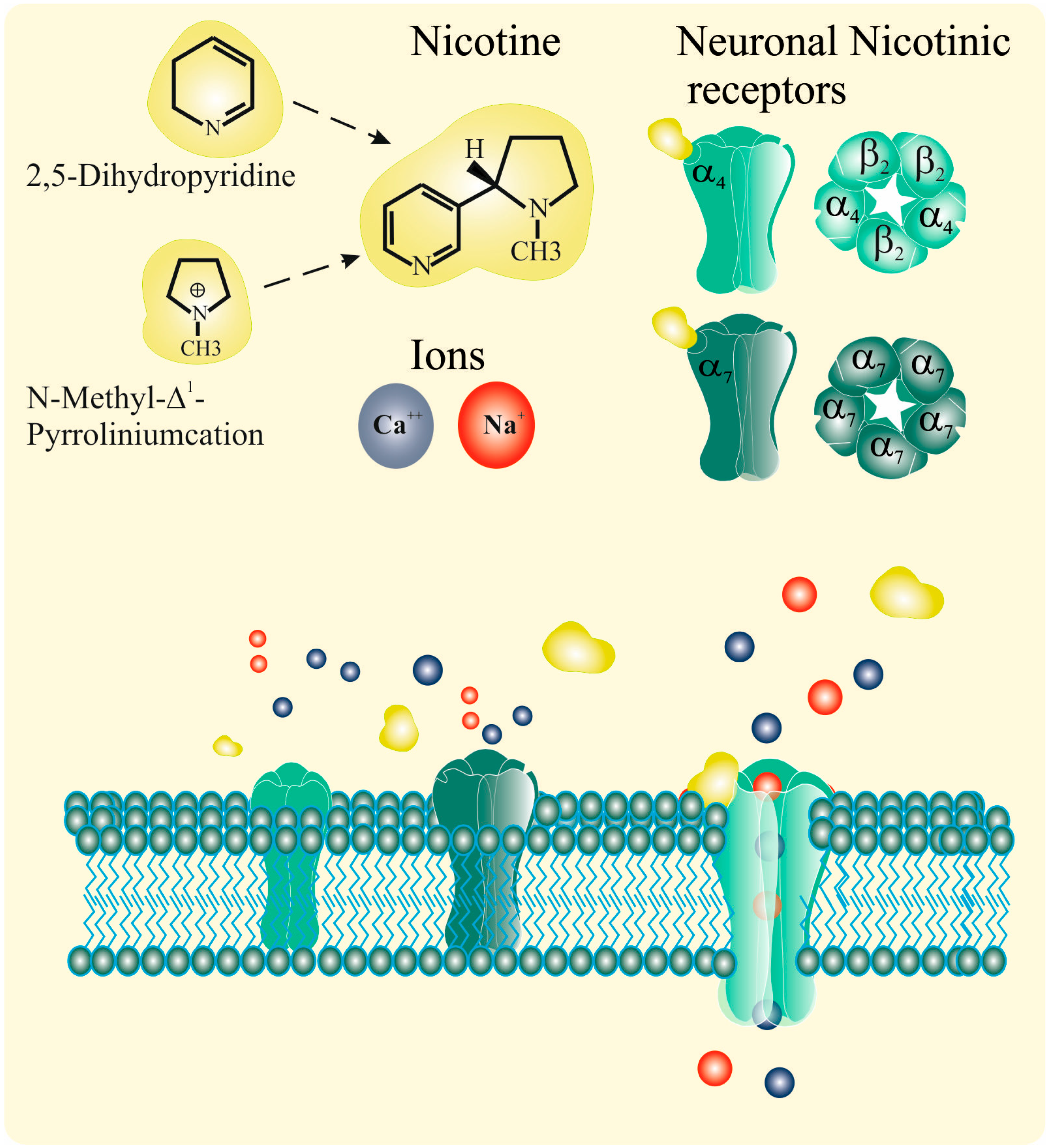
2.1. Nicotinic Acetylcholine Receptors (nAChRs)
2.2. Nicotine and nAChRs
2.3. Nicotine and Biological Effects
- Proanthocyanidins (PCs) and anthocyanins (ACNs) are the predominant flavonoid pigments that are widely distributed in plants, and are known for their therapeutic potential in addressing certain chronic diseases. Treatment with non-toxic concentrations of PCs and ACNs exhibits diverse effects against nicotine-induced non-small-cell lung cancer (NSCLC), encompassing anti-proliferative, anti-migratory, anti-metastatic, anti-invasive, and anti-angiogenic effects, as well as induction of apoptosis and autophagy. The utilization of PC-rich extracts derived from grape seeds and/or Cinnamomi Cortex, in conjunction with radiation or chemotherapy, holds promise for yielding anti-proliferative, anti-inflammatory, and apoptotic benefits against nicotine-induced NSCLC. Moreover, compounds such as delphinidin and cyanidin exhibit the potential to enhance apoptotic and autophagic activity by augmenting the chemosensitivity and/or radiosensitivity of NSCLC cells [93].
- Quercetin stands as a safe and natural compound with substantial potential to address cigarette-smoking-induced chronic obstructive pulmonary disease (CS-COPD). Quercetin prevents CS-COPD and mitigates airway remodeling through a range of mechanisms, including its antioxidant, anti-inflammatory, and immunomodulatory properties, as well as anti-cellular senescence, modulation of mitochondrial autophagy, and regulation of gut microbiota. Quercetin exhibits potential synergistic effects when combined with beta-agonists and M-receptor antagonists, corticosteroids and roflumilast, antibiotics, and N-acetylcysteine (NAC). This collaboration enhances bronchodilatory, anti-inflammatory, antibacterial, and antiviral effects [94].
