Nuciferine Inhibits Oral Squamous Cell Carcinoma Partially through Suppressing the STAT3 Signaling Pathway
Abstract
:1. Introduction
2. Results
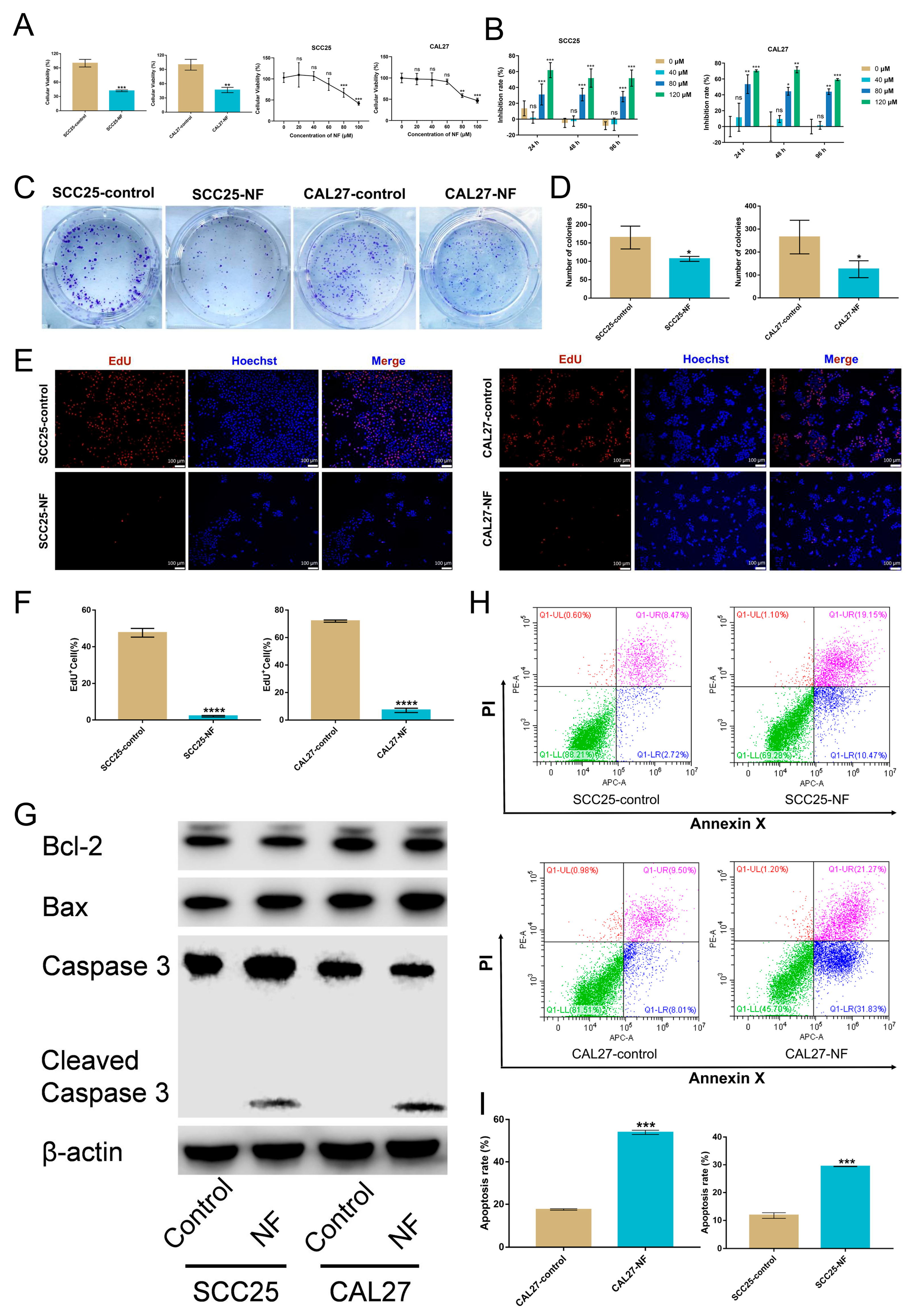
2.1. Nuciferine Inhibited the Proliferation and Promoted the Apoptosis of OSCC Cells
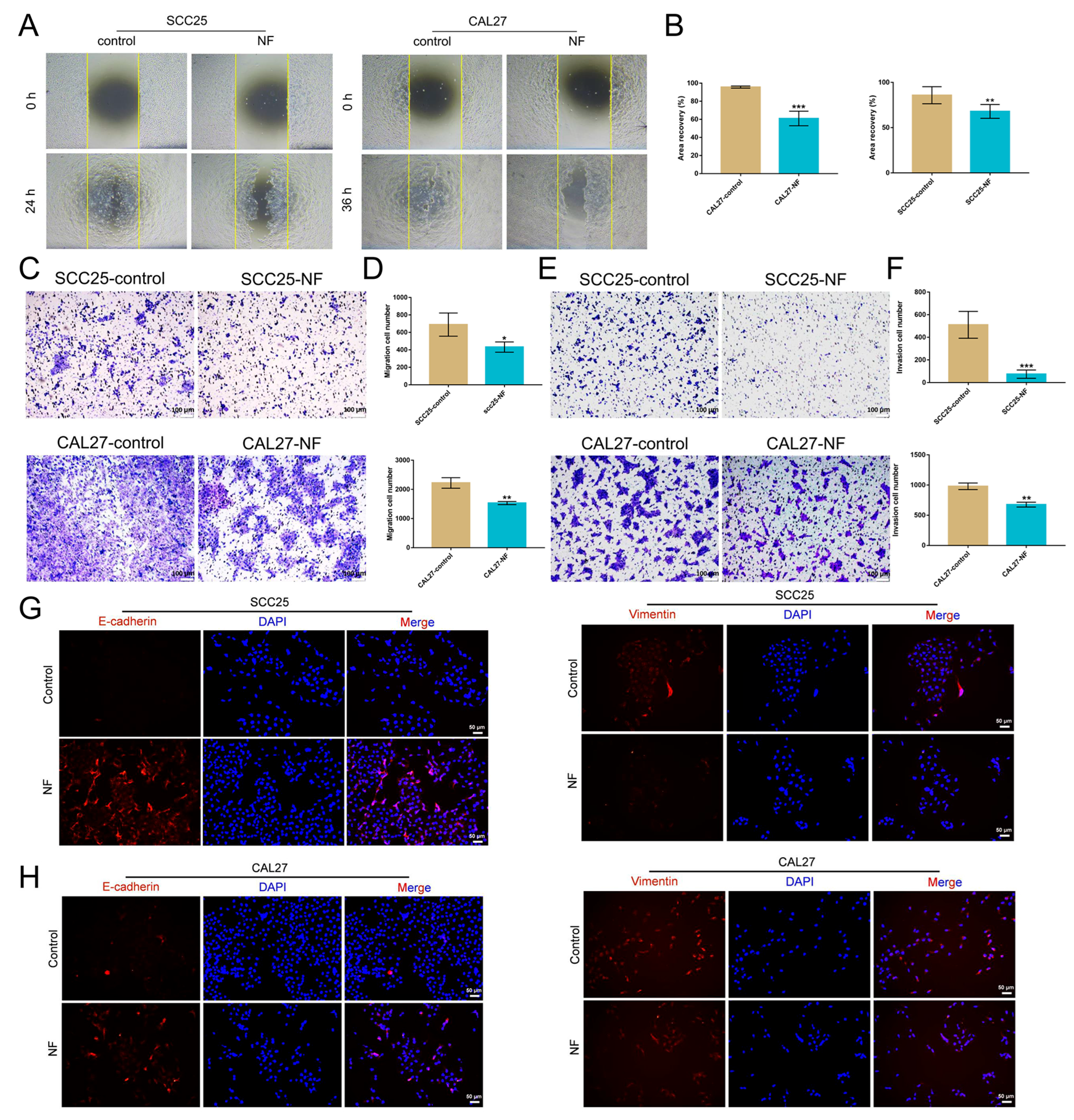
2.2. Nuciferine Inhibited the Migration and Invasion of OSCC Cells
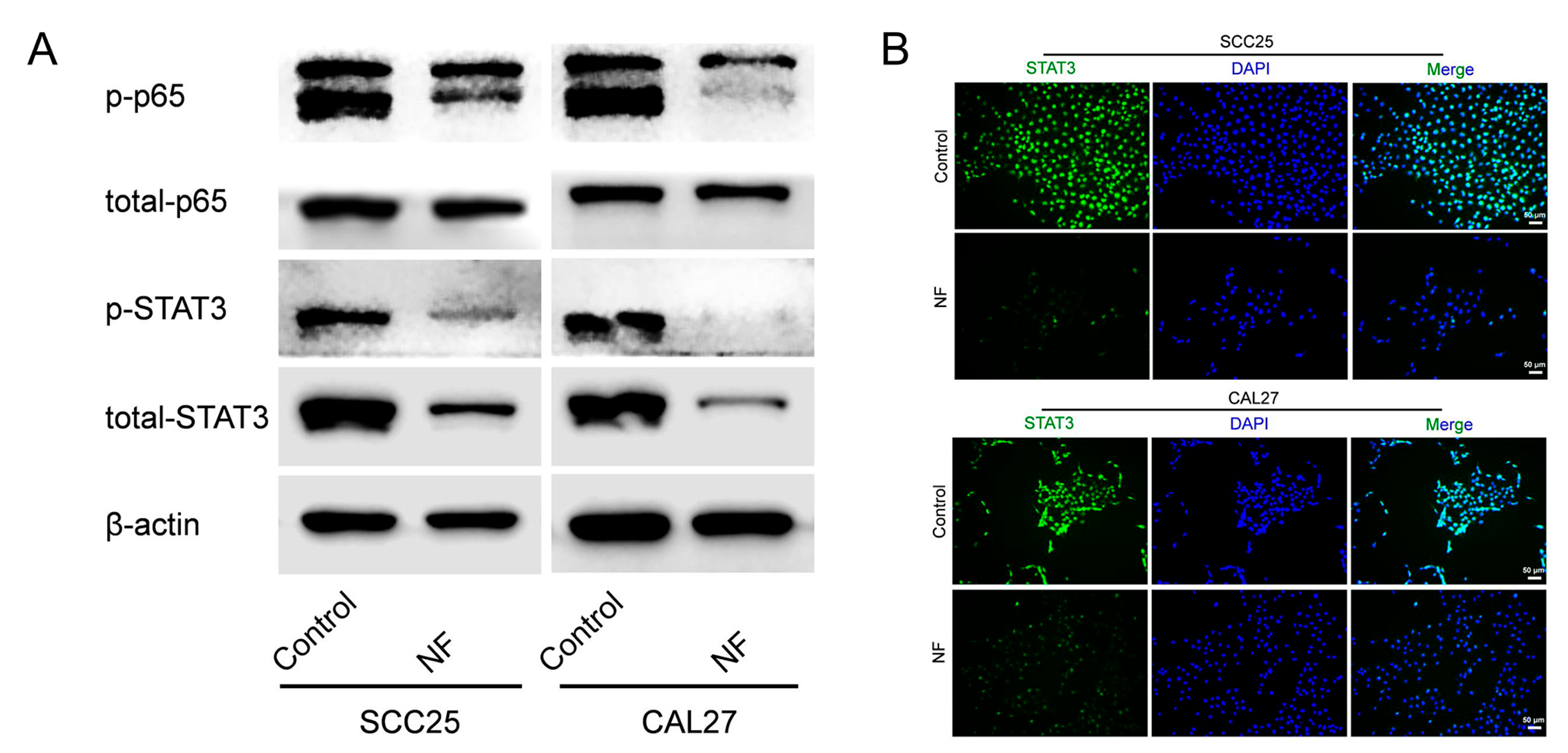
2.3. STAT3 Signaling Pathway Was Suppressed in Nuciferine-Treated OSCC Cells
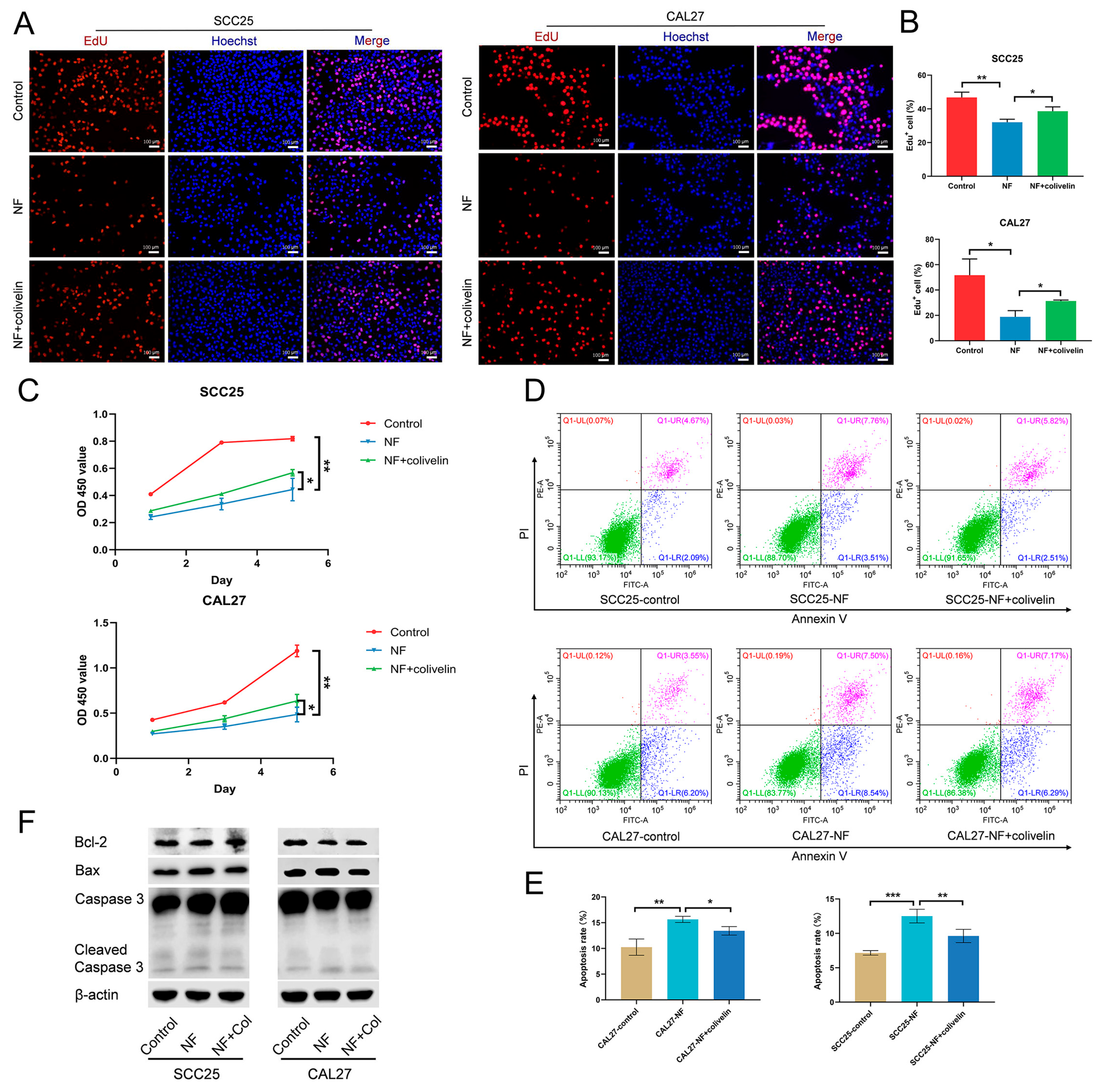
2.4. Nuciferine Inhibited the Proliferation and Promoted the Apoptosis of OSCC Cells via Suppressing the STAT3 Signaling Pathway
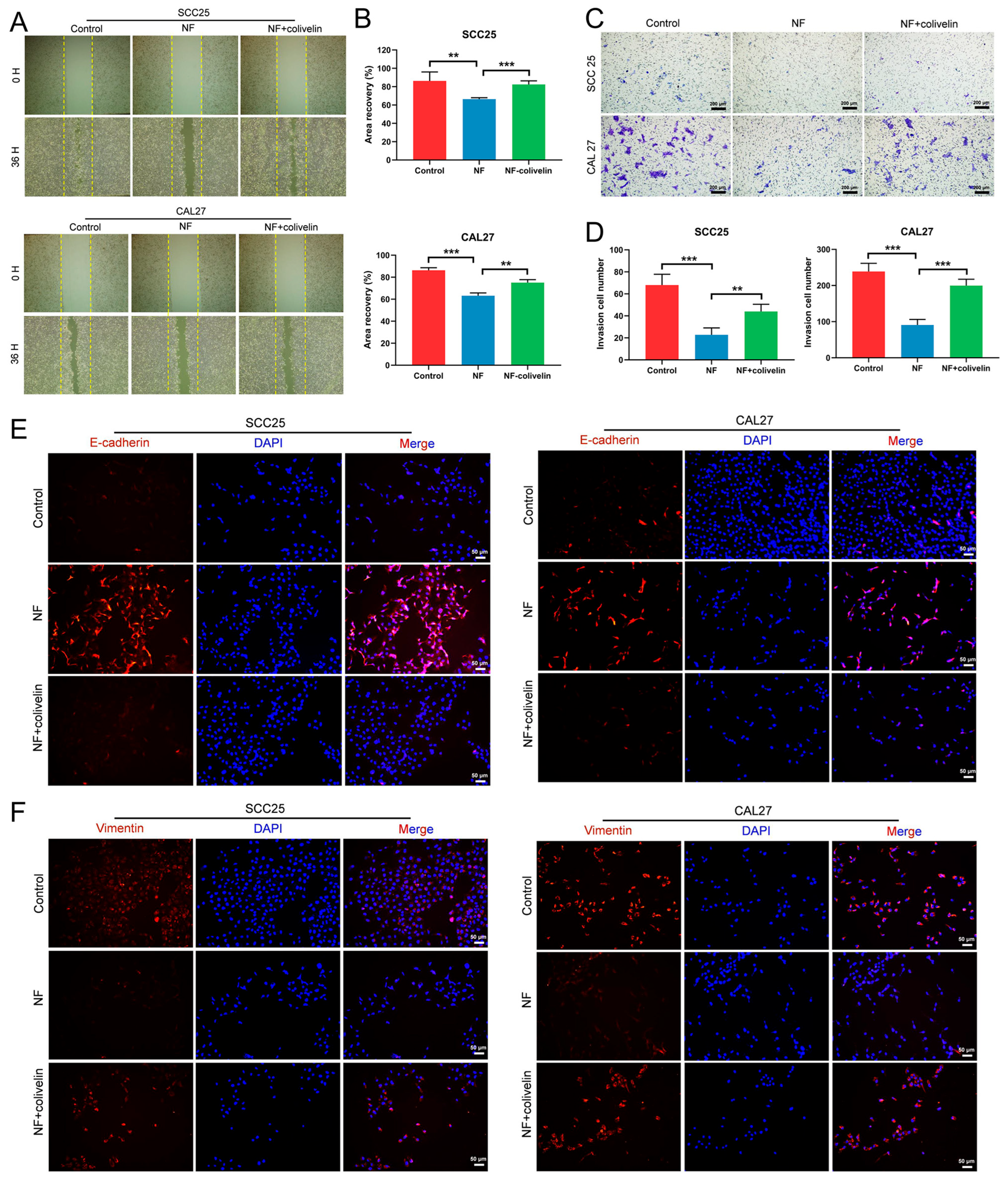
2.5. Nuciferine Inhibited the Migration and Invasion of OSCC Cells via Suppressing the STAT3 Signaling Pathway
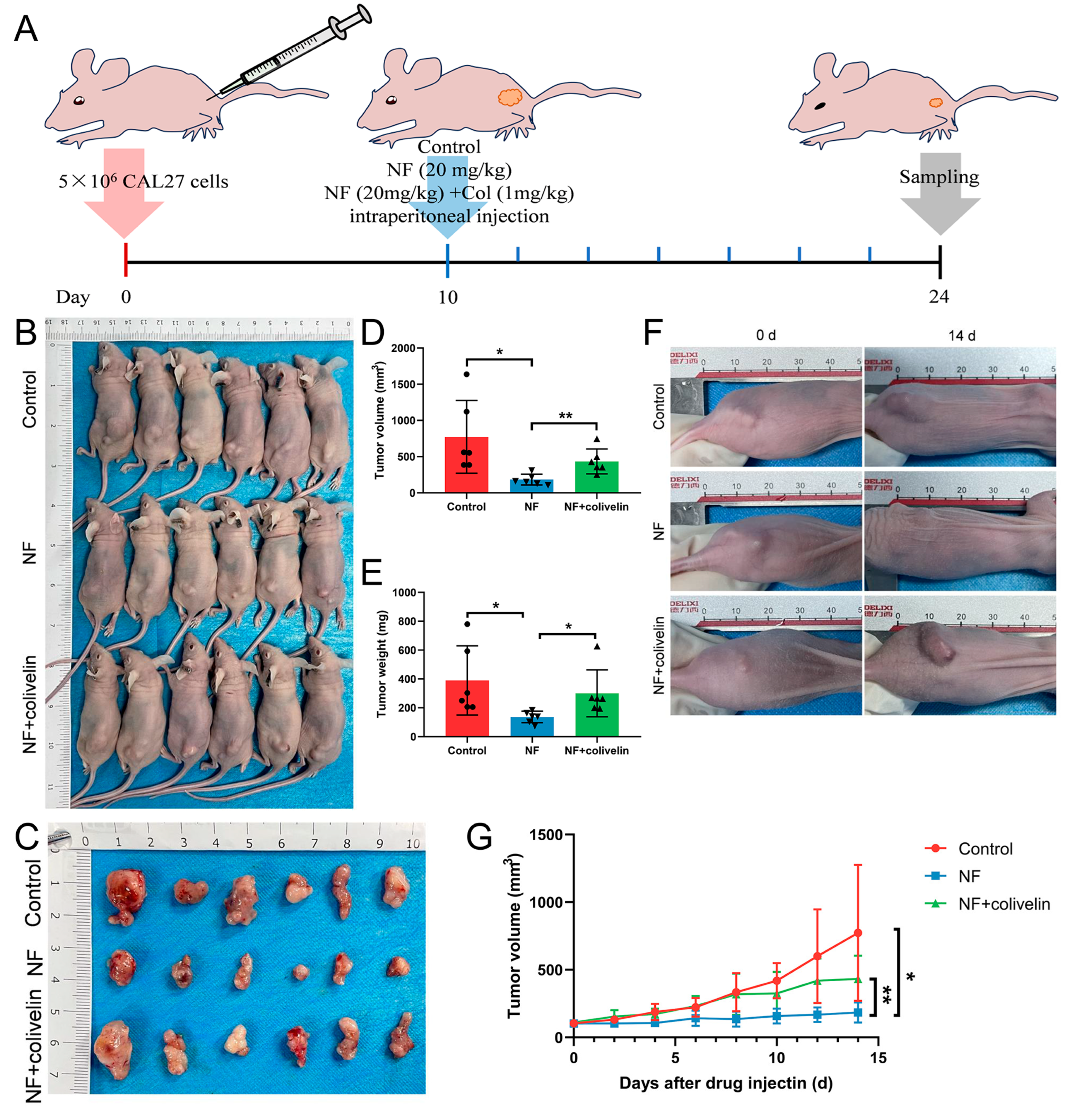
2.6. Nuciferine Inhibited OSCC Growth via Suppressing the STAT3 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents Antibodies and Cell Culture
4.2. Cell Counting Kit-8 (CCK-8) Assay
4.3. Plate Colony-Forming Assay
4.4. Ethynyl-2-Deoxyuridine (EdU) Assay
4.5. Flow Cytometry
4.6. Wound-Healing Assay
4.7. Transwell Assay
4.8. Western Blot
4.9. Immunofluorescence
4.10. Cell-Derived Xenograft (CDX) Tumor Model
