Dual-Species Biofilms: Biomass, Viable Cell Ratio/Cross-Species Interactions, Conjugative Transfer
Abstract
1. Introduction
2. Results
2.1. Biofilm Biomass
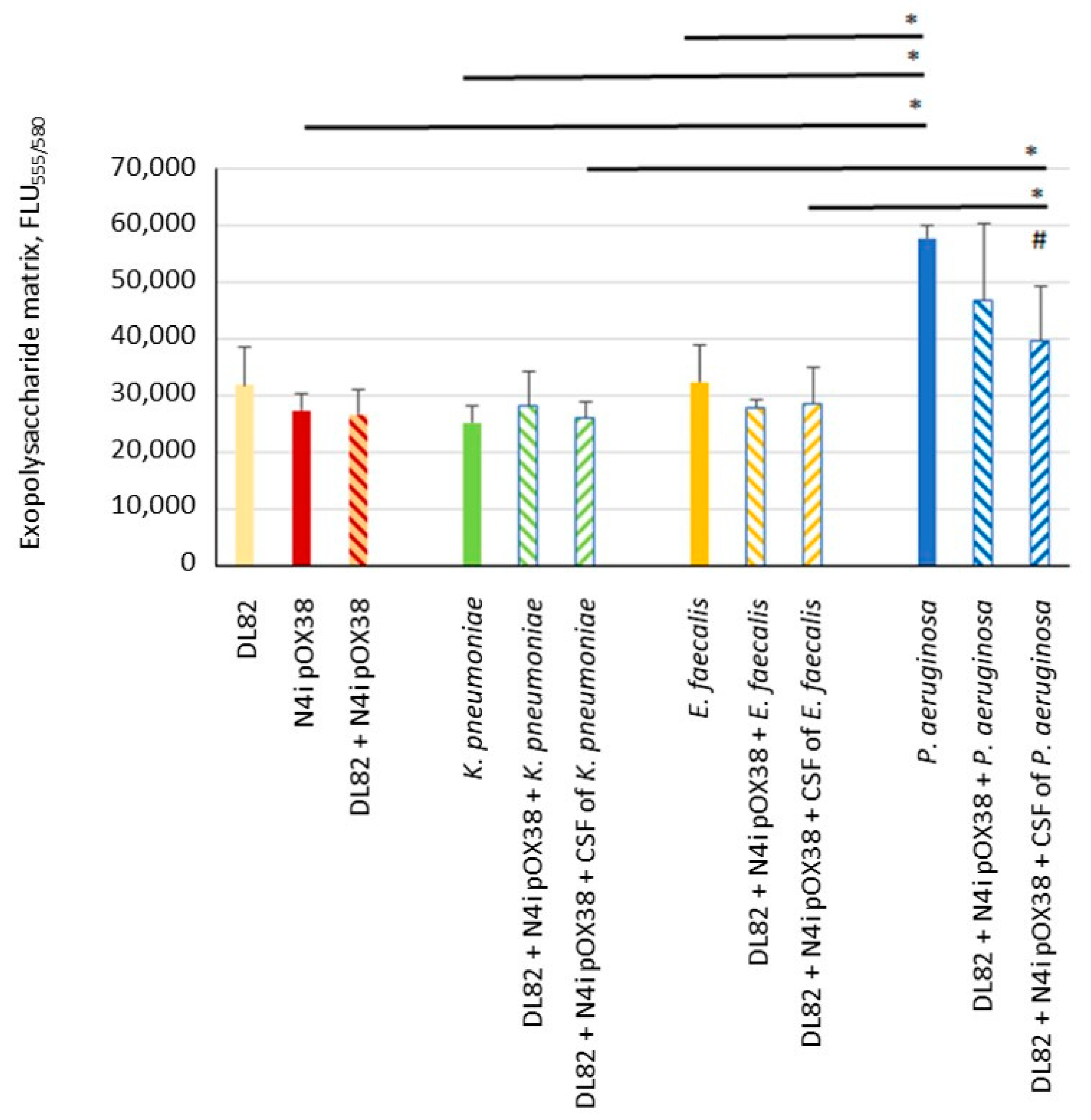
2.2. Biofilm Exopolysaccharide Matrix
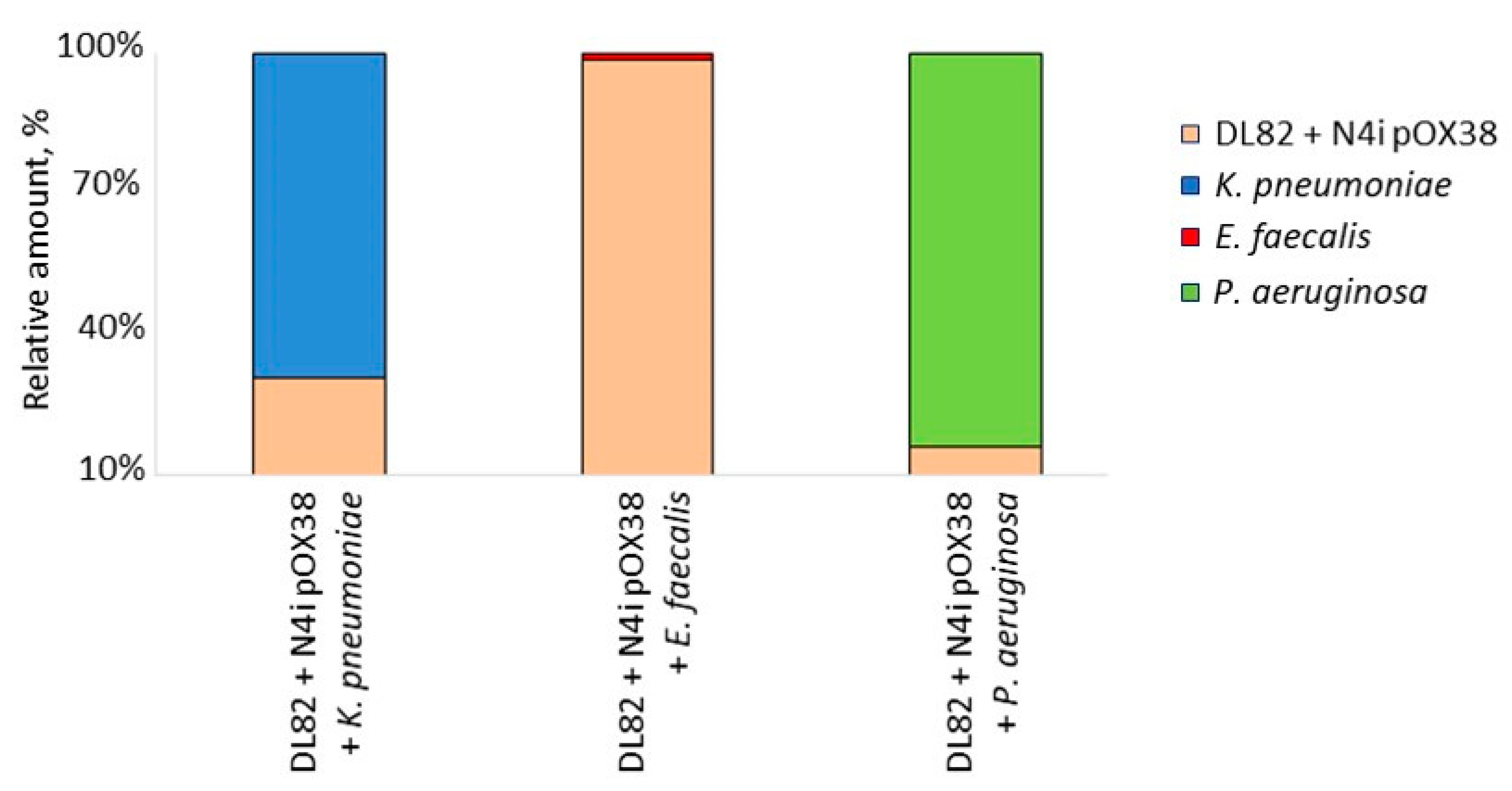
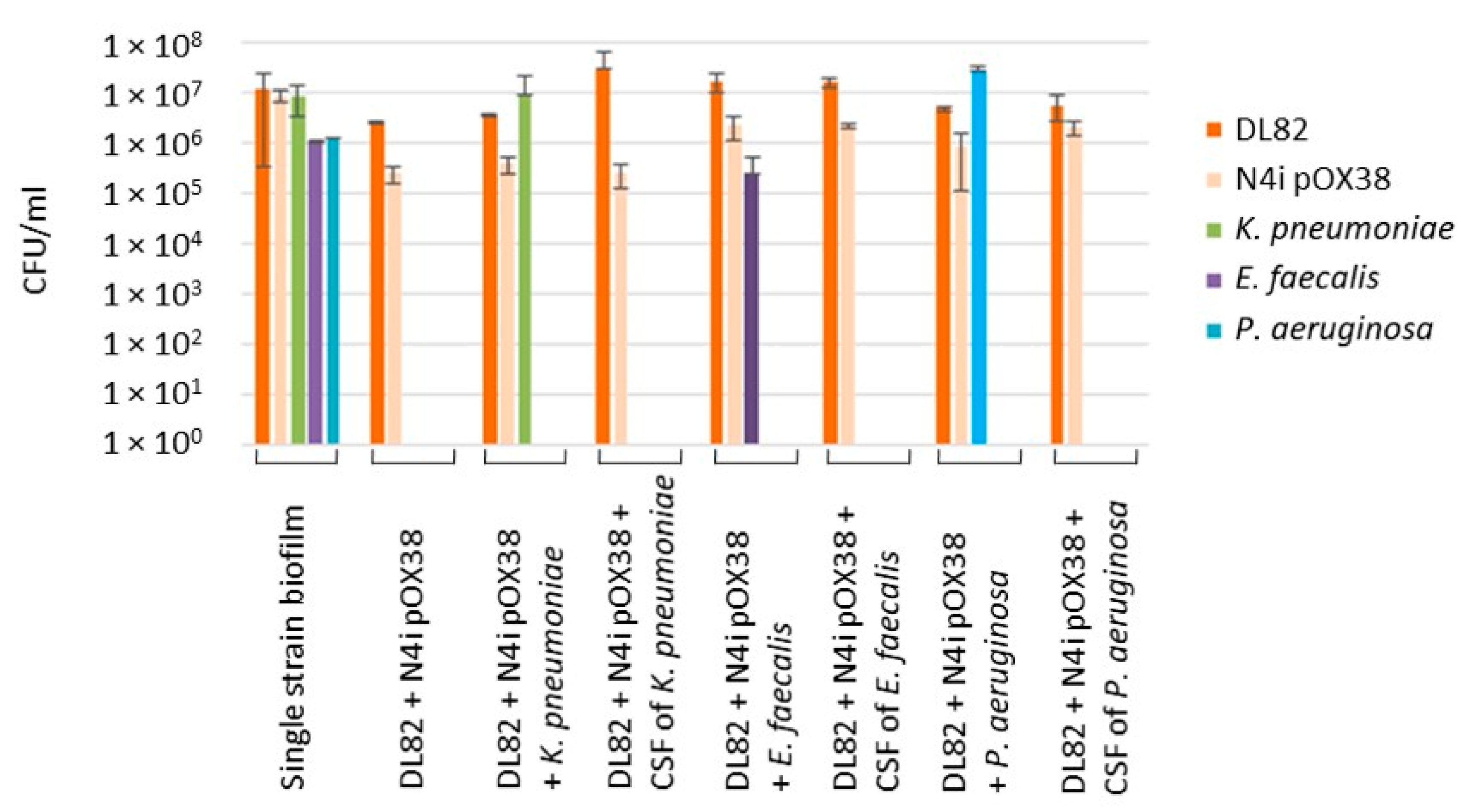
2.3. The Viability of Recipient E. coli DL82, Donor E. coli N4i pOX38 and Opportunistic Pathogenic Bacteria within Dual-Species Biofilms
2.4. The Frequency of Conjugation within Dual-Species Biofilm
2.5. AI-2 Activity in CFSs of Conjugation Mixtures
3. Discussion
4. Materials and Methods
4.1. Strains and Media
4.2. Cell-Free Supernatant (CFS)
4.3. Conjugation Assay
4.4. Biofilm Biomass Analysis
4.5. Biofilm Exopolysaccharide Matrix Analysis
4.6. AI-2 Production Assay
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Besemer, K. Biodiversity, community structure and function of biofilms in stream ecosystems. Res. Microbiol. 2015, 10, 774–781. [Google Scholar] [CrossRef]
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial biofilm formation: A need to act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Mizan, M.F.; Jahid, I.K.; Ha, S.D. Microbial biofilms in seafood: A food-hygiene challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Larsen, L.H.; Lorenzen, J.; Hall-Stoodley, L.; Kikhney, J.; Moter, A.; Thomsen, T.R. Microbiological diagnosis of device-related biofilm infections. APMIS 2017, 125, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.M.; Pracser, N.; Thalguter, S.; Fischel, K.; Rammer, N.; Pospíšilová, L.; Alispahic, M.; Wagner, M.; Rychli, K. Identification of biofilm hotspots in a meat processing environment: Detection of spoilage bacteria in multi-species biofilms. Int. J. Food Microbiol. 2020, 328, 108668. [Google Scholar] [CrossRef]
- McLean, R.J.; Kakirde, K.S. Enhancing metagenomics investigations of microbial interactions with biofilm technology. Int. J. Mol. Sci. 2013, 14, 22246–22257. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell. 2021, 8, 28–56. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary tract infections: The current scenario and future prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Rajab, A.A.H.; Abdallah, M.H.; Rizvi, S.M.D.; Moin, A.; Khafagy, E.S.; Tabrez, S.; Hegazy, W.A.H. Biofilm lifestyle in recurrent urinary tract infections. Life 2023, 13, 148. [Google Scholar] [CrossRef]