- Scutellaria baicalensis and its flavone compounds exhibit therapeutic effects in nicotine-induced NSCLC. These therapeutic effects against NSCLC cells, activated by nicotine via α7nAChR, stem from their capacity to impede proliferation, invasion, migration, metastasis, and angiogenesis. Furthermore, they induce apoptosis, halt cell cycle progression, and trigger autophagy by inhibiting the signaling pathways implicated in NSCLC development. Consequently, targeting α7nAChR and its downstream signaling pathways using flavone compounds holds promise for the development of drugs to counter nicotine-induced NSCLC cells and the treatment of NSCLC in smokers. Combining flavone compounds with chemotherapeutic agents such as cisplatin, which can modulate NSCLC-related signaling pathways, presents a potential strategy for enhancing the anti-NSCLC efficacy of these agents. As such, flavone compounds alone or in synergy with chemotherapeutics could emerge as approved medicinal interventions for NSCLC in smokers [95].
- Increase in α7 nAChR expression at both mRNA and protein levels [14].
- Reduction in markers of senescence, such as SA-β-Gal activity and induction of apoptosis markers, including p53/phospho-p53 [54].
- Increase in markers of neo-angiogenesis, such as VEGF [30,54]. The downstream pathways activated by nicotine, promoting the proliferation, migration, and invasion of airway epithelial cancer cells, as well as of other cancer cell type (i.e., pancreatic [31]). ultimately resulting in a transition toward a more severe neoplastic phenotype.
2.4. Ultrastructure of Human Adenocarcinoma Cell Line A549 Treated with Nicotine
3. Discussion
4. Materials and Methods
- The biosynthesis and accumulation of nicotine in tobacco plants with a focus on its role as a defense against predatory insects.
- The interaction of nicotine with nicotinic acetylcholine receptors (nAChRs) and its downstream effects.
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5-Tr), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Hall, B.J.; Wells, C.; Allenby, C.; Lin, M.Y.; Hao, I.; Marshall, L.; Rose, J.E.; Levin, E.D. Differential Effects of Non-Nicotine Tobacco Constituent Compounds on Nicotine Self-Administration in Rats. Pharmacol. Biochem. Behav. 2014, 120, 103–108. [Google Scholar] [CrossRef]
- Russo, P.; Nastrucci, C.; Alzetta, G.; Szalai, C. Tobacco Habit: Historical, Cultural, Neurobiological, and Genetic Features of People’s Relationship with an Addictive Drug. Perspect. Biol. Med. 2011, 54, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, R.E.; Wolfman, S.L.; De Biasi, M.; Dani, J.A. Nicotinic Acetylcholine Receptors and Nicotine Addiction: A Brief Introduction. Neuropharmacology 2020, 177, 108256. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, B.; Piper, M.E.; Fowler, C.D.; Tonstad, S.; Bierut, L.; Lu, L.; Jha, P.; Hall, W.D. Tobacco and Nicotine Use. Nat. Rev. Dis. Primers 2022, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Sharma, V.; Pandey, R.K.; Shukla, S.S. Nicotine Addiction: Neurobiology and Mechanism. J. Pharmacopunct. 2020, 23, 1–7. [Google Scholar] [CrossRef]
- Prochaska, J.J.; Benowitz, N.L. The Past, Present, and Future of Nicotine Addiction Therapy. Annu. Rev. Med. 2016, 67, 467–486. [Google Scholar] [CrossRef]
- Konno, S.; Chiao, J.W.; Wu, J.M. Effects of Nicotine on Cellular Proliferation, Cell Cyclephase Distribution, and Macromolecular Synthesis in Human Promyelocytic HL-60 Leukemia Cells. Cancer Lett. 1986, 33, 91–97. [Google Scholar] [CrossRef]
- Bishun, N.P.; Lloyd, N.; Raven, R.W.; Williams, D.C. The in Vitro and in Vivo Cytogenetic Effects of Nicotine. Acta Biol. Acad. Sci. Hung. 1972, 23, 175–180. [Google Scholar]
- Riebe, M.; Westphal, K. Studies on the Induction of Sister-Chromatid Exchanges in Chinese Hamster Ovary Cells by Various Tobacco Alkaloids. Mutat. Res./Genet. Toxicol. 1983, 124, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.H.; Dave, B.J.; Adhvaryu, S.G. Assessment of Genotoxicity of Nicotine Employing in Vitro Mammalian Test System. Cancer Lett. 1990, 54, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Nastrucci, C.; Cesario, A.; Russo, P. Nicotine: Specific Role in Angiogenesis, Proliferation and Apoptosis. Crit. Rev. Toxicol. 2012, 42, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Catassi, A.; Servent, D.; Paleari, L.; Cesario, A.; Russo, P. Multiple Roles of Nicotine on Cell Proliferation and Inhibition of Apoptosis: Implications on Lung Carcinogenesis. Mutat. Res./Rev. Mutat. Res. 2008, 659, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Cardinale, A.; Margaritora, S.; Cesario, A. Nicotinic Receptor and Tobacco-Related Cancer. Life Sci. 2012, 91, 1087–1092. [Google Scholar] [CrossRef]
- Russo, P.; Bufalo, A.; Milic, M.; Salinaro, G.; Fini, M.; Cesario, A. Cholinergic Receptors as Target for Cancer Therapy in a Systems Medicine Perspective. CMM 2014, 14, 1126–1138. [Google Scholar] [CrossRef]
- Trombino, S.; Bisio, A.; Catassi, A.; Cesario, A.; Falugi, C.; Russo, P. Role of the Non-Neuronal Human Cholinergic System in Lung Cancer and Mesothelioma: Possibility of New Therapeutic Strategies. CMCACA 2004, 4, 535–542. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, S.; Wu, K.; Wu, S.-Y.; Xing, F.; Liu, Y.; Zhao, D.; Deshpande, R.P.; D’Agostino, R.B.; Watabe, K. Nicotine Promotes Breast Cancer Metastasis by Stimulating N2 Neutrophils and Generating Pre-Metastatic Niche in Lung. Nat. Commun. 2021, 12, 474. [Google Scholar] [CrossRef]