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, A.W.Y.; Lim, K.P.; Cheong, S.C. Translational genomics and recent advances in oral squamous cell carcinoma. Semin. Cancer Biol. 2020, 61, 71–83. [Google Scholar] [CrossRef]
- Amirchaghmaghi, M.; Mohtasham, N.; Delavarian, Z.; Shakeri, M.T.; Taghizadeh, A.; Khazaeni, K.; Hatami, M. Analyzing the relationship between tissue color observed in VELscope examination and histopathological factors in OSCC patients. Photodiagnosis Photodyn. Ther. 2023, 41, 103248. [Google Scholar] [CrossRef]
- Mumtaz, M.; Bijnsdorp, I.V.; Böttger, F.; Piersma, S.R.; Pham, T.V.; Mumtaz, S.; Brakenhoff, R.H.; Akhtar, M.W.; Jimenez, C.R. Secreted protein markers in oral squamous cell carcinoma (OSCC). Clin. Proteom. 2022, 19, 4. [Google Scholar] [CrossRef]
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K.-I. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018, 119, 7350–7362. [Google Scholar] [CrossRef]
- Celentano, A.; McCullough, M.; Cirillo, N. Glucocorticoids reduce chemotherapeutic effectiveness on OSCC cells via glucose-dependent mechanisms. J. Cell. Physiol. 2019, 234, 2013–2020. [Google Scholar] [CrossRef]