- Oliveira, A.; Sousa, J.C.; Silva, A.C.; Melo, L.D.R.; Sillankorva, S. Chestnut honey and bacteriophage application to control Pseudomonas aeruginosa and Escherichia coli biofilms: Evaluation in an ex vivo wound model. Front. Microbiol. 2018, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Juarez, G.E.; Galván, E.M. Role of nutrient limitation in the competition between uropathogenic strains of Klebsiella pneumoniae and Escherichia coli in mixed biofilms. Biofouling 2018, 34, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Galván, E.M.; Mateyca, C.; Ielpi, L. Role of interspecies interactions in dual-species biofilms developed in vitro by uropathogens isolated from polymicrobial urinary catheter-associated bacteriuria. Biofouling 2016, 32, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Travier, L.; Latour-Lambert, P.; Fontaine, T.; Magnus, J.; Denamur, E.; Ghigo, J.M. Screening of Escherichia coli species biodiversity reveals new biofilm-associated antiadhesion polysaccharides. MBio 2011, 2, e00043-11. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.J.; Ning, S.J.; Gao, Y.L. The response of type 2 quorum sensing in Klebsiella pneumoniae to a fluctuating culture environment. DNA Cell Biol. 2012, 31, 455–459. [Google Scholar] [CrossRef]
- Brito, P.H.; Rocha, E.P.; Xavier, K.B.; Gordo, I. Natural genome diversity of AI-2 quorum sensing in Escherichia coli: Conserved signal production but labile signal reception. Genome Biol. Evol. 2013, 1, 16–30. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, Z.; Fu, Y.; Ai, Q.; Dong, Y.; Yu, J. Autoinducer-2 regulates Pseudomonas aeruginosa PAO1 biofilm formation and virulence production in a dose-dependent manner. BMC Microbiol. 2015, 15, 192. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Hou, B.; Zhang, C. Quorum sensing LuxS/autoinducer-2 inhibits Enterococcus faecalis biofilm formation ability. J. Appl. Oral. Sci. 2018, 26, e20170566. [Google Scholar] [CrossRef]
