Enhanced Astrocyte Activity and Excitatory Synaptic Function in the Hippocampus of Pentylenetetrazole Kindling Model of Epilepsy
Abstract
:1. Introduction
2. Results
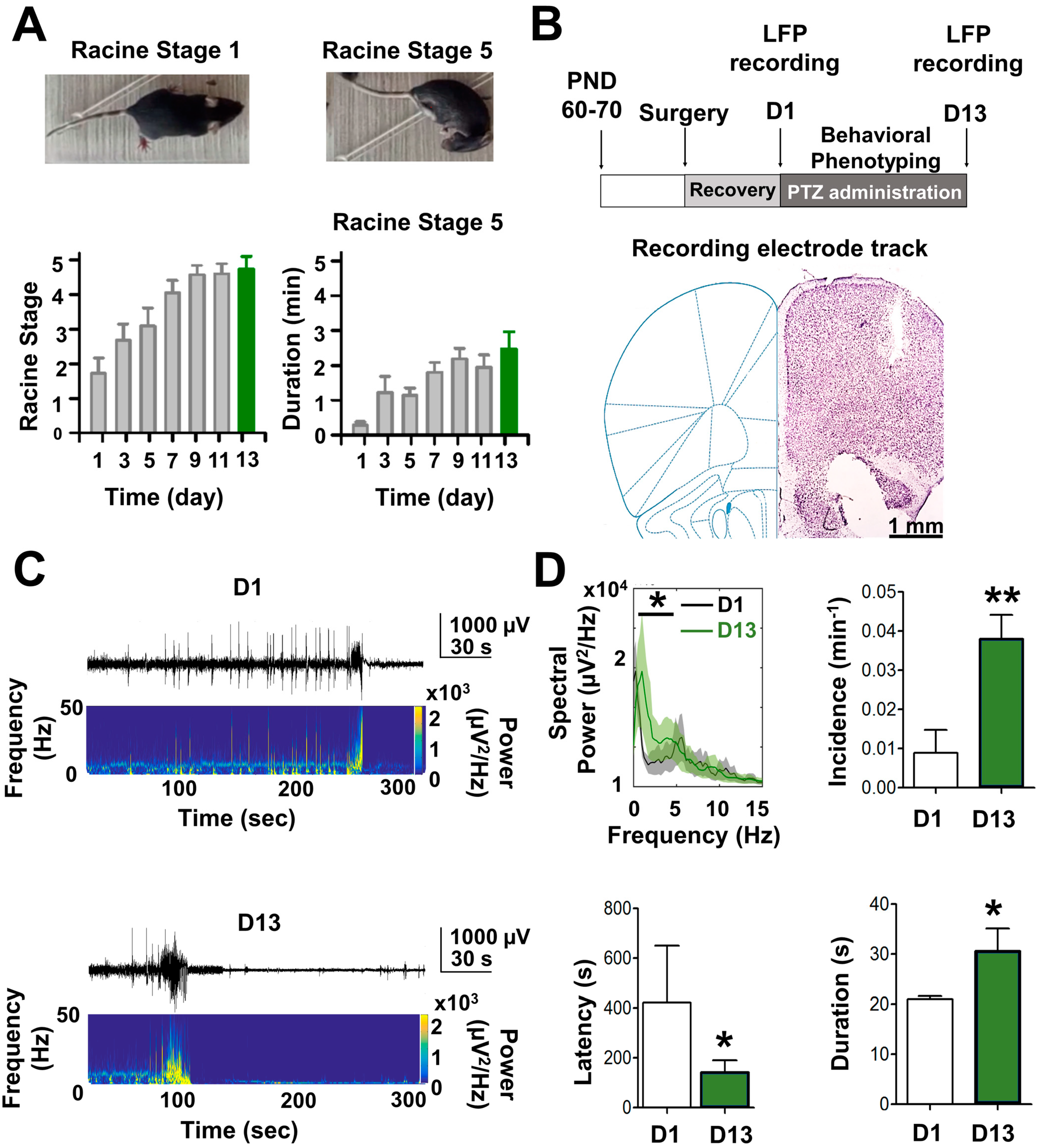
2.1. In Vivo Epileptogenic Progression the in PTZ-Kindling Model of Epilepsy
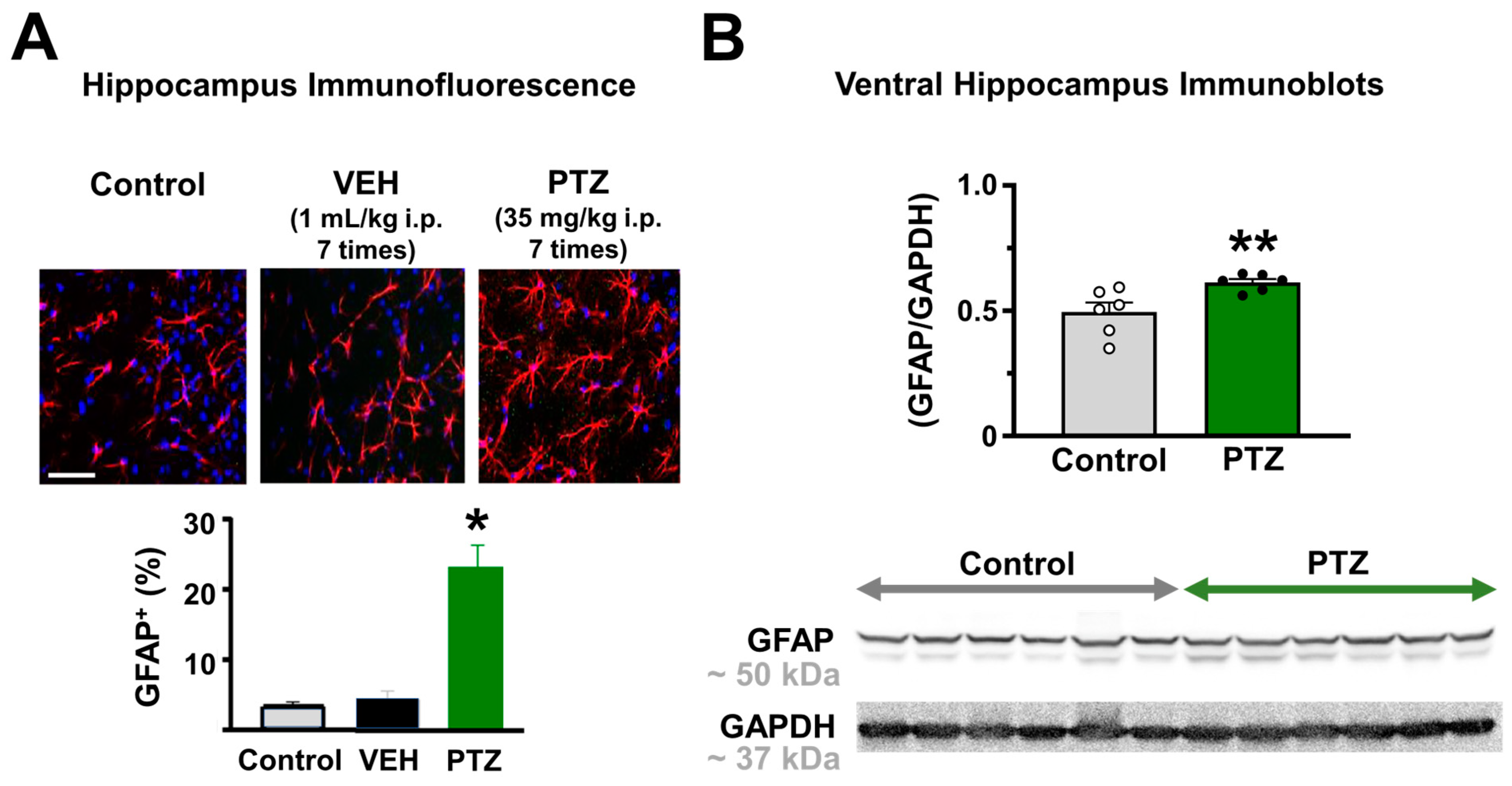
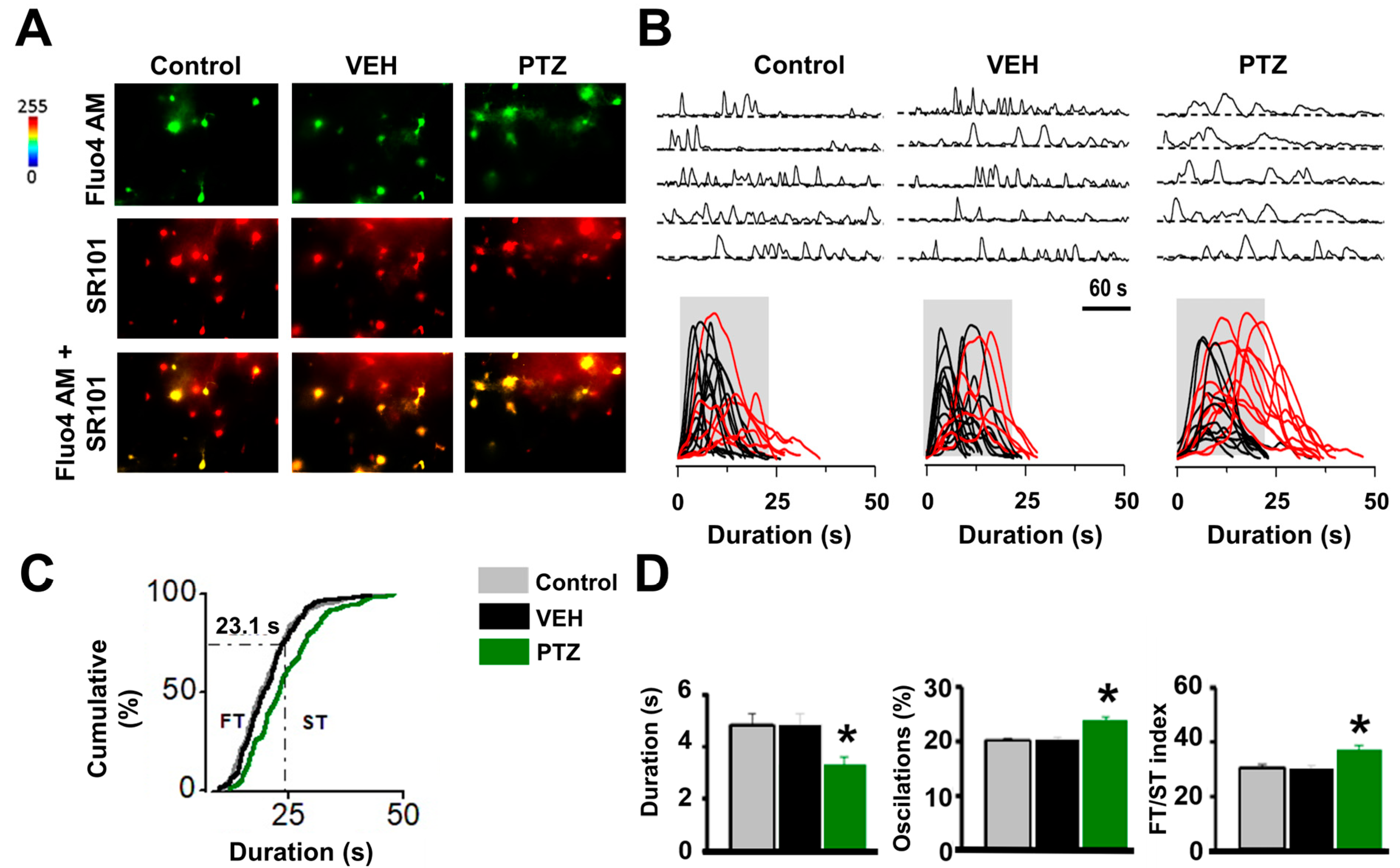
2.2. Astrogliosis and Astrocyte Calcium Wave in the Hippocampus of the PTZ-Kindling Model
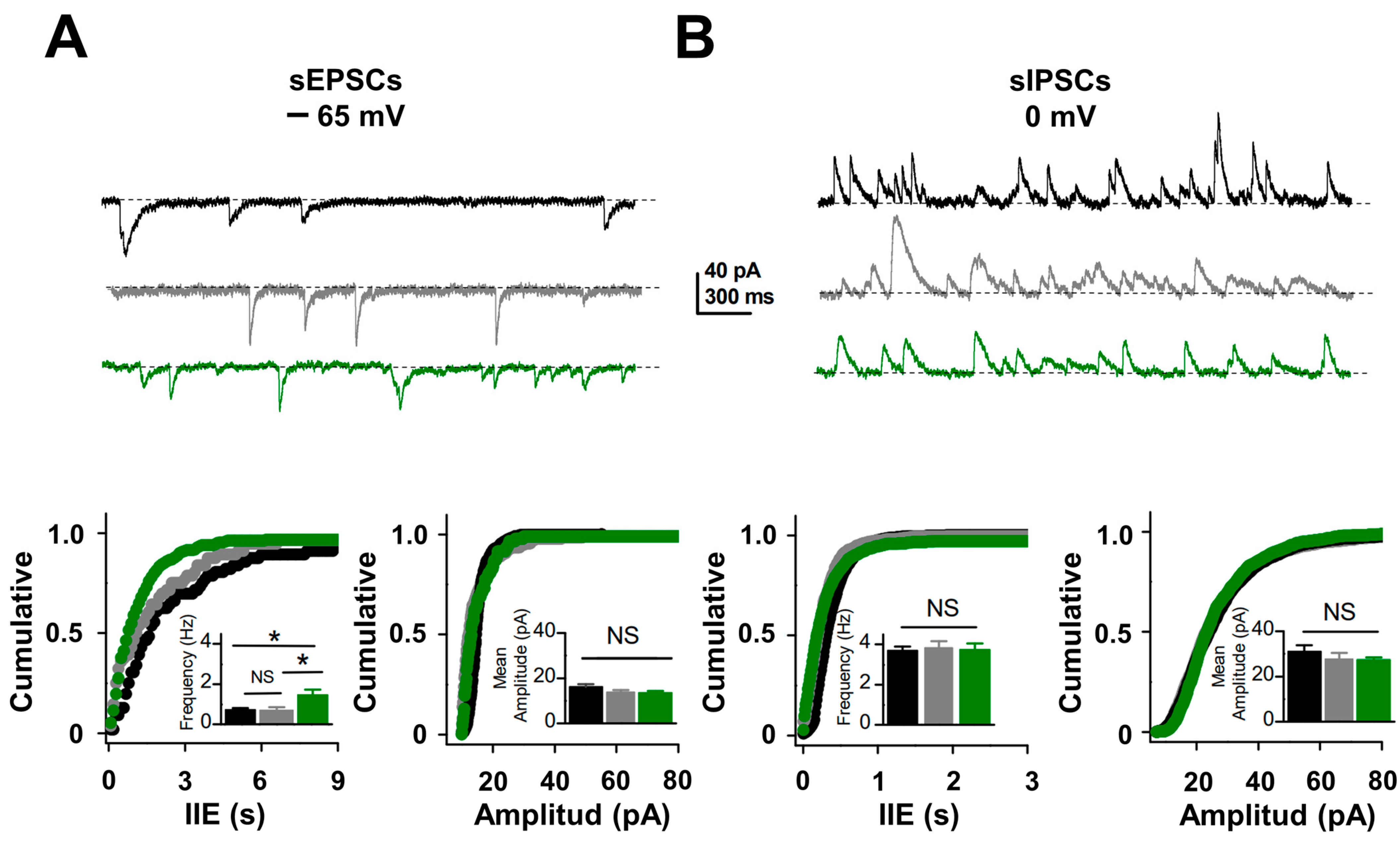
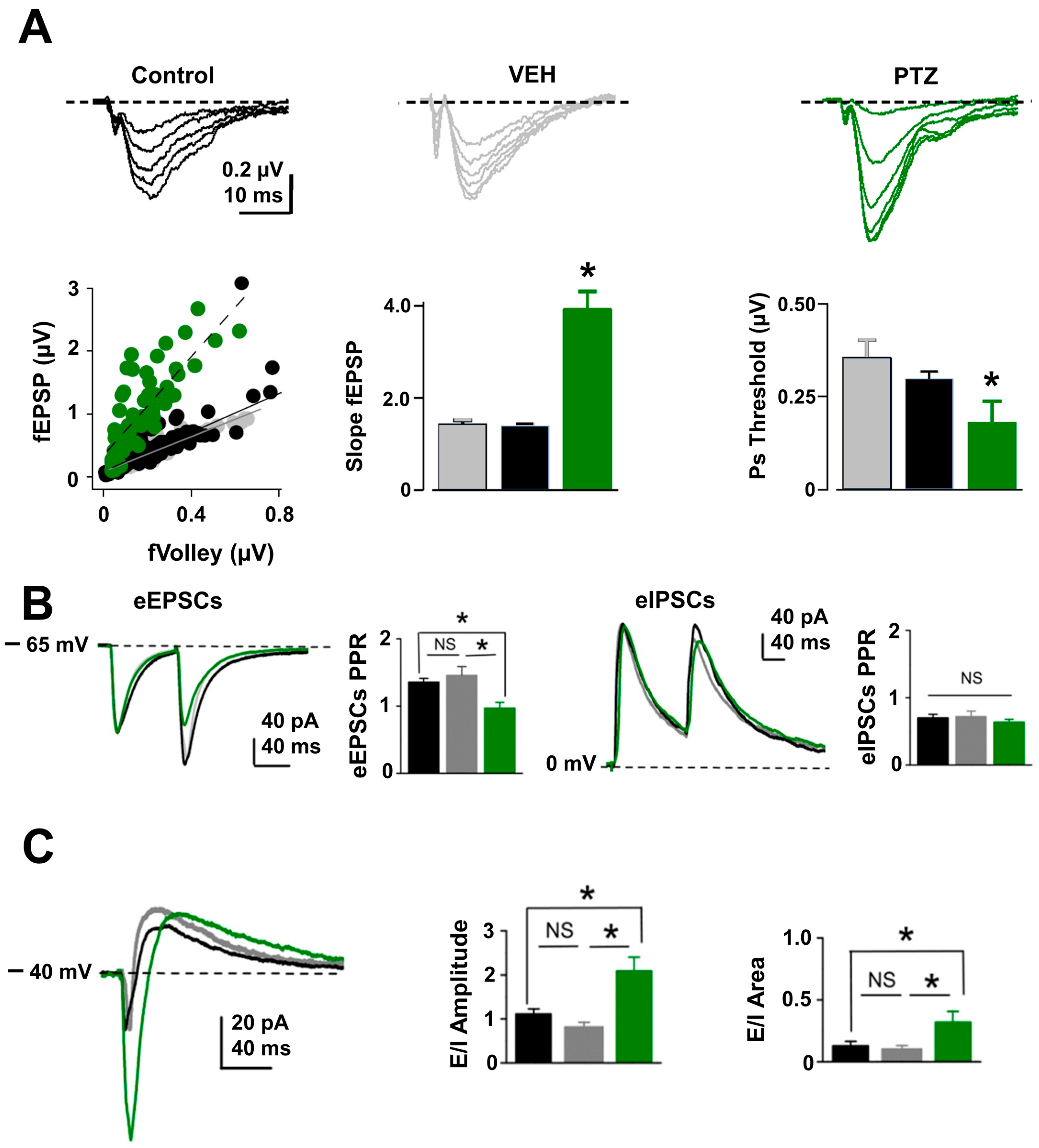
2.3. Excitatory Synaptic and Neuronal Excitability Function Is Enhanced in PTZ-Kindling Model
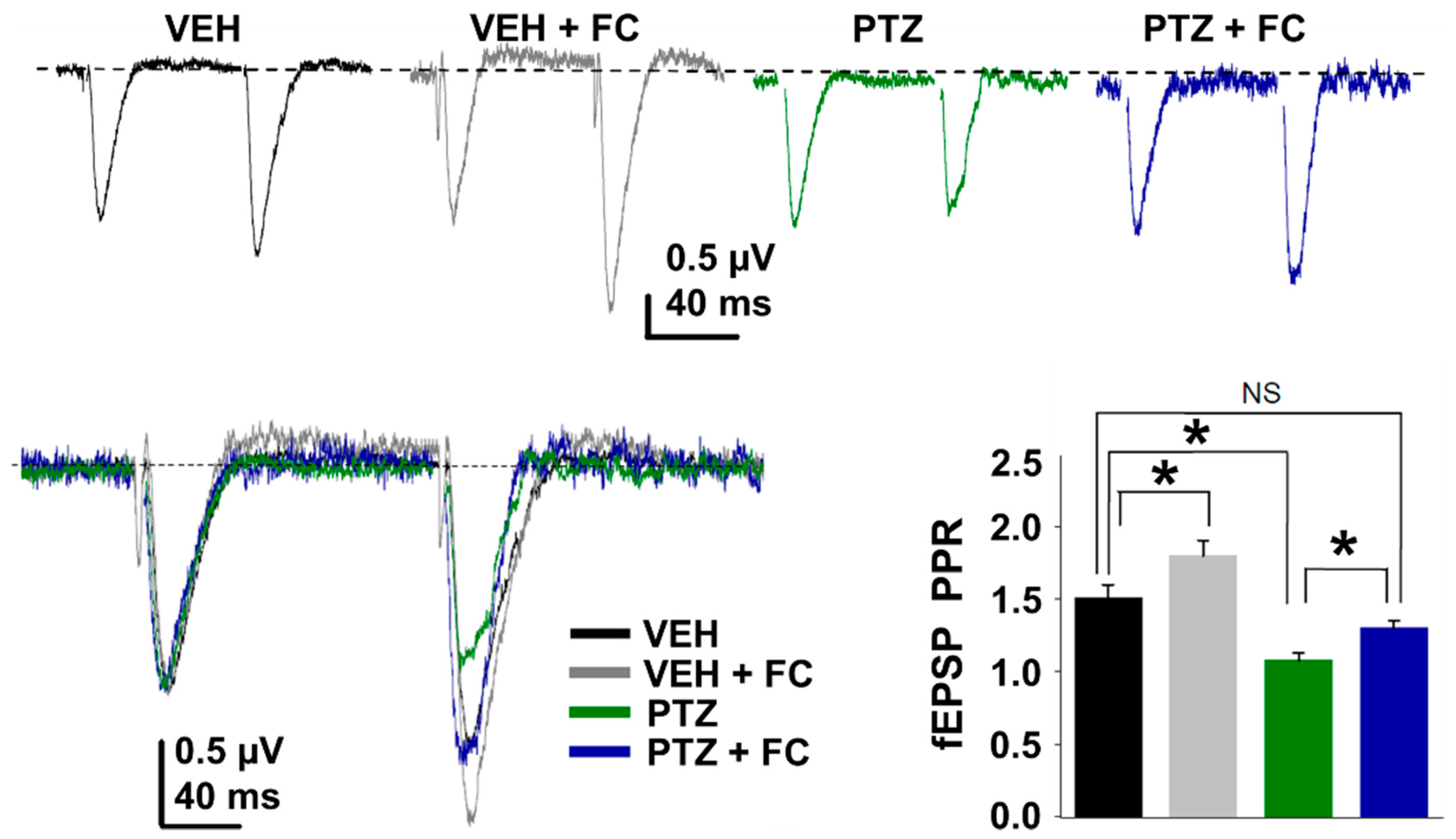
2.4. Metabolic Arrest of Abnormal Astrocyte Restores the Release Probability at Excitatory Synapse in the PTZ-Kindling Model
3. Discussion
3.1. Ca2+-Dependent Astroglial Hyper-Excitability in the Epileptic Hippocampus
3.2. Increase in Excitatory Synaptic Activity Yields E/I Unbalances in the Hippocampal Network of PTZ-Kindled Mice
4. Materials and Methods
4.1. Animals
4.2. Tracing Epileptogenesis in the PTZ-Kindling Mouse Model
4.3. Electrophysiology in Hippocampal Slices
4.4. Ca2+ Imaging in Astrocytes
4.5. Western Blot
4.6. Immunohistochemistry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitkanen, A.; Lukasiuk, K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009, 14 (Suppl. S1), 16–25. [Google Scholar] [PubMed]