- Shriwas, O.; Priyadarshini, M.; Samal, S.K.; Rath, R.; Panda, S.; Majumdar, S.K.D.; Muduly, D.K.; Botlagunta, M.; Dash, R. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m6A-demethylation of FOXM1 and NANOG. Apoptosis 2020, 25, 233–246. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; Lleonart, M.E. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, S.; Sohi, H.; Dang, S. Advances in Delivery of Chemotherapeutic Agents for Cancer Treatment. AAPS PharmSciTech 2021, 23, 25. [Google Scholar] [CrossRef]
- Khan, S.; Khan, H.U.; Khan, F.A.; Shah, A.; Wadood, A.; Ahmad, S.; Almehmadi, M.; Alsaiari, A.A.; Shah, F.U.; Kamran, N. Anti-Alzheimer and Antioxidant Effects of Nelumbo nucifera L. Alkaloids, Nuciferine and Norcoclaurine in Alloxan-Induced Diabetic Albino Rats. Pharmaceuticals 2022, 15, 1205. [Google Scholar] [CrossRef]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A Comprehensive Review on Chemical Profiling of Nelumbo Nucifera: Potential for Drug Development. Phytother. Res. 2017, 31, 3–26. [Google Scholar] [CrossRef]
- Wan, Y.; Xia, J.; Xu, J.-F.; Chen, L.; Yang, Y.; Wu, J.-J.; Tang, F.; Ao, H.; Peng, C. Nuciferine, an active ingredient derived from lotus leaf, lights up the way for the potential treatment of obesity and obesity-related diseases. Pharmacol. Res. 2022, 175, 106002. [Google Scholar] [CrossRef]