- Murphy, S.E. Biochemistry of Nicotine Metabolism and Its Relevance to Lung Cancer. J. Biol. Chem. 2021, 296, 100722. [Google Scholar] [CrossRef]
- Santoro, A.; Tomino, C.; Prinzi, G.; Lamonaca, P.; Cardaci, V.; Fini, M.; Russo, P. Tobacco Smoking: Risk to Develop Addiction, Chronic Obstructive Pulmonary Disease, and Lung Cancer. Recent Pat. Anti-Cancer Drug Discov. 2019, 14, 39–52. [Google Scholar] [CrossRef]
- Grozio, A.; Catassi, A.; Cavalieri, Z.; Paleari, L.; Cesario, A.; Russo, P. Nicotine, Lung and Cancer. Anti-Cancer Agents Med. Chem. 2007, 7, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Regulatory Role of the α7nAChR in Cancer. Curr. Drug Targets 2012, 13, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Khodabandeh, Z.; Valilo, M.; Velaei, K.; Pirpour Tazehkand, A. The Potential Role of Nicotine in Breast Cancer Initiation, Development, Angiogenesis, Invasion, Metastasis, and Resistance to Therapy. Breast Cancer 2022, 29, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine Induces Cell Proliferation, Invasion and Epithelial-Mesenchymal Transition in a Variety of Human Cancer Cell Lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef]
- Carlisle, D.L.; Liu, X.; Hopkins, T.M.; Swick, M.C.; Dhir, R.; Siegfried, J.M. Nicotine Activates Cell-Signaling Pathways through Muscle-Type and Neuronal Nicotinic Acetylcholine Receptors in Non-Small Cell Lung Cancer Cells. Pulm. Pharmacol. Ther. 2007, 20, 629–641. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Zhang, J.; Xiao, M.; Cui, S.; Wu, S.; Jin, C.; Yang, J.; Lu, X. Transcription Factor SP1 and Oncoprotein PPP1R13L Regulate Nicotine-Induced Epithelial-Mesenchymal Transition in Lung Adenocarcinoma via a Feedback Loop. Biochem. Pharmacol. 2022, 206, 115344. [Google Scholar] [CrossRef]
- Jasper, A.E.; Sapey, E.; Thickett, D.R.; Scott, A. Understanding Potential Mechanisms of Harm: The Drivers of Electronic Cigarette-Induced Changes in Alveolar Macrophages, Neutrophils, and Lung Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L336–L348. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, D.; Raja, A.; Grunig, G.; Zelikoff, J.; Jin, C. Downregulation of Stem-Loop Binding Protein by Nicotine via A7-Nicotinic Acetylcholine Receptor and Its Role in Nicotine-Induced Cell Transformation. Toxicol. Sci. 2022, 189, 186–202. [Google Scholar] [CrossRef]
- Schaal, C.; Chellappan, S.P. Nicotine-Mediated Cell Proliferation and Tumor Progression in Smoking-Related Cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef]
- Schaal, C.; Padmanabhan, J.; Chellappan, S. The Role of nAChR and Calcium Signaling in Pancreatic Cancer Initiation and Progression. Cancers 2015, 7, 1447–1471. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Naghipour, B.; Shahabi, P.; Dastmalchi, N.; Alipour, M.R. The role of microRNAs in nicotine signaling. EXCLI J. 2023, 22, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.J. Epigenetic and Long-Term Effects of Nicotine on Biology, Behavior, and Health. Pharmacol. Res. 2023, 192, 106741. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, V.B. A Cross-Cultural and Historical Survey of Tobacco Use Among Various Ethnic Groups. J. Ethn. Subst. Abus. 2008, 6, 9–80. [Google Scholar] [CrossRef] [PubMed]
- Buckland, P.C.; Panagiotakopulu, E. Ramses II and the Tobacco Beetle. Antiquity 2001, 75, 549–556. [Google Scholar] [CrossRef]
- Parsche, F.; Nerlich, A. Presence of Drugs in Different Tissues of an Egyptian Mummy. Fresenius J. Anal. Chem. 1995, 352, 380–384. [Google Scholar] [CrossRef]
- Pearsall, D.M. The Origins of Plant Cultivation in South America. In The Origins of Agriculture: An International Perspective; Cowan, C.W., Watson, P.J., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1992; pp. 173–204. [Google Scholar]
- Carmody, S.B.; Kassabaum, M.C.; Hunt, R.K.; Prodanovich, N.; Elliott, H.; Russ, J. Residue Analysis of Smoking Pipe Fragments from the Feltus Archaeological Site, Southeastern North America. J. Archaeol. Sci. Rep. 2018, 17, 640–649. [Google Scholar] [CrossRef]
- Tushingham, S.; Snyder, C.M.; Brownstein, K.J.; Damitio, W.J.; Gang, D.R. Biomolecular Archaeology Reveals Ancient Origins of Indigenous Tobacco Smoking in North American Plateau. Proc. Natl. Acad. Sci. USA 2018, 115, 11742–11747. [Google Scholar] [CrossRef] [PubMed]
- Loughmiller-Cardinal, J.; Eppich, K. (Eds.) Breath and Smoke Tobacco Use among the Maya; University of New Mexico Press: Albuquerque, NM, USA, 2019; 280p. [Google Scholar]
- Pane, R. An Account of the Antiquities of the Indians; Griswold, S.C., Translator; Duke Univ. Press: Durham, NC, USA, 1999. [Google Scholar]
- Gilman, S.L.; Zhou, X. Smoke: A Global History of Smoking; Reakton Books: London, UK, 2004. [Google Scholar]
- TOBACCO. Available online: Https://Www.Who.Int/News-Room/Fact-Sheets/Detail/Tobacco (accessed on 7 September 2023).
- Tobacco Plant Species. Available online: Https://Www.Britannica.Com/Plant/Common-Tobacco (accessed on 7 September 2023).