- Wang, Y.M.; Dong, W.L.; Odah, K.A.; Kong, L.C.; Ma, H.X. Transcriptome analysis reveals AI-2 relevant genes of multi-drug resistant Klebsiella pneumoniae in response to Eugenol at sub-MIC. Front. Microbiol. 2019, 10, 1159. [Google Scholar] [CrossRef]
- Chen, L.; Wilksch, J.J.; Liu, H.; Zhang, X.; Torres, V.V.L.; Bi, W.; Mandela, E.; Cao, J.; Li, J.; Lithgow, T.; et al. Investigation of LuxS-mediated quorum sensing in Klebsiella pneumoniae. J. Med. Microbiol. 2020, 69, 402–413. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signaling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef] [PubMed]
- Laganenka, L.; Sourjik, V. Autoinducer 2-dependent Escherichia coli biofilm formation is enhanced in a dual-species coculture. Appl. Environ. Microbiol. 2018, 84, e02638-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wu, H.; Hóiby, N.; Molin, S.; Song, Z.J. Current understanding of multi-species biofilms. Int. J. Oral. Sci. 2011, 3, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Ghigo, J.M. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.S.; Hammer, B.K. Quorum-sensing autoinducer molecules produced by members of a multispecies biofilm promote horizontal gene transfer to Vibrio cholerae. FEMS Microbiol. Lett. 2011, 1, 68–76. [Google Scholar] [CrossRef]
- Hola, V.; Ruzicka, F. The Formation of Poly-Microbial Biofilms on Urinary Catheters. In Urinary Tract Infections, 2nd ed.; Tenke, P., Ed.; Rendelőintézet: Budapest, Hungary, 2011; pp. 153–172. [Google Scholar] [CrossRef][Green Version]
- Reisner, A.; Höller, B.M.; Molin, S.; Zechner, E.L. Synergistic effects in mixed Escherichia coli biofilms: Conjugative plasmid transfer drives biofilm expansion. J. Bacteriol. 2006, 188, 3582–3588. [Google Scholar] [CrossRef]
- Koraimann, G. Spread and persistence of virulence and antibiotic resistance genes: A ride on the F plasmid conjugation module. EcoSal Plus 2018, 8, 1–23. [Google Scholar] [CrossRef]
