Postnatal Overfeeding during Lactation Induces Endothelial Dysfunction and Cardiac Insulin Resistance in Adult Rats
Abstract
1. Introduction
2. Results
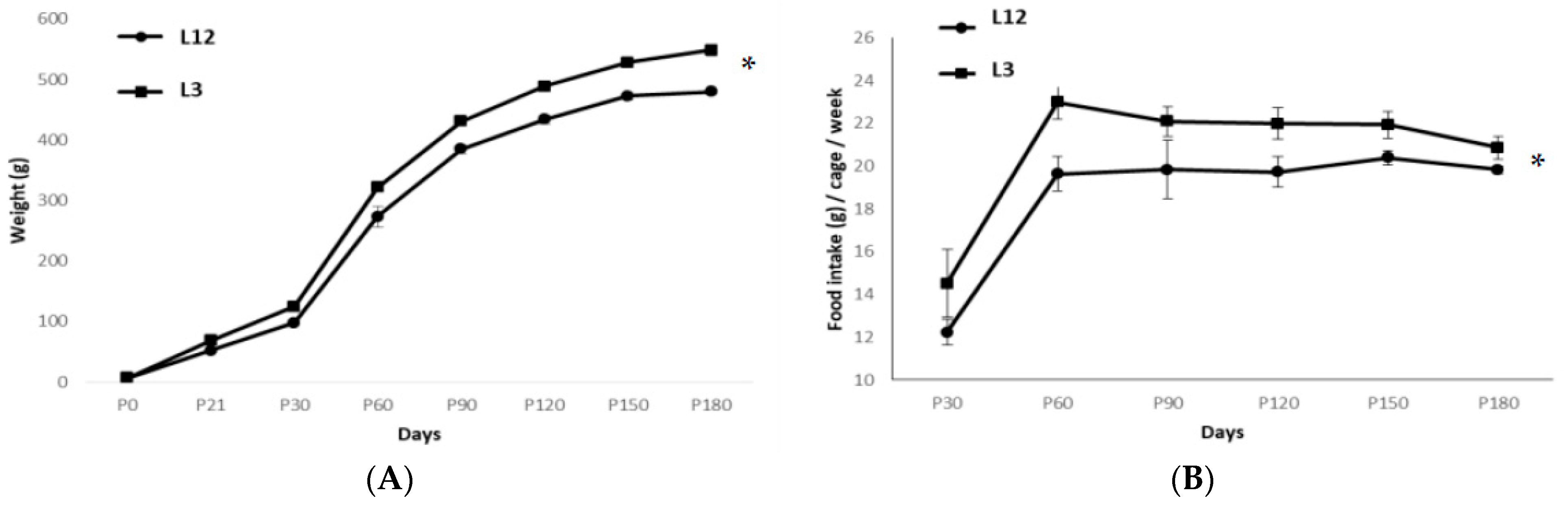
2.1. Body and Organ Weight
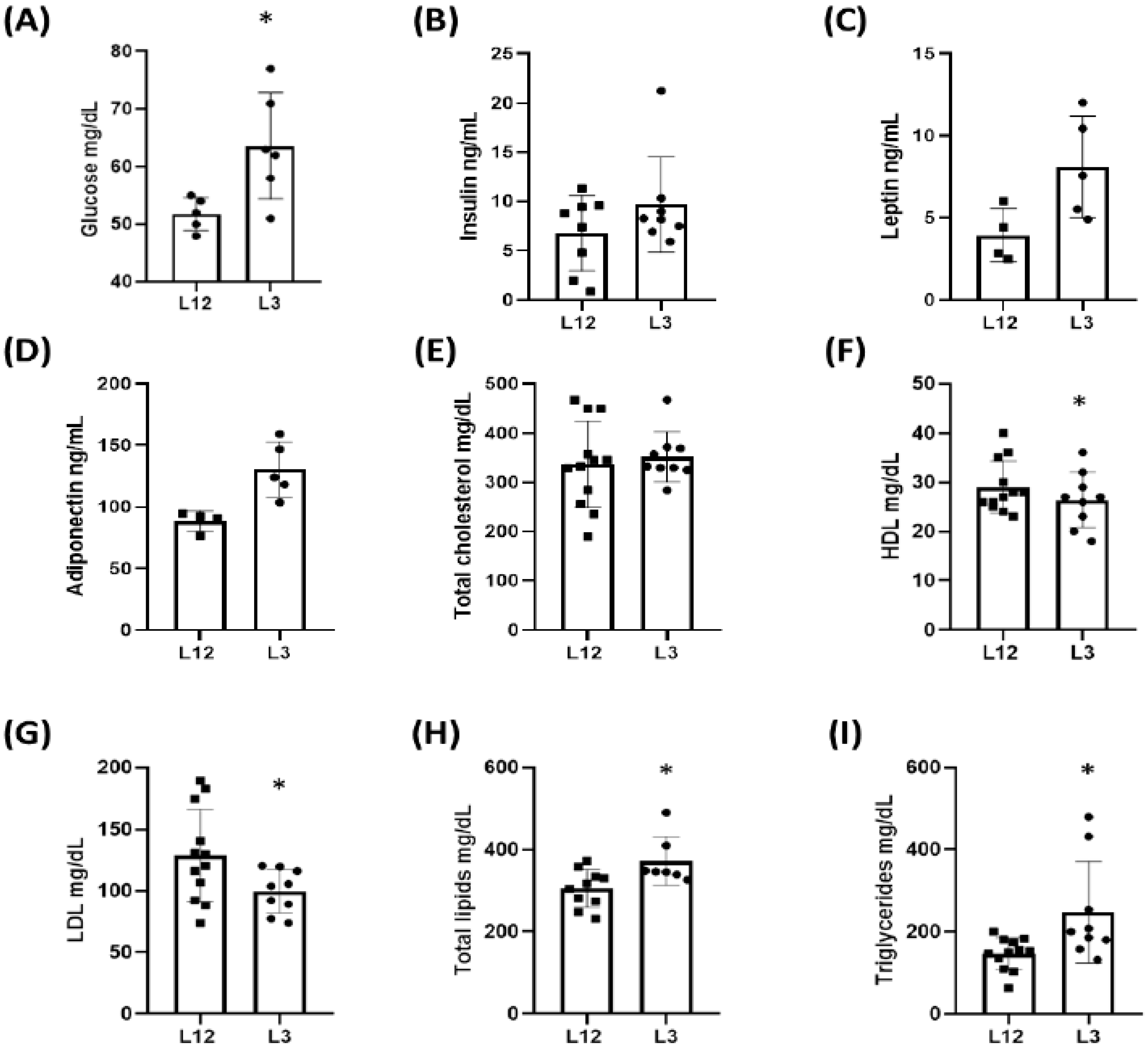
2.2. Glycemia, Lipid Profile, and Plasma Concentrations of Metabolic Hormones: Insulin, Leptin, and Adiponectin