- Samuels, J. Biodiversity of Food Species of the Solanaceae Family: A Preliminary Taxonomic Inventory of Subfamily Solanoideae. Resources 2015, 4, 277–322. [Google Scholar] [CrossRef]
- Gately, I. Tobacco: A Cultural History of How an Exotic Plant Seduced Civilization; Simon & Schuster: New York, NY, USA, 2001. [Google Scholar]
- Knapp, S.; Chase, M.W.; Clarkson, J.J. Nomenclatural Changes and a New Sectional Classification in Nicotiana (Solanaceae). Taxon 2004, 53, 73–82. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological Roles and Biological Activities of Specialized Metabolites from the Genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G. The Nicotiana attenuata LECTIN RECEPTOR KINASE 1 Is Involved in the Perception of Insect Feeding. Plant Signal. Behav. 2011, 6, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Baudry, J.; Berenbaum, M.R.; Schuler, M.A. Structural and Functional Divergence of Insect CYP6B Proteins: From Specialist to Generalist Cytochrome P450. Proc. Natl. Acad. Sci. USA 2004, 101, 2939–2944. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. In Nicotine Psychopharmacology; Henningfield, J.E., London, E.D., Pogun, S., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 192, pp. 29–60. ISBN 978-3-540-69246-1. [Google Scholar]
- Lupacchini, L.; Maggi, F.; Tomino, C.; De Dominicis, C.; Mollinari, C.; Fini, M.; Bonassi, S.; Merlo, D.; Russo, P. Nicotine Changes Airway Epithelial Phenotype and May Increase the SARS-CoV-2 Infection Severity. Molecules 2020, 26, 101. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 89594, Nicotine.” PubChem. Available online: Https://Pubchem.Ncbi.Nlm.Nih.Gov/Compound/Nicotine. (accessed on 20 July 2023).
- Alkam, T.; Nabeshima, T. Molecular Mechanisms for Nicotine Intoxication. Neurochem. Int. 2019, 125, 117–126. [Google Scholar] [CrossRef]
- Mayer, B. How Much Nicotine Kills a Human? Tracing Back the Generally Accepted Lethal Dose to Dubious Self-Experiments in the Nineteenth Century. Arch. Toxicol. 2014, 88, 5–7. [Google Scholar] [CrossRef]
- Matta, S.G.; Balfour, D.J.; Benowitz, N.L.; Boyd, R.T.; Buccafusco, J.J.; Caggiula, A.R.; Craig, C.R.; Collins, A.C.; Damaj, M.I.; Donny, E.C.; et al. Guidelines on Nicotine Dose Selection for in Vivo Research. Psychopharmacology 2007, 190, 269–319. [Google Scholar] [CrossRef]
- Garduño-Sánchez, M.A.; de Jesus-Bonilla, V.; Perea, S.; Miranda-Gamboa, R.; Herrera-García, A.; De la Maza Benignos, M.; Ornelas-García, C.P. Mitochondrial Phylogeography and Molecular Evolution of the Rhodopsin Visual Pigment in Troglobitic Populations of Astyanax Mexicanus (De Filippi, 1853). Zool. Res. 2023, 44, 761–775. [Google Scholar] [CrossRef]
- Ho, T.N.T.; Abraham, N.; Lewis, R.J. Structure-Function of Neuronal Nicotinic Acetylcholine Receptor Inhibitors Derived From Natural Toxins. Front. Neurosci. 2020, 14, 609005. [Google Scholar] [CrossRef]
- Pawson, A.J.; Sharman, J.L.; Benson, H.E.; Faccenda, E.; Alexander, S.P.H.; Buneman, O.P.; Davenport, A.P.; McGrath, J.C.; Peters, J.A.; Southan, C.; et al. The IUPHAR/BPS Guide to PHARMACOLOGY: An Expert-Driven Knowledgebase of Drug Targets and Their Ligands. Nucl. Acids Res. 2014, 42, D1098–D1106. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond Neurons: The Non-Neuronal Cholinergic System in Humans: Non-Neuronal Cholinergic System in Humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef]
- Alexander, S.P.; Peters, J.A.; Kelly, E.; Marrion, N.V.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand-gated Ion Channels. Br. J Pharmacol. 2017, 174, S130–S159. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, D.; Terry, A.V. The Wonderland of Neuronal Nicotinic Acetylcholine Receptors. Biochem. Pharmacol. 2018, 151, 214–225. [Google Scholar] [CrossRef]
- Dani, J.A. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 124, pp. 3–19. ISBN 978-0-12-801583-4. [Google Scholar]
- Delgado-Vélez, M.; Quesada, O.; Villalobos-Santos, J.C.; Maldonado-Hernández, R.; Asmar-Rovira, G.; Stevens, R.C.; Lasalde-Dominicci, J.A. Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades. Molecules 2021, 26, 5753. [Google Scholar] [CrossRef] [PubMed]
- Elgoyhen, A.B. The A9α10 Acetylcholine Receptor: A Non-Neuronal Nicotinic Receptor. Pharmacol. Res. 2023, 190, 106735. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.I.; Krasteva-Christ, G. Nicotinic Acetylcholine Receptors in the Respiratory Tract. Molecules 2021, 26, 6097. [Google Scholar] [CrossRef]
- Grando, S.A.; Kawashima, K.; Kirkpatrick, C.J.; Meurs, H.; Wessler, I. The Non-Neuronal Cholinergic System: Basic Science, Therapeutic Implications and New Perspectives. Life Sci. 2012, 91, 969–972. [Google Scholar] [CrossRef]