- Rattka, M.; Brandt, C.; Loscher, W. Do proconvulsants modify or halt epileptogenesis? Pentylenetetrazole is ineffective in two rat models of temporal lobe epilepsy. Eur. J. Neurosci. 2012, 36, 2505–2520. [Google Scholar] [PubMed]
- Shigetomi, E.; Saito, K.; Sano, F.; Koizumi, S. Aberrant Calcium Signals in Reactive Astrocytes: A Key Process in Neurological Disorders. Int. J. Mol. Sci. 2019, 20, 996. [Google Scholar] [CrossRef] [PubMed]
- Bonansco, C.; Couve, A.; Perea, G.; Ferradas, C.A.; Roncagliolo, M.; Fuenzalida, M. Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity. Eur. J. Neurosci. 2011, 33, 1483–1492. [Google Scholar] [CrossRef]
- Tang, F.R.; Lee, W.L.; Yeo, T.T. Expression of the group I metabotropic glutamate receptor in the hippocampus of patients with mesial temporal lobe epilepsy. J. Neurocytol. 2001, 30, 403–411. [Google Scholar] [CrossRef]
- Wellmann, M.; Alvarez-Ferradas, C.; Maturana, C.J.; Saez, J.C.; Bonansco, C. Astroglial Ca(2+)-Dependent Hyperexcitability Requires P2Y(1) Purinergic Receptors and Pannexin-1 Channel Activation in a Chronic Model of Epilepsy. Front. Cell Neurosci. 2018, 12, 446. [Google Scholar] [CrossRef]
- Aronica, E.; van Vliet, E.A.; Mayboroda, O.A.; Troost, D.; da Silva, F.H.; Gorter, J.A. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur. J. Neurosci. 2000, 12, 2333–2344. [Google Scholar] [CrossRef]