- Lim, S.H.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Saikosaponin A and D Inhibit Adipogenesis via the AMPK and MAPK Signaling Pathways in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef]
- Kim, S.-M.; Park, E.-J.; Lee, H.-J. Nuciferine attenuates lipopolysaccharide-stimulated inflammatory responses by inhibiting p38 MAPK/ATF2 signaling pathways. Inflammopharmacology 2022, 30, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- HarishKumar, R.; Selvaraj, C.I. Nuciferine from Nelumbo nucifera Gaertn. attenuates isoproterenol-induced myocardial infarction in Wistar rats. Biotechnol. Appl. Biochem. 2022, 69, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.S.; McCorvy, J.D.; Huang, X.-P.; Urban, D.J.; White, K.L.; Giguere, P.M.; Doak, A.K.; Bernstein, A.I.; Stout, K.A.; Park, S.M.; et al. In Vitro and In Vivo Characterization of the Alkaloid Nuciferine. PLoS ONE 2016, 11, e0150602. [Google Scholar] [CrossRef] [PubMed]
- Harishkumar, R.; Christopher, J.G.; Ravindran, R.; Selvaraj, C.I. Nuciferine Attenuates Doxorubicin-Induced Cardiotoxicity: An In Vitro and In Vivo Study. Cardiovasc. Toxicol. 2021, 21, 947–963. [Google Scholar] [CrossRef]