- Paleari, L.; Grozio, A.; Cesario, A.; Russo, P. The Cholinergic System and Cancer. Semin. Cancer Biol. 2008, 18, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nastrucci, C.; Russo, P. 7 nAChR in Airway Respiratory Epithelial Cells. Curr. Drug Targets 2012, 13, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Olds, J.L.; Kabbani, N. Is Nicotine Exposure Linked to Cardiopulmonary Vulnerability to COVID-19 in the General Population? FEBS J. 2020, 287, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Sifat, A.E.; Nozohouri, S.; Villalba, H.; Vaidya, B.; Abbruscato, T.J. The Role of Smoking and Nicotine in the Transmission and Pathogenesis of COVID-19. J. Pharmacol. Exp. Ther. 2020, 375, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Bosetti, C.; Gorini, G.; Stival, C.; Boffi, R.; Lugo, A.; Carreras, G.; Veronese, C.; Santucci, C.; Pacifici, R.; et al. The Association of Tobacco Smoking, Second-Hand Smoke, and Novel Tobacco Products With COVID-19 Severity and Mortality in Italy: Results From the COSMO-IT Study. J. Epidemiol. 2023, 33, 367–371. [Google Scholar] [CrossRef]
- Gallus, S.; Scala, M.; Possenti, I.; Jarach, C.M.; Clancy, L.; Fernandez, E.; Gorini, G.; Carreras, G.; Malevolti, M.C.; Commar, A.; et al. The Role of Smoking in COVID-19 Progression: A Comprehensive Meta-Analysis. Eur. Respir. Rev. 2023, 32, 220191. [Google Scholar] [CrossRef]
- Leung, J.M.; Niikura, M.; Yang, C.W.T.; Sin, D.D. COVID-19 and COPD. Eur. Respir. J. 2020, 56, 2002108. [Google Scholar] [CrossRef]
- Sansone, L.; De Iure, A.; Cristina, M.; Belli, M.; Vitiello, L.; Marcolongo, F.; Rosellini, A.; Macera, L.; Spezia, P.G.; Tomino, C.; et al. Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity. Int. J. Mol. Sci. 2022, 23, 9488. [Google Scholar] [CrossRef]
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of Native Nicotinic Receptor Subtypes in Mammalian Brain. Neuropharmacology 2015, 96, 302–311. [Google Scholar] [CrossRef]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef]
- Natarajan, K.; Mukhtasimova, N.; Corradi, J.; Lasala, M.; Bouzat, C.; Sine, S.M. Mechanism of Calcium Potentiation of the A7 Nicotinic Acetylcholine Receptor. J. Gen. Physiol. 2020, 152, e202012606. [Google Scholar] [CrossRef]
- Centner, A.M.; Bhide, P.G.; Salazar, G. Nicotine in Senescence and Atherosclerosis. Cells 2020, 9, 1035. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Liakoni, E. Tobacco Use Disorder and Cardiovascular Health. Addiction 2022, 117, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L. The Vagus Nerve and the Nicotinic Anti-Inflammatory Pathway. Nat. Rev. Drug Discov. 2005, 4, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, W.; Wang, H.; Geng, P.; Sun, Y.; Zhang, J.; Wang, W.; Jin, X. Nicotine’s Effect on Cognition, a Friend or Foe? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 124, 110723. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Porter Papke, J.K. Comparative Pharmacology of Rat and Human A7 nAChR Conducted with Net Charge Analysis: Pharmacology of Rat and Human α7AChR. Br. J. Pharmacol. 2002, 137, 49–61. [Google Scholar] [CrossRef]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of α 7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef]
- Burns, L.H.; Pei, Z.; Wang, H. Targeting A7 Nicotinic Acetylcholine Receptors and Their Protein Interactions in Alzheimer’s Disease Drug Development. Drug Dev. Res. 2023, 84, ddr.22085. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob, P.; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob. Res. 2020, 22, 1086–1097. [Google Scholar] [CrossRef]
- Maggi, F.; Rosellini, A.; Spezia, P.G.; Focosi, D.; Macera, L.; Lai, M.; Pistello, M.; De Iure, A.; Tomino, C.; Bonassi, S.; et al. Nicotine Upregulates ACE2 Expression and Increases Competence for SARS-CoV-2 in Human Pneumocytes. ERJ Open Res. 2021, 7, 00713–02020. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Insights into the Mechanisms of Action of Proanthocyanidins and Anthocyanins in the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Jiang, W.; Zhan, W.; Xiong, C.; Chen, J.; Wang, Y.; Jia, H.; Lei, M. The Therapeutic Potential of Quercetin for Cigarette Smoking–Induced Chronic Obstructive Pulmonary Disease: A Narrative Review. Ther. Adv. Respir. Dis. 2023, 17, 175346662311708. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Scutellaria Baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. [Google Scholar] [CrossRef]
- Riley, B.; Williamson, M.; Collier, D.; Wilkie, H.; Makoff, A. A 3-Mb Map of a Large Segmental Duplication Overlapping the A7-Nicotinic Acetylcholine Receptor Gene (CHRNA7) at Human 15q13–Q14. Genomics 2002, 79, 197–209. [Google Scholar] [CrossRef]
- Locke, D.P.; Archidiacono, N.; Misceo, D.; Cardone, M.; Deschamps, S.; Roe, B.; Rocchi, M.; Eichler, E.E. Refinement of a chimpanzee pericentric inversion breakpoint to a segmental duplication cluster. Genome Biol. 2003, 4, R50. [Google Scholar] [CrossRef]
- Villiger, Y.; Szanto, I.; Jaconi, S.; Blanchet, C.; Buisson, B.; Krause, K.-H.; Bertrand, D.; Romand, J.-A. Expression of an A7 Duplicate Nicotinic Acetylcholine Receptor-Related Protein in Human Leukocytes. J. Neuroimmunol. 2002, 126, 86–98. [Google Scholar] [CrossRef]
- De Lucas-Cerrillo, A.M.; Maldifassi, M.C.; Arnalich, F.; Renart, J.; Atienza, G.; Serantes, R.; Cruces, J.; Sánchez-Pacheco, A.; Andrés-Mateos, E.; Montiel, C. Function of Partially Duplicated Human A7 Nicotinic Receptor Subunit CHRFAM7A Gene. J. Biol. Chem. 2011, 286, 594–606. [Google Scholar] [CrossRef]
- Flomen, R.H.; Shaikh, M.; Walshe, M.; Schulze, K.; Hall, M.-H.; Picchioni, M.; Rijsdijk, F.; Toulopoulou, T.; Kravariti, E.; Murray, R.M.; et al. Association between the 2-Bp Deletion Polymorphism in the Duplicated Version of the Alpha7 Nicotinic Receptor Gene and P50 Sensory Gating. Eur. J. Hum. Genet. 2013, 21, 76–81. [Google Scholar] [CrossRef]