- Stephens, C.; Arismendi, T.; Wright, M.; Hartman, A.; Gonzalez, A.; Gill, M.; Pandori, M.; Hess, D. F Plasmids are the major carriers of antibiotic resistance genes in human-associated commensal Escherichia coli. mSphere 2020, 5, e00709-20. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Maslennikova, I.L.; Pospelova, J.S.; Žgur Bertok, D.; Starčič Erjavec, M. Differences in recipient ability of uropathogenic Escherichia coli strains in relation with their pathogenic potential. Infect. Genet. Evol. 2022, 97, 105160. [Google Scholar] [CrossRef]
- Smith, R.S.; Iglewski, B.H. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003, 6, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Laganenka, L.; Lee, J.W.; Malfertheiner, L.; Dieterich, C.L.; Fuchs, L.; Piel, J.; von Mering, C.; Sourjik, V.; Hardt, W.D. Chemotaxis and autoinducer-2 signalling mediate colonization and contribute to co-existence of Escherichia coli strains in the murine gut. Nat. Microbiol. 2023, 2, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Tchebotar, I.V.; Mayanskiy, A.N.; Mayanskiy, N.A. Matrix of microbial biofilms. Clin. Microbiol. Antimicrobial. Chemother. 2016, 18, 9–19. (In Russian) [Google Scholar]
- Sørensen, S.J.; Bailey, M.; Hansen, L.H.; Kroer, N.; Wuertz, S. Studying plasmid horizontal transfer in situ: A critical review. Nat. Rev. Microbiol. 2005, 3, 700–710. [Google Scholar] [CrossRef]
- Król, J.E.; Wojtowicz, A.J.; Rogers, L.M.; Heuer, H.; Smalla, K.; Krone, S.M.; Top, E.M. Invasion of E. coli biofilms by antibiotic resistance plasmids. Plasmid 2013, 70, 110–119. [Google Scholar] [CrossRef]
- Cook, L.C.; Dunny, G.M. The influence of biofilms in the biology of plasmids. Microbiol Spectr. 2014, 2, 0012. [Google Scholar] [CrossRef]
- Ferrières, L.; Hancock, V.; Klemm, P. Specific selection for virulent urinary tract infectious Escherichia coli strains during catheter-associated biofilm formation. FEMS Immunol. Med. Microbiol. 2007, 51, 212–219. [Google Scholar] [CrossRef]
- Keogh, D.; Tay, W.H.; Ho, Y.Y.; Dale, J.L.; Chen, S.; Umashankar, S.; Williams, R.B.H.; Chen, S.L.; Dunny, G.M.; Kline, K.A. Enterococcal Metabolite Cues Facilitate Interspecies Niche Modulation and Polymicrobial Infection. Cell Host Microbe 2016, 20, 493–503. [Google Scholar] [CrossRef]