- Charoensin, S.; Weera, W. Preventive Effect of Nuciferine on H2O2-Induced Fibroblast Senescence and Pro-Inflammatory Cytokine Gene Expression. Molecules 2022, 27, 8148. [Google Scholar] [CrossRef]
- Xu, J.; Ying, A.; Shi, T. Nuciferine Inhibits Skin Cutaneous Melanoma Cell Growth by Suppressing TLR4/NF-κB Signaling. Anti-cancer Agents Med. Chem. 2020, 20, 2099–2105. [Google Scholar] [CrossRef]
- Bai, X.; Liu, X.; Li, S.; An, H.; Kang, X.; Guo, S. Nuciferine Inhibits TMEM16A in Dietary Adjuvant Therapy for Lung Cancer. J. Agric. Food Chem. 2022, 70, 3687–3696. [Google Scholar] [CrossRef]
- Manogaran, P.; Beeraka, N.M.; Paulraj, R.S.; Sathiyachandran, P.; Thammaiappa, M. Impediment of Cancer by Dietary Plant-derived Alkaloids Through Oxidative Stress: Implications of PI3K/AKT Pathway in Apoptosis, Autophagy, and Ferroptosis. Curr. Top. Med. Chem. 2023, 23, 860–877. [Google Scholar] [CrossRef]
- Buyel, J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef]
- Zubair, H.; Khan, M.A.; Anand, S.; Srivastava, S.K.; Singh, S.; Singh, A.P. Modulation of the tumor microenvironment by natural agents: Implications for cancer prevention and therapy. Semin. Cancer Biol. 2022, 80, 237–255. [Google Scholar] [CrossRef]