- Drisdel, R.C.; Green, W.N. Neuronal α-Bungarotoxin Receptors Are A7 Subunit Homomers. J. Neurosci. 2000, 20, 133–139. [Google Scholar] [CrossRef]
- Kabbani, N.; Nichols, R.A. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol. Sci. 2018, 39, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Taly, A. A7-Nicotinic Acetylcholine Receptors: An Old Actor for New Different Roles. Curr. Drug Targets 2012, 13, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.; Corradi, J.; Sine, S.M.; Bouzat, C. Stoichiometry for Activation of Neuronal A7 Nicotinic Receptors. Proc. Natl. Acad. Sci. USA. 2013, 110, 20819–20824. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Tang, P.; Mikkelsen, J.D.; Shen, J.; Whiteaker, P.; Yakel, J.L. Heteromeric A7β2 Nicotinic Acetylcholine Receptors in the Brain. Trends Pharmacol. Sci. 2016, 37, 562–574. [Google Scholar] [CrossRef]
- Uteshev, V.V.; Meyer, E.M.; Papke, R.L. Regulation of Neuronal Function by Choline and 4OH-GTS-21 Through A7 Nicotinic Receptors. J. Neurophysiol. 2003, 89, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.K.; Peng, C.; Kimbrell, M.R.; Papke, R.L. Intrinsically Low Open Probability of A7 Nicotinic Acetylcholine Receptors Can Be Overcome by Positive Allosteric Modulation and Serum Factors Leading to the Generation of Excitotoxic Currents at Physiological Temperatures. Mol. Pharmacol. 2012, 82, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Steinbach, J.H. The Neuronal Nicotinic A4β2 Receptor Has a High Maximal Probability of Being Open: ACh Is Highly Efficacious at the A4β2 Receptor. Br. J. Pharmacol. 2010, 160, 1906–1915. [Google Scholar] [CrossRef]
- Lansdell, S.J.; Gee, V.J.; Harkness, P.C.; Doward, A.I.; Baker, E.R.; Gibb, A.J.; Millar, N.S. RIC-3 Enhances Functional Expression of Multiple Nicotinic Acetylcholine Receptor Subtypes in Mammalian Cells. Mol. Pharmacol. 2005, 68, 1431–1438. [Google Scholar] [CrossRef]
- Alexander, J.K.; Sagher, D.; Krivoshein, A.V.; Criado, M.; Jefford, G.; Green, W.N. Ric-3 Promotes A7 Nicotinic Receptor Assembly and Trafficking through the ER Subcompartment of Dendrites. J. Neurosci. 2010, 30, 10112–10126. [Google Scholar] [CrossRef]
- Halevi, S.; Yassin, L.; Eshel, M.; Sala, F.; Sala, S.; Criado, M.; Treinin, M. Conservation within the RIC-3 Gene Family. J. Biol. Chem. 2003, 278, 34411–34417. [Google Scholar] [CrossRef]
- Matta, J.A.; Gu, S.; Davini, W.B.; Lord, B.; Siuda, E.R.; Harrington, A.W.; Bredt, D.S. NACHO Mediates Nicotinic Acetylcholine Receptor Function throughout the Brain. Cell Rep. 2017, 19, 688–696. [Google Scholar] [CrossRef]
- Kuryatov, A.; Mukherjee, J.; Lindstrom, J. Chemical Chaperones Exceed the Chaperone Effects of RIC-3 in Promoting Assembly of Functional A7 AChRs. PLoS ONE 2013, 8, e62246. [Google Scholar] [CrossRef]
- Drisdel, R.C.; Manzana, E.; Green, W.N. The Role of Palmitoylation in Functional Expression of Nicotinic A7 Receptors. J. Neurosci. 2004, 24, 10502–10510. [Google Scholar] [CrossRef]
- Li, S.; Nai, Q.; Lipina, T.V.; Roder, J.C.; Liu, F. α7nAchR/NMDAR Coupling Affects NMDAR Function and Object Recognition. Mol. Brain 2013, 6, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Li, T.; Li, S.; Liu, F. Cross-Talk between A7 nAchR and NMDAR Revealed by Protein Profiling. J. Proteom. 2016, 131, 113–121. [Google Scholar] [CrossRef]
- Yang, Y.; Paspalas, C.D.; Jin, L.E.; Picciotto, M.R.; Arnsten, A.F.T.; Wang, M. Nicotinic A7 Receptors Enhance NMDA Cognitive Circuits in Dorsolateral Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2013, 110, 12078–12083. [Google Scholar] [CrossRef] [PubMed]
- Lozada, A.F.; Wang, X.; Gounko, N.V.; Massey, K.A.; Duan, J.; Liu, Z.; Berg, D.K. Glutamatergic Synapse Formation Is Promoted by A7-Containing Nicotinic Acetylcholine Receptors. J. Neurosci. 2012, 32, 7651–7661. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Shulepko, M.A.; Buldakova, S.L.; Kasheverov, I.E.; Shenkarev, Z.O.; Reshetnikov, R.V.; Filkin, S.Y.; Kudryavtsev, D.S.; Ojomoko, L.O.; Kryukova, E.V.; et al. Water-Soluble LYNX1 Residues Important for Interaction with Muscle-Type and/or Neuronal Nicotinic Receptors. J. Biol. Chem. 2013, 288, 15888–15899. [Google Scholar] [CrossRef]
- Slominski, A. Nicotinic Receptor Signaling in Nonexcitable Epithelial Cells: Paradigm Shifting from Ion Current to Kinase Cascade. Focus on “Upregulation of Nuclear Factor-κB Expression by SLURP-1 Is Mediated by α 7 -Nicotinic Acetylcholine Receptor and Involves Both Ionic Events and Activation of Protein Kinases”. Am. J. Physiol.-Cell Physiol. 2010, 299, C885–C887. [Google Scholar] [CrossRef]
- Grando, S.A.; Kawashima, K.; Wessler, I. A Historic Perspective on the Current Progress in Elucidation of the Biologic Significance of Non-Neuronal Acetylcholine. Int. Immunopharmacol. 2020, 81, 106289. [Google Scholar] [CrossRef]