- Lopes, S.P.; Machado, I.; Pereira, M.O. Role of planktonic and sessile extracellular metabolic byproducts on Pseudomonas aeruginosa and Escherichia coli intra and interspecies relationships. J. Ind. Microbiol. Biotechnol. 2011, 38, 133–140. [Google Scholar] [CrossRef]
- Machado, I.; Lopes, S.P.; Sousa, A.M.; Pereira, M.O. Adaptive response of single and binary Pseudomonas aeruginosa and Escherichia coli biofilms to benzalkonium chloride. J. Basic Microbiol. 2012, 52, 43–52. [Google Scholar] [CrossRef]
- Cerqueira, L.; Oliveira, J.A.; Nicolau, A.; Azevedo, N.F.; Vieira, M.J. Biofilm formation with mixed cultures of Pseudomonas aeruginosa/Escherichia coli on silicone using artificial urine to mimic urinary catheters. Biofouling 2013, 29, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, M.H.; van der Schaaf, S.; Koester, W.; Krooneman, J.; van der Meer, W.; Harmsen, H.; Landini, P. Biofilm formation by Escherichia coli is stimulated by synergistic interactions and co-adhesion mechanisms with adherence-proficient bacteria. Res. Microbiol. 2006, 5, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, M.V.; Maslennikova, I.L.; Karpunina, T.I.; Nesterova, L.Y.; Demakov, V.A. Interactions of Pseudomonas aeruginosa in predominant biofilm or planktonic forms of existence in mixed culture with Escherichia coli in vitro. Can. J. Microbiol. 2013, 59, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, V.A.; Mrugova, T.M.; Kurlayev, P.P.; Belozertseva, Y.P.; Borisov, S.D. Antagonistic relationship Pseudomonas aeruginosa with gram-negative bacteria. Bull. OSC 2016, 4, 1–5. (In Russian) [Google Scholar]
- González Barrios, A.F.; Zuo, R.; Hashimoto, Y.; Yang, L.; Bentley, W.E.; Wood, T.K. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 2006, 1, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Goh, S.G.; You, L.; Yuan, Q.; Mohapatra, S.; Gin, K.Y.; Chen, B. Low concentration quaternary ammonium compounds promoted antibiotic resistance gene transfer via plasmid conjugation. Sci. Total Environ. 2023, 887, 163781. [Google Scholar] [CrossRef]
- Cho, J.; Jenneson, S.; Lane, M.; Macfadyen, A.; Van Rietschoten, S. The effects of altering autoinducer-2 concentration on transfer efficiencies of the F and RPI plasmids to the Quorum sensing recipient Escherichia coli strain AB 1157. JEMI 2003, 3, 8–14. [Google Scholar]