- Steinhauser, C.; Grunnet, M.; Carmignoto, G. Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience 2016, 323, 157–169. [Google Scholar]
- Morales, J.C.; Alvarez-Ferradas, C.; Roncagliolo, M.; Fuenzalida, M.; Wellmann, M.; Nualart, F.J.; Bonansco, C. A new rapid kindling variant for induction of cortical epileptogenesis in freely moving rats. Front. Cell Neurosci. 2014, 8, 200. [Google Scholar] [CrossRef]
- Alvarez-Ferradas, C.; Morales, J.C.; Wellmann, M.; Nualart, F.; Roncagliolo, M.; Fuenzalida, M.; Bonansco, C. Enhanced astroglial Ca2+ signaling increases excitatory synaptic strength in the epileptic brain. Glia 2015, 63, 1507–1521. [Google Scholar] [CrossRef]
- Delekate, A.; Fuchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Gomez-Villafuertes, R.; Delanty, N.; Farrell, M.A.; O’Brien, D.F.; Miras-Portugal, M.T.; Hernandez, M.D.; Henshall, D.C.; Engel, T. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia 2017, 58, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Hirayama, Y.J.; Ikenaka, K.; Tanaka, K.F.; Koizumi, S. Role of Purinergic Receptor P2Y1 in Spatiotemporal Ca(2+) Dynamics in Astrocytes. J. Neurosci. 2018, 38, 1383–1395. [Google Scholar] [CrossRef]
- Gomez-Gonzalo, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Cammarota, M.; Brondi, M.; Vetri, F.; Uva, L.; Pozzan, T.; de Curtis, M.; et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010, 8, e1000352. [Google Scholar] [CrossRef]