- Kulhari, U.; Kundu, S.; Mugale, M.N.; Sahu, B.D. Nuciferine alleviates intestinal inflammation by inhibiting MAPK/NF-κB and NLRP3/Caspase 1 pathways in vivo and in vitro. Int. Immunopharmacol. 2023, 115, 109613. [Google Scholar] [CrossRef]
- Li, Q.-H.; Sui, L.-P.; Zhao, Y.-H.; Chen, B.-G.; Li, J.; Ma, Z.-H.; Hu, Z.-H.; Tang, Y.-L.; Guo, Y.-X. Tripartite Motif-Containing 44 is Involved in the Tumorigenesis of Laryngeal Squamous Cell Carcinoma, and its Expression is Downregulated by Nuciferine. Tohoku J. Exp. Med. 2021, 254, 17–23. [Google Scholar] [CrossRef]
- Sinto, M.S.; Thomas, S.; Kannan, S. Combinatorial treatment with Gefitinib and Bay11-7085 sensitizes primary Gefitinib-resistant OSCC cells by influencing the EGFR-NFκB signaling axis. Med. Oncol. 2021, 38, 110. [Google Scholar] [CrossRef] [PubMed]
- Gkouveris, I.; Nikitakis, N.; Avgoustidis, D.; Karanikou, M.; Rassidakis, G.; Sklavounou, A. ERK1/2, JNK and STAT3 activation and correlation with tumor differentiation in oral SCC. Histol. Histopathol. 2017, 32, 1065–1076. [Google Scholar] [PubMed]
- Jeon, Y.-J.; Cho, J.H.; Lee, S.-Y.; Choi, Y.H.; Park, H.; Jung, S.; Shim, J.-H.; Chae, J.-I. Esculetin Induces Apoptosis Through EGFR/PI3K/Akt Signaling Pathway and Nucleophosmin Relocalization. J. Cell. Biochem. 