- Rijavec, M.; Rijavec, M.; Starčič Erjavec, M.; Ambrožič Avguštin, J.; Reissbrodt, R.; Fruth, A.; Križan-Hergouth, V.; Žgur-Bertok, D. High prevalence of multidrug resistance and random distribution of mobile genetic elements among uropathogenic Escherichia coli (UPEC) of the four major phylogenetic groups. Curr. Microbiol. 2006, 53, 158–162. [Google Scholar] [CrossRef]
- Starčič Erjavec, M.; Petkovšek, Ž.; Kuznetsova, M.V.; Maslennikova, I.L.; Žgur-Bertok, D. Strain ŽP—The first bacterial conjugation-based “kill”-“anti-kill” antimicrobial system. Plasmid 2015, 82, 28–34. [Google Scholar] [CrossRef]
- Bassler, B.L.; Greenberg, E.P.; Stevens, A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997, 179, 4043–4045. [Google Scholar] [CrossRef]
- Guglielmetti, E.; Korhonen, J.M.; Heikkinen, J.; Morelli, L.; Von Wright, A. Transfer of plasmid-mediated resistance to tetracycline in pathogenic bacteria from fish and aquaculture environments. FEMS Microbiol. Lett. 2009, 293, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005. [Google Scholar] [CrossRef]
- Surette, M.G.; Bassler, B.L. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1998, 95, 7046–7050. [Google Scholar] [CrossRef]
- Zorina, A.S.; Maksimova, Y.G.; Demakov, V.A. Biofilm formation by monocultures and mixed cultures of Alcaligenes faecalis 2 and Rhodococcus ruber GT 1. Microbiology 2019, 88, 164–171. [Google Scholar] [CrossRef]


| Conjugation Mixture | № | Frequency of Conjugation | |
|---|---|---|---|
| R + D + Opportunistic Pathogenic Bacteria | R + D + CFSs of Opportunistic Pathogenic Bacteria | ||
| E. coli DL82 + E. coli N4i pOX38 | 1 | 4.72 × 10−4 ± 2.28 × 10−4 | |
| E. coli DL82 + E. coli N4i pOX38+ K. pneumoniae | 2 | 2.69 × 10−4 ± 1.01 × 10−4 | 4.93 × 10−5 ± 3.66 × 10−5 P1–2 = 0.0495 1 |
| E. coli DL82 + E. coli N4i pOX38 + E. faecalis | 3 | 1.78 × 10−5 ± 5.38 × 10−6 P1–3 = 0.0495 P2–3 = 0.0495 | 1.93 × 10−5 ± 4.17 × 10−7 P1–3 = 0.0495 |
| E. coli DL82 + E. coli N4i pOX38+ P. aeruginosa | 4 | 2.93 × 10−5 ± 3.07 × 10−5 P1–4 = 0.0495 P2–4 = 0.0495 | 0.00 × 100 P1–4 = 0.0495 P2–4 = 0.0495 P3–4 = 0.0495 |