- Loscher, W. Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse events in humans. Eur. J. Pharmacol. 2009, 610, 1–11. [Google Scholar] [PubMed]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, 12, e56573. [Google Scholar]
- Singh, T.; Mishra, A.; Goel, R.K. PTZ kindling model for epileptogenesis, refractory epilepsy, and associated comorbidities: Relevance and reliability. Metab. Brain Dis. 2021, 36, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Musto, A.E.; Samii, M.S.; Hayes, J.F. Different phases of afterdischarge during rapid kindling procedure in mice. Epilepsy Res. 2009, 85, 199–205. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Becker, A.; Grecksch, G.; Ruthrich, H.L.; Pohle, W.; Marx, B.; Matthies, H. Kindling and its consequences on learning in rats. Behav. Neural Biol. 1992, 57, 37–43. [Google Scholar] [CrossRef]
- Dhir, A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr. Protoc. Neurosci. 2012, 58, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Purnell, B.S.; Alves, M.; Boison, D. Astrocyte-neuron circuits in epilepsy. Neurobiol. Dis. 2023, 179, 106058. [Google Scholar]
- Riquelme, J.; Wellmann, M.; Sotomayor-Zarate, R.; Bonansco, C. Gliotransmission: A Novel Target for the Development of Antiseizure Drugs. Neuroscientist 2020, 26, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.; Wellmann, M.; Guiffa, F.; Fuenzalida, M.; Bonansco, C. P2Y1 receptor inhibition rescues impaired synaptic plasticity and astroglial Ca(2+)-dependent activity in the epileptic hippocampus. Neurobiol. Dis. 2020, 146, 105132. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Silfvenius, H.; Sundberg, S.H.; Sveen, O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J. Physiol. 1980, 307, 273–299. [Google Scholar] [CrossRef]
- Swanson, R.A.; Graham, S.H. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 1994, 664, 94–100. [Google Scholar] [CrossRef]
- Berg-Johnsen, J.; Paulsen, R.E.; Fonnum, F.; Langmoen, I.A. Changes in evoked potentials and amino acid content during fluorocitrate action studied in rat hippocampal cortex. Exp. Brain Res. 1993, 96, 241–246. [Google Scholar] [CrossRef]
- Hassel, B.; Sonnewald, U.; Unsgard, G.; Fonnum, F. NMR spectroscopy of cultured astrocytes: Effects of glutamine and the gliotoxin fluorocitrate. J. Neurochem. 1994, 62, 2187–2194. [Google Scholar] [CrossRef]
- Ueno, H.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Takahashi, Y.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Pentylenetetrazol kindling induces cortical astrocytosis and increased expression of extracellular matrix molecules in mice. Brain Res. Bull. 2020, 163, 120–134. [Google Scholar] [CrossRef]
- Kaur, H.; Patro, I.; Tikoo, K.; Sandhir, R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015, 89, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Anissian, D.; Ghasemi-Kasman, M.; Khalili-Fomeshi, M.; Akbari, A.; Hashemian, M.; Kazemi, S.; Moghadamnia, A.A. Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in the chemical kindling model of epilepsy. Int. J. Biol. Macromol. 2018, 107 Pt A, 973–983. [Google Scholar] [CrossRef]
- Seifert, G.; Schilling, K.; Steinhauser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Smith, J.; Alves, M. Targeting Neuroinflammation via Purinergic P2 Receptors for Disease Modification in Drug-Refractory Epilepsy. J. Inflamm. Res. 2021, 14, 3367–3392. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, M.S.; Whyte-Fagundes, P.; Lukewich, M.K.; Zhang, L.; Bardakjian, B.L.; Zoidl, G.R.; Carlen, P.L. Pannexin-1 Deficiency Decreases Epileptic Activity in Mice. Int. J. Mol. Sci. 2020, 21, 7510. [Google Scholar] [CrossRef] [PubMed]
- Bonansco, C.; Fuenzalida, M. Plasticity of Hippocampal Excitatory-Inhibitory Balance: Missing the Synaptic Control in the Epileptic Brain. Neural Plast. 2016, 2016, 8607038. [Google Scholar] [CrossRef] [PubMed]
- Stief, F.; Zuschratter, W.; Hartmann, K.; Schmitz, D.; Draguhn, A. Enhanced synaptic excitation-inhibition ratio in hippocampal interneurons of rats with temporal lobe epilepsy. Eur. J. Neurosci. 2007, 25, 519–528. [Google Scholar] [CrossRef]
- Perea, G.; Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 2007, 317, 1083–1086. [Google Scholar] [CrossRef]