2.3. Long-Term Cardiovascular Effects of Early Overnutrition
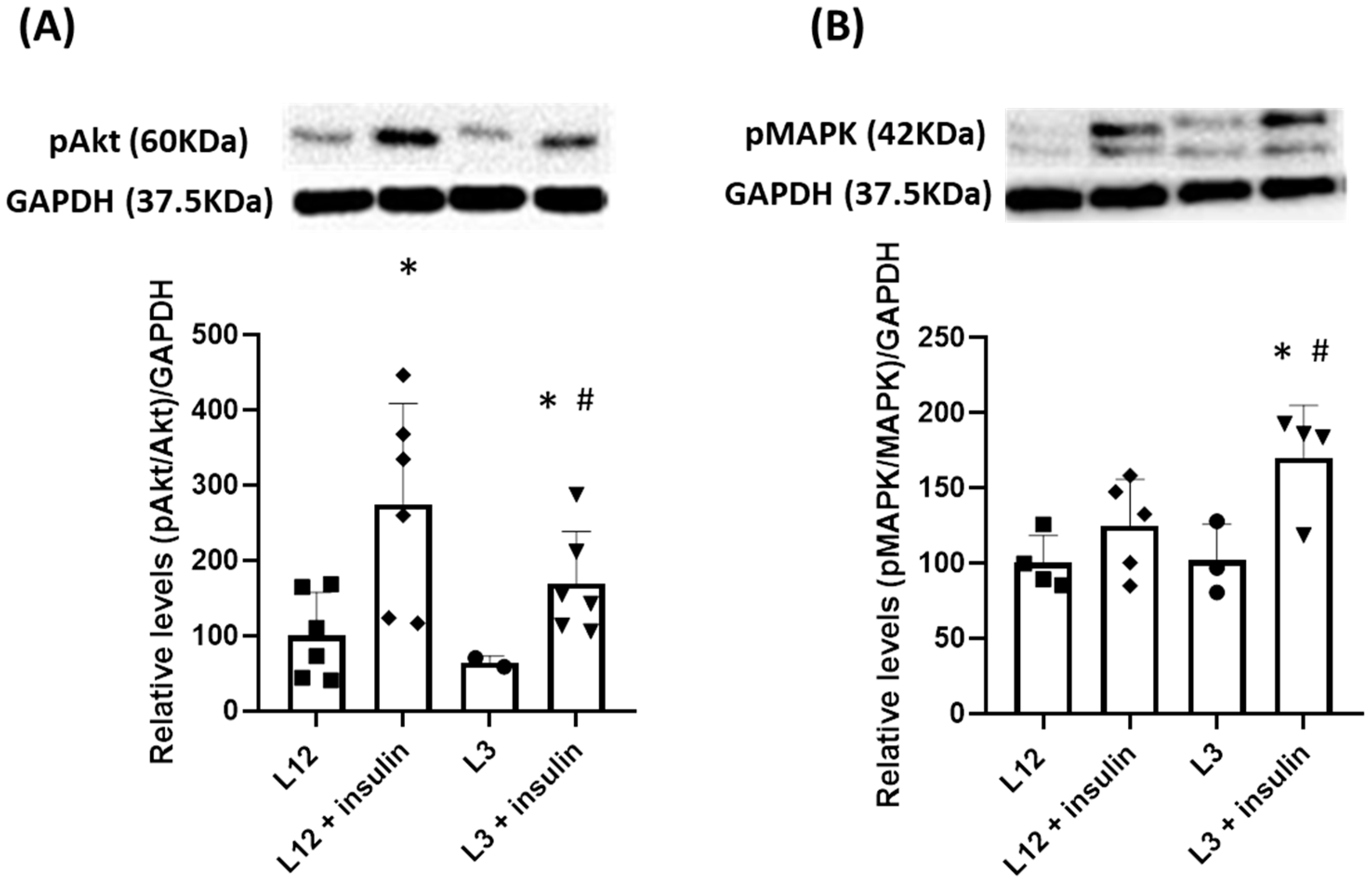
2.3.1. Myocardial Changes Triggered by Litter Reduction: mRNA Expression, Hemodynamic Parameters, and Activation of Molecular Pathways
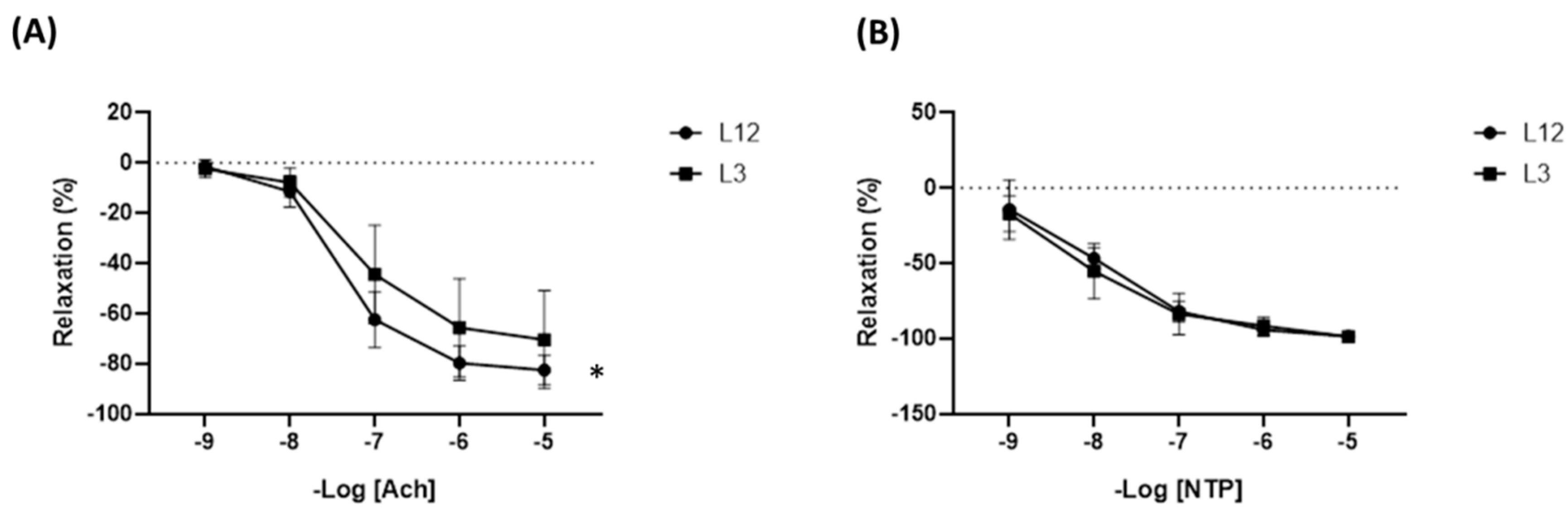
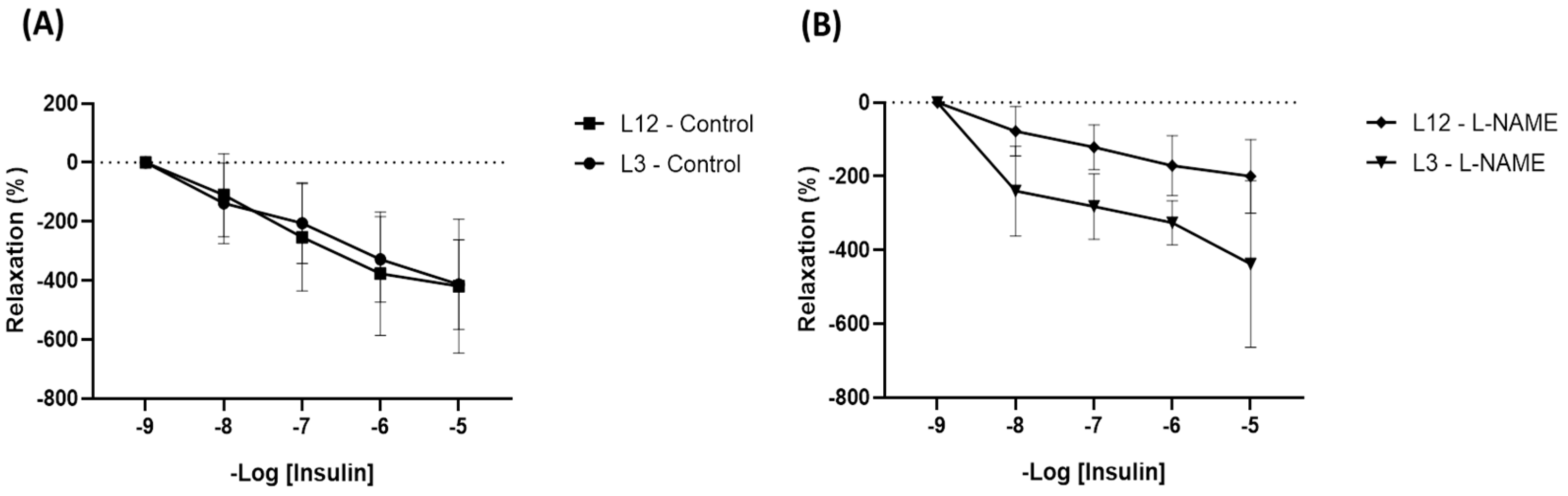
2.3.2. Arterial Changes Induced by Litter Reduction: mRNA Expression, Hemodynamic Parameters, and Activation of Molecular Pathways
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Plasma Measurements
4.3. Experiments of Vascular Reactivity
4.4. Experiments of Cardiac Function: Langendorff
4.5. Nitrite and Nitrate Determination in the Culture Medium
4.6. Protein Quantification by Western Blot
4.7. RNA Extraction and Quantitative RT Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 4 August 2023).
- World Health Organization, Regional Office for Europe. WHO European Regional Obesity Report 2022. 2022. Available online: https://apps.who.int/iris/handle/10665/353747 (accessed on 4 August 2023).
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Causes and Consequences of Childhood Obesity. 2022. Available online: https://www.cdc.gov/obesity/basics/consequences.html (accessed on 4 August 2023).