| Cell-Free Supernatant (CSF) of Strains/Conjugation Mixtures | № | Induction of Luminescence, % |
|---|---|---|
| V. harveyi BB152 | 1 | 100.0 |
| E. coli K12 | 2 | 386.8 ± 42.9 P1–2 = 0.012 1 |
| E. coli DL82 | 3 | 3.2 ± 0.7 |
| E. coli N4i pOX38 | 4 | 1.3 ± 0.2 |
| E. coli DL82 + E. coli N4i pOX38 | 5 | 73.7 ± 35.5 P3–5 = 0.027 P4–5 = 0.016 |
| K. pneumoniae | 6 | 190.1 ± 57.9 P3–5 = 0.023 |
| E. coli DL82 + E. coli N4i pOX38+ K. pneumoniae | 7 | 77.4 ± 42.3 |
| E. coli DL82 + E. coli N4i pOX38 + CFS of K. pneumoniae | 8 | 96.2 ± 6.3 |
| E. faecalis | 9 | 0.1 ± 0.05 |
| E. coli DL82+ E. coli N4i pOX38 + E. faecalis | 10 | 0.7 ± 0.1 P5–10 = 0.027 |
| E. coli DL82+ E. coli N4i pOX38 + CFS of E. faecalis | 11 | 37.0 ± 4.7 P9–11 = 0.005 |
| P. aeruginosa | 12 | 1.5 ± 1.0 P2–12 = 0.010 |
| E. coli DL82 + E. coli N4i pOX38+ P. aeruginosa | 13 | 23.5 ± 14.6 P5–13 = 0.040 |
| E. coli DL82 + E. coli N4i pOX38+ CFS of P. aeruginosa | 14 | 29.4 ± 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, M.V.; Pospelova, J.S.; Maslennikova, I.L.; Starčič Erjavec, M. Dual-Species Biofilms: Biomass, Viable Cell Ratio/Cross-Species Interactions, Conjugative Transfer. Int. J. Mol. Sci. 2023, 24, 14497. https://doi.org/10.3390/ijms241914497
Kuznetsova MV, Pospelova JS, Maslennikova IL, Starčič Erjavec M. Dual-Species Biofilms: Biomass, Viable Cell Ratio/Cross-Species Interactions, Conjugative Transfer. International Journal of Molecular Sciences. 2023; 24(19):14497. https://doi.org/10.3390/ijms241914497
Chicago/Turabian StyleKuznetsova, Marina V., Julia S. Pospelova, Irina L. Maslennikova, and Marjanca Starčič Erjavec. 2023. "Dual-Species Biofilms: Biomass, Viable Cell Ratio/Cross-Species Interactions, Conjugative Transfer" International Journal of Molecular Sciences 24, no. 19: 14497. https://doi.org/10.3390/ijms241914497
APA StyleKuznetsova, M. V., Pospelova, J. S., Maslennikova, I. L., & Starčič Erjavec, M. (2023). Dual-Species Biofilms: Biomass, Viable Cell Ratio/Cross-Species Interactions, Conjugative Transfer. International Journal of Molecular Sciences, 24(19), 14497. https://doi.org/10.3390/ijms241914497