- Covelo, A.; Araque, A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 2018, 7, e32237. [Google Scholar] [CrossRef]
- Carmignoto, G.; Fellin, T. Glutamate release from astrocytes as a non-synaptic mechanism for neuronal synchronization in the hippocampus. J. Physiol. Paris. 2006, 99, 98–102. [Google Scholar] [CrossRef]
- Caudal, L.C.; Gobbo, D.; Scheller, A.; Kirchhoff, F. The Paradox of Astroglial Ca(2+) Signals at the Interface of Excitation and Inhibition. Front. Cell Neurosci. 2020, 14, 609947. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, R.E.; Contestabile, A.; Villani, L.; Fonnum, F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: The use of fluorocitrate. J. Neurochem. 1987, 48, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D. Fluoroacetate and fluorocitrate: Mechanism of action. Neurochem. Res. 1991, 16, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Kafitz, K.W.; Meier, S.D.; Stephan, J.; Rose, C.R. Developmental profile and properties of sulforhodamine 101--Labeled glial cells in acute brain slices of rat hippocampus. J. Neurosci. Methods 2008, 169, 84–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, F.; Aguilar, F.; Wellmann, M.; Martorell, A.; González-Arancibia, C.; Chacana-Véliz, L.; Negrón-Oyarzo, I.; Chávez, A.E.; Fuenzalida, M.; Nualart, F.; et al. Enhanced Astrocyte Activity and Excitatory Synaptic Function in the Hippocampus of Pentylenetetrazole Kindling Model of Epilepsy. Int. J. Mol. Sci. 2023, 24, 14506. https://doi.org/10.3390/ijms241914506