- Barton, M. Childhood obesity: A life-long health risk. Acta Pharmacol. Sin. 2012, 33, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Deng, M.Q.; Zhang, Q.; Xiao, X.H. Early-life nutrition and metabolic disorders in later life: A new perspective on energy metabolism. Chin. Med. J. 2020, 133, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Velkoska, E. Mechanisms behind early life nutrition and adult disease outcome. World J. Diabetes 2011, 2, 127. [Google Scholar] [CrossRef]
- Forsdahl, A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? J. Epidemiol. Community Health 1977, 31, 91–95. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Ozanne, S.E. Early life programming of obesity and metabolic disease. Physiol. Behav. 2008, 94, 17–28. [Google Scholar] [CrossRef]
- Bouret, S.G. Early life origins of obesity: Role of hypothalamic programming. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. 1), S31–S38. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Huang, W.; Jaspan, J.B.; Maness, L.M. Leptin enters the brain by a saturable system independent of insulin. Peptides 1996, 17, 305–311. [Google Scholar] [CrossRef]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Pantazi, K.; Karlafti, E.; Bekiaridou, A.; Didagelos, M.; Ziakas, A.; Didangelos, T. Insulin Receptors and Insulin Action in the Heart: The Effects of Left Ventricular Assist Devices. Biomolecules 2022, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, B.; Kahn, C.R. Insulin Action and the Insulin Signaling Network. Endocr. Rev. 1995, 16, 117–142. [Google Scholar] [PubMed]
- Montagnani, M.; Chen, H.; Barr, V.A.; Quon, M.J. Insulin-stimulated Activation of eNOS Is Independent of Ca2+ but Requires Phosphorylation by Akt at Ser1179. J. Biol. Chem. 2001, 276, 30392–30398. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Kahn, A.M.; Seidel, C.L.; Allen, J.C.; O’Neil, R.G.; Shelat, H.; Song, T. Insulin reduces contraction and intracellular calcium concentration in vascular smooth muscle. Hypertension 1993, 22, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Sundell, J. Insulin and myocardial blood flow. Cardiovasc. Res. 2003, 57, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.W. Insulin as a mitogenic factor: Role in the pathogenesis of cardiovascular disease. Am. J. Med. 1991, 90, S62–S65. [Google Scholar] [CrossRef]
- González-Hedström, D.; Guerra-Menéndez, L.; Tejera-Muñoz, A.; Amor, S.; de la Fuente-Fernández, M.; Martín-Carro, B.; Arriazu, R.; García-Villalón, Á.L.; Granado, M. Overfeeding During Lactation in Rats is Associated with Cardiovascular Insulin Resistance in the Short-Term. Nutrients 2020, 12, 549. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Morris, M.J.; Velkoska, E.; Cole, T.J. Central and peripheral contributions to obesity-associated hypertension: Impact of early overnourishment: Cardiovascular consequences of obesity. Exp. Physiol. 2005, 90, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.E.; Yoo, K.H.; Bae, I.S.; Hong, Y.S.; Lee, J.W. Postnatal early overnutrition causes long-term renal decline in aging male rats. Pediatr. Res. 2014, 75, 259–265. [Google Scholar] [CrossRef][Green Version]
- Josse, M.; Rigal, E.; Rosenblatt-Velin, N.; Rochette, L.; Zeller, M.; Guenancia, C.; Vergely, C. Programming of Cardiovascular Dysfunction by Postnatal Overfeeding in Rodents. Int. J. Mol. Sci. 2020, 21, 9427. [Google Scholar] [CrossRef]
- Habbout, A.; Li, N.; Rochette, L.; Vergely, C. Postnatal Overfeeding in Rodents by Litter Size Reduction Induces Major Short- and Long-Term Pathophysiological Consequences. J. Nutr. 2013, 143, 553–562. [Google Scholar] [CrossRef]