- Wessler, I.K.; Kirkpatrick, C.J. Activation of Muscarinic Receptors by Non-Neuronal Acetylcholine. In Muscarinic Receptors; Fryer, A.D., Christopoulos, A., Nathanson, N.M., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 208, pp. 469–491. ISBN 978-3-642-23273-2. [Google Scholar]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Non-Neuronal Cholinergic System in Regulation of Immune Function with a Focus on A7 nAChRs. Int. Immunopharmacol. 2015, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-C. Insights from Modelling the 3D Structure of the Extracellular Domain of A7 Nicotinic Acetylcholine Receptor. Biochem. Biophys. Res. Commun. 2004, 319, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, K.; Chen, Y.; Jiang, Y.; Zhou, Z.; Liu, J.; Du, Y.; Wang, L.; Han, X.; Wu, X.; et al. Impaired Autophagy Flux by lncRNA NEAT1 Is Critical for Inflammation Factors Production in Human Periodontal Ligament Stem Cells with Nicotine Treatment. J. Periodontal Res. 2023, 58, 70–82. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Description | References |
| CAS Name | (-)-Nicotine | [54] |
| CAS Registry Number® | 54-11-5 | [54] |
| Other Names for This Substance | Pyridine, 3-[(2S)-1-methyl-2-pyrrolidinyl]-. Pyridine, 3-(1-methyl-2-pyrrolidinyl)-, (S)-. 3-[(2S)-1-Methyl-2-pyrrolidinyl]pyridine. (-)-3-(1-Methyl-2-pyrrolidyl)pyridine. Nicotine. | [54] |
| Chemical Specification and Classes | Bicyclic molecule characterized by a pyridine cycle and a pyrrolidine cycle existing in natures only in the S shape (i.e., levogyre). Biological Agents -> Plant Toxins. | [54] |
| Physical Description | Nicotine appears as a colorless to light yellow or brown liquid. Combustible. Produces toxic oxides of nitrogen during combustion. Fish-like odor when warm. Chemical formula: C10H14N2, M W: 162.234 | [55] |
| Physical Characteristics | Boiling Point: 247 °C; 125 deg at 18 mm Hg or 476.1 °F at 745 mmHg. Melting Point: −79 °C or 110 °F. Flash Point: 95 °C or 203 °F. Solubility: miscible with water below 60 °C; very sol in alcohol, chloroform, ether, petroleum ether, kerosene, oils. Density: 1.00925 at 20 °C/4 °C or 1.0097 at 68 °F. Vapor Density: 5.61 (Air = 1). Vapor Pressure: 0.038 mm Hg at 25 °C or 1 mmHg at 143.24 °F. Autoignition Temperature: 240 °C or 471 °F. | [55] |
| SMILES | CN1CCC[C@H]1C1=CC=CN=C1 | [55] |
| IUPAC (InChI) | InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2, 4, 6, 8, 10H, 3, 5, 7H2, 1H3/t10-/m0/s1 check Key: SNICXCGAKADSCV-JTQLQIEISA-N | [55] |
| Natural Source and Chemical Isolation | Synthesized as secondary metabolite by plants of the family Solanaceae, genus Nicotiana, species Nicotiana tabacum. | [55] |
| Absorption | Across biological membranes depends on pH. Nicotine is a weak base, with a pKa of 8.0. | [55] |
| Metabolism | Metabolized in the liver, principally to cotinine, which in turn is metabolized to trans-30 -hydroxycotinine excreted renally. In smokers, more than 90% of the nicotine dose is accounted for by eight metabolites: nicotine N-oxide, nicotine glucuronide, cotinine, cotinine glucuronide, cotinine N-oxide, 3′-hydroxycotinine, 3′-hydroxycotinine glucuronide. | [55] |
| Human Lethal Dose (LD100) | No consensus on the human LD100 for adults of 60 mg, resulting in approximately 180 µg L−1 plasma concentration. | [56,57] |
| Nicotine in One Tobacco Cigarette | 11.9–14.5 mg of nicotine. On average, a person only absorbs 1–1.5 mg of nicotine from a single stick. | [58] |
| Biological Effects | Comments | References |
|---|---|---|
| SUDs: Addictive Properties | Involve the integration of contrasting signals from multiple brain regions that process reward and aversion, attributed to the mesolimbic pathway. | [4,5,6,7,8,9] |
| Senescence and Atherosclerosis | Nicotine increases MAPK signaling, inflammation, and oxidative stress through NADPH oxidase 1 (Nox1) to induce vascular smooth muscle cell (VSMC) senescence. The accumulation of senescent VSMCs increases the pathogenesis of atherosclerosis by promoting an unstable plaque phenotype. | [83] |
| Vascular Dysfunction | Nicotine induces vascular remodeling through its effects on proliferation, migration and matrix reduction. Acute effects: myocardial infarction, stroke and sudden cardiac death. Chronic effects: inflammation, thrombogenesis, endothelial dysfunction, hemodynamic stress, arrythmogenesis, insulin resistance and lipid abnormalities. | [84] |
| COVID-19 | Smoking is a potential risk factor for COVID-19 since nicotine upregulates ACE2 expression. | [74,75,76,77,78,79] |
| Memory and Cognition | Nicotine administration can improve cognitive impairment in Alzheimer’s disease and Parkinson’s disease. Nicotine may also activate thyroid receptor signaling pathways to improve memory impairment caused by hypothyroidism. In healthy individuals, nicotine improves memory impairment caused by sleep deprivation by enhancing the phosphorylation of calmodulin-dependent protein kinase II. | [85,86] |
| Immune System | Inhibits innate and acquired immunity. Chronic exposure of nicotine plays a critical role in initiating neutrophil recruitment and premetastatic niche formation by skewing neutrophil toward a tumor-supporting phenotype. | [85] |
| Anti-Inflammatory Function of the Vagus Nerve | Recent studies indicate that the vagus nerve can modulate the immune response and control inflammation through a ‘nicotinic anti-inflammatory pathway’ dependent on the α7 nAChR. Nicotine has been used in clinical trials for the treatment of ulcerative colitis. | [85] |