2016, 117, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Shriwas, O.; Samal, S.K.; Priyadarshini, M.; Rath, R.; Panda, S.; Majumdar, S.K.D.; Muduly, D.K.; Dash, R. STAT3- and GSK3β-mediated Mcl-1 regulation modulates TPF resistance in oral squamous cell carcinoma. Carcinogenesis 2019, 40, 173–183. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.; Karanikou, M.; Rassidakis, G.; Sklavounou, A. JNK1/2 expression and modulation of STAT3 signaling in oral cancer. Oncol. Lett. 2016, 12, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Furuta, H.; Osawa, K.; Shin, M.; Ishikawa, A.; Matsuo, K.; Khan, M.; Aoki, K.; Ohya, K.; Okamoto, M.; Tominaga, K.; et al. Selective inhibition of NF-κB suppresses bone invasion by oral squamous cell carcinoma in vivo. Int. J. Cancer 2012, 131, E625–E635. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jue, S.-S.; Kang, S.-K.; Bae, W.-J.; Kim, Y.-C.; Kim, E.-C. Cudraxanthone H Induces Growth Inhibition and Apoptosis in Oral Cancer Cells via NF-κB and PIN1 Pathways. Am. J. Chin. Med. 2015, 43, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, H.; Wang, F.; Zhao, S. Oct4 downregulation-induced inflammation increases the migration and invasion rate of oral squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2021, 53, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, R.; Li, H.