Díaz F, Aguilar F, Wellmann M, Martorell A, González-Arancibia C, Chacana-Véliz L, Negrón-Oyarzo I, Chávez AE, Fuenzalida M, Nualart F, et al. Enhanced Astrocyte Activity and Excitatory Synaptic Function in the Hippocampus of Pentylenetetrazole Kindling Model of Epilepsy. International Journal of Molecular Sciences. 2023; 24(19):14506. https://doi.org/10.3390/ijms241914506
Chicago/Turabian StyleDíaz, Franco, Freddy Aguilar, Mario Wellmann, Andrés Martorell, Camila González-Arancibia, Lorena Chacana-Véliz, Ignacio Negrón-Oyarzo, Andrés E. Chávez, Marco Fuenzalida, Francisco Nualart, and et al. 2023. "Enhanced Astrocyte Activity and Excitatory Synaptic Function in the Hippocampus of Pentylenetetrazole Kindling Model of Epilepsy" International Journal of Molecular Sciences 24, no. 19: 14506. https://doi.org/10.3390/ijms241914506
APA StyleDíaz, F., Aguilar, F., Wellmann, M., Martorell, A., González-Arancibia, C., Chacana-Véliz, L., Negrón-Oyarzo, I., Chávez, A. E., Fuenzalida, M., Nualart, F., Sotomayor-Zárate, R., & Bonansco, C. (2023). Enhanced Astrocyte Activity and Excitatory Synaptic Function in the Hippocampus of Pentylenetetrazole Kindling Model of Epilepsy. International Journal of Molecular Sciences, 24(19), 14506. https://doi.org/10.3390/ijms241914506