| Autoimmune Disease | Multiple sclerosis: nicotine slows down the demyelination process. Rheumatoid arthritis: treatment with nicotine could reduce some of the hematological and biochemical parameters of rats. Sarcoidosis: nicotine increases T.reg levels and decreases TLR2 and TLR9 expression. Inflammatory bowel disease: decreases inflammatory cytokines. Type I diabetes: balance Th1/Th2 ratio. Increases Th1 related cytokines. | [85] |
| Cancer | Nicotine promotes angiogenesis, proliferation, and epithelial–mesenchymal transition and growth and metastasis of tumors. Different reviews have explored this association in detail. | [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] |
| α7nAChR | References |
|---|---|
| CHRNA7 (Homo sapiens, NACHRA7) is located on chromosome 15q12.13 | [96] |
| CHRNA7 is partially duplicated with FAM7A (exons A–E), forming the chimera gene CHRFAM7A. Simultaneous transcription of CHRNA7 and CHRFAM7A generates α7 and dupα7 proteins. dupα7 may modulate α7-mediated synaptic transmission or cholinergic anti-inflammatory reaction. | [97,98,99] |
| CHRFAM7A exists in two orientations with respect to CHRNA7. Expression of CHRFAM7A alone generates protein expression but no functional receptor. | [100] |
| α7 may be considered a primordial type of receptor because it apparently evolved without additional gene duplications. | [101] |
| α7 may operate both in ionotropic and metabotropic modes, leading to CICR and G-protein-associated inositol trisphosphate-induced calcium release. | [102] |
| α7 shows high permeability to Ca2+. α7 activates multiple Ca2+ amplification pathways. α7 is modulated by extracellular Ca2+ concentrations. | [103] |
| α7 may bind two–five molecules of agonist and modulates cellular functions via phosphorylation and/or via Ca2+-dependent serine/threonine kinases. | [103] |
| α7 is functional without co-assembling with specialized accessory subunits as required by other nAChR subtypes. | [103] |
| α7 may co-assemble with β2, forming the functional α7β2 receptors expressed in human basal forebrain neurons and cerebral cortical neurons. | [104,105] |
| Choline is the least potent agonist for α7, with a potency approximately 10-fold lower than Ach. α7 choline-activated current may play an important role in Ca2+ homeostasis regulation in α7-expressing cells. | [87,106] |
| The low probability of α7 being open can be overcome by positive allosteric modulation and serum factors, leading to the generation of excitotoxic currents at physiological temperatures. | [107,108] |
| The activity of RIC-3 is critical for the folding, maturation and functional expression of nAChR. α7 needs RIC-3 activity for biogenesis and cell-surface expression. | [109,110,111] |
| α7 requires NACHO, a small multi-pass transmembrane protein enriched in neuronal ER, in combination with RIC-3 for proper assembly. | [112] |
| Since great amounts of α7 also remain improperly assembled in the presence of RIC-3, it has been suggested that additional chaperone such as cholinergic ligands may promote the α7 assembly. | [113] |
| α7 is palmitoylated with a stoichiometry of approximately one palmitate/subunit during the assembly in the ER. | [114] |
| α7 regulates NMDAR, forming a complex α7nAchR/NMDAR via protein–protein interaction. | [115,116] |
| α7 stimulation is needed for NMDA actions. | [117] |
| α7 promotes the formation of glutamatergic synapses during development. | [118] |
| The endogenous “prototoxin” LYNX1, belonging to the Ly6 protein family, binds α7 within the extracellular domain, leaving the classical binding site for agonists and competitive antagonists of α7 nAChR unoccupied. | [119] |
| The prototoxin SLURP-1 is a positive allosteric modulator of α7. | [120] |
| α7nAChR is the major nicotinic subtype that is highly expressed in the brain (olfactory bulb, cerebral cortex, hippocampus, hypothalamus and amygdale), as well as in non-neuronal cells (epithelial, immunological, etc.). | [71,121,122,123] |
| The 3D structure of human α7nAChR is still to be elucidated. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansone, L.; Milani, F.; Fabrizi, R.; Belli, M.; Cristina, M.; Zagà, V.; de Iure, A.; Cicconi, L.; Bonassi, S.; Russo, P. Nicotine: From Discovery to Biological Effects. Int. J. Mol. Sci. 2023, 24, 14570. https://doi.org/10.3390/ijms241914570
Sansone L, Milani F, Fabrizi R, Belli M, Cristina M, Zagà V, de Iure A, Cicconi L, Bonassi S, Russo P. Nicotine: From Discovery to Biological Effects. International Journal of Molecular Sciences. 2023; 24(19):14570. https://doi.org/10.3390/ijms241914570
Chicago/Turabian StyleSansone, Luigi, Francesca Milani, Riccardo Fabrizi, Manuel Belli, Mario Cristina, Vincenzo Zagà, Antonio de Iure, Luca Cicconi, Stefano Bonassi, and Patrizia Russo. 2023. "Nicotine: From Discovery to Biological Effects" International Journal of Molecular Sciences 24, no. 19: 14570. https://doi.org/10.3390/ijms241914570
APA StyleSansone, L., Milani, F., Fabrizi, R., Belli, M., Cristina, M., Zagà, V., de Iure, A., Cicconi, L., Bonassi, S., & Russo, P. (2023). Nicotine: From Discovery to Biological Effects. International Journal of Molecular Sciences, 24(19), 14570. https://doi.org/10.3390/ijms241914570