- de Andrade Silva, S.C.; da Silva, A.I.; Braz, G.R.F.; da Silva Pedroza, A.A.; de Lemos, M.D.T.; Sellitti, D.F.; Lagranha, C. Overfeeding during development induces temporally-dependent changes in areas controlling food intake in the brains of male Wistar rats. Life Sci. 2021, 285, 119951. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, R.; Rossetti, M.F.; Lazzarino, G.P.; Canesini, G.; García, A.P.; Stoker, C.; Andreoli, M.F.; Ramos, J.G. Temporary effects of neonatal overfeeding on homeostatic control of food intake involve alterations in POMC promoter methylation in male rats. Mol. Cell Endocrinol. 2021, 522, 111123. [Google Scholar] [CrossRef]
- Roberts, B.L.; Bennett, C.M.; Carroll, J.M.; Lindsley, S.R.; Kievit, P. Early overnutrition alters synaptic signaling and induces leptin resistance in arcuate proopiomelanocortin neurons. Physiol. Behav. 2019, 206, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Enes-Marques, S.; Rojas, V.C.T.; Batista, T.H.; Vitor-Vieira, F.; Novais, C.O.; Vilela, F.C.; Rafacho, A.; Giusti-Paiva, A. Neonatal overnutrition programming impairs cholecystokinin effects in adultmale rats. J. Nutr. Biochem. 2020, 86, 108494. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Rocha, M.; Amaro, A.; Ferreira-Junior, M.D.; Cavalcante, K.V.N.; Monteiro-Alfredo, T.; Barra, C.; Rosendo-Silva, D.; Saavedra, L.P.J.; Magalhães, J.; et al. Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models. Nutrients 2023, 15, 1281. [Google Scholar] [CrossRef]
- Du, Q.; Hosoda, H.; Umekawa, T.; Kinouchi, T.; Ito, N.; Miyazato, M.; Kangawa, K.; Ikeda, T. Postnatal weight gain induced by overfeeding pups and maternal high-fat diet during the lactation period modulates glucose metabolism and the production of pancreatic and gastrointestinal peptides. Peptides 2015, 70, 23–31. [Google Scholar] [CrossRef]
- Portella, A.K.; Silveira, P.P.; Laureano, D.P.; Cardoso, S.; Bittencourt, V.; Noschang, C.; Werlang, I.; Fontella, F.U.; Dalmaz, C.; Goldani, M.Z. Litter size reduction alters insulin signaling in the ventral tegmental area and influences dopamine-related behaviors in adult rats. Behav. Brain Res. 2015, 278, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Granado, M.; Fernández, N.; Monge, L.; Carreño-Tarragona, G.; Figueras, J.C.; Amor, S.; García-Villalón, Á.L. Long-Term Effects of Early Overnutrition in the Heart of Male Adult Rats: Role of the Renin-Angiotensin System. PLoS ONE. 2013, 8, e65172. [Google Scholar] [CrossRef] [PubMed]
- Conceição, E.P.S.; Trevenzoli, I.H.; Oliveira, E.; Franco, J.G.; Carlos, A.S.; Nascimento-Saba, C.C.A.; Moura, E.G.; Lisboa, P.C. Higher White Adipocyte Area and Lower Leptin Production in Adult Rats Overfed During Lactation. Horm. Metab. Res. 2011, 43, 513–516. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Zhang, Y. Leptin resistance: A prediposing factor for diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R493–R500. [Google Scholar] [CrossRef]
- Argente-Arizón, P.; Ros, P.; Díaz, F.; Fuente-Martin, E.; Castro-González, D.; Sánchez-Garrido, M.Á.; Barrios, V.; Tena-Sempere, M.; Argente, J.; Chowen, J.A. Age and Sex Dependent Effects of Early Overnutrition on Metabolic Parameters and the Role of Neonatal Androgens. Biol. Sex Differ. 2016, 7, 26. [Google Scholar] [CrossRef]
- Blanco, N.; Fernández-García, J.M.; Carrillo, B.; Ballesta, A.; García-Úbeda, R.; Collado, P.; Pinos, H. Prenatal Low-Protein and Low-Calorie Diets Differentially Alter Arcuate Nucleus Morphology in Newborn Male Rats. Front. Neuroanat. 2022, 16, 896732. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.L.; Moura, E.G.; Lisboa, P.C. Litter Size Reduction as a Model of Overfeeding during Lactation and Its Consequences for the Development of Metabolic Diseases in the Offspring. Nutrients 2022, 14, 2045. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yang, F.; Zhou, N.; Sha, L.; Zhou, S.; Wang, J.; Li, X. A post-weaning fish oil dietary intervention reverses adverse metabolic outcomes and 11 β -hydroxysteroid dehydrogenase type 1 expression in postnatal overfed rats. Br. J. Nutr. 2016, 116, 1519–1529. [Google Scholar] [CrossRef]