-Y.; Cao, Y.-B.; Bai, M.; Fan, X.-J.; Wang, S.-Y.; Zhang, B.; Li, S. Identification of the anti-tumor activity and mechanisms of nuciferine through a network pharmacology approach. Acta Pharmacol. Sin. 2016, 37, 963–972. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.-R.; Chen, X.-J.; Zhou, G. Nuciferine Inhibits Oral Squamous Cell Carcinoma Partially through Suppressing the STAT3 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 14532. https://doi.org/10.3390/ijms241914532
Xie J-R, Chen X-J, Zhou G. Nuciferine Inhibits Oral Squamous Cell Carcinoma Partially through Suppressing the STAT3 Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(19):14532. https://doi.org/10.3390/ijms241914532
Chicago/Turabian StyleXie, Ji-Rong, Xiao-Jie Chen, and Gang Zhou. 2023. "Nuciferine Inhibits Oral Squamous Cell Carcinoma Partially through Suppressing the STAT3 Signaling Pathway" International Journal of Molecular Sciences 24, no. 19: 14532. https://doi.org/10.3390/ijms241914532
APA StyleXie, J.-R., Chen, X.-J., & Zhou, G. (2023). Nuciferine Inhibits Oral Squamous Cell Carcinoma Partially through Suppressing the STAT3 Signaling Pathway. International Journal of Molecular Sciences, 24(19), 14532. https://doi.org/10.3390/ijms241914532