- Leite, R.D.; Kraemer-Aguiar, L.G.; Boa, B.C.D.S.; Cyrino, F.Z.G.A.; Nivoit, P.; Bouskela, E. Muscle endothelial-dependent microvascular dysfunction in adulthood due to early postnatal overnutrition. Microvasc. Res. 2012, 84, 94–98. [Google Scholar] [CrossRef]
- Azemi, A.K.; Siti-Sarah, A.R.; Mokhtar, S.S.; Rasool, A.H.G. Time-Restricted Feeding Improved Vascular Endothelial Function in a High-Fat Diet-Induced Obesity Rat Model. Vet. Sci. 2022, 9, 217. [Google Scholar] [CrossRef]
- Kwitek, A.E. Rat Models of Metabolic Syndrome. Rat Genom. 2019, 2018, 269–285. [Google Scholar]
- Velkoska, E.; Cole, T.J.; Morris, M.J. Early dietary intervention: Long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1236–E1243. [Google Scholar] [CrossRef]
- Moreira, A.S.B.; Teixeira, M.T.; da Silveira Osso, F.; Pereira, R.O.; de Oliveira Silva-Junior, G.; de Souza, E.G.; de Lacerda, C.M.; Moura, A.S. Left ventricular hypertrophy induced by overnutrition early in life. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 805–810. [Google Scholar] [CrossRef]
- Junior, M.D.F.; Cavalcante, K.V.N.; Ferreira, L.A.; Lopes, P.R.; Pontes, C.N.R.; de Bessa, A.D.S.M.; Neves, Â.R.; Francisco, F.A.; Pedrino, G.R.; Xavier, C.H.; et al. Postnatal early overfeeding induces cardiovascular dysfunction by oxidative stress in adult male Wistar rats. Life Sci. 2019, 226, 173–184. [Google Scholar] [CrossRef]
- Martins, M.R.; Vieira, A.K.G.; De Souza, É.P.G.; Moura, A.S. Early overnutrition impairs insulin signaling in the heart of adult Swiss mice. J. Endocrinol. 2008, 198, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.K.G.; Soares, V.M.; Bernardo, A.F.; Neves, F.A.; Mattos, A.B.M.; Guedes, R.M.; Cortez, E.; Andrade, D.C.; Lacerda-Miranda, G.; Garcia-Souza, E.P.; et al. Overnourishment during lactation induces metabolic and haemodynamic heart impairment during adulthood. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.F.; Cortez, E.; Neves, F.A.; Vieira, A.K.; Soares, V.D.M.; Rodrigues-Cunha, A.C.D.S.; Andrade, D.C.; Thole, A.A.; Gabriel-Costa, D.; Brum, P.C.; et al. Overnutrition during lactation leads to impairment in insulin signaling, up-regulation of GLUT1 and increased mitochondrial carbohydrate oxidation in heart of weaned mice. J. Nutr. Biochem. 2016, 29, 124–132. [Google Scholar] [CrossRef]
- Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells 2022, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, A.K.; Sack, M.N.; Mjøs, O.D.; Yellon, D.M. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ. Res. 2001, 89, 1191–1198. [Google Scholar] [CrossRef]
- Brito Díaz, B.; Alemán Sánchez, J.J.; Cabrera de León, A. Frecuencia cardiaca en reposo y enfermedad cardiovascular. Med. Clínica 2014, 143, 34–38. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, M.S.; Li, Y.; Wang, A.; Chadipiralla, K.; Tian, R.; Raij, L. Oral nicotine aggravates endothelial dysfunction and vascular inflammation in diet-induced obese rats: Role of macrophage TNFα. PLoS ONE. 2017, 12, e0188439. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, G.; Del Bosque-Plata, L.; Hong, E. Postnatal overnutrition affects metabolic and vascular function reflected by physiological and histological changes in the aorta of adult Wistar rats. Clin. Exp. Hypertens. 2018, 40, 452–460. [Google Scholar] [CrossRef] [PubMed]


| L12 | L3 | |
|---|---|---|
| Visceral Fat | 1354.12 ± 51.9 | 1655.10 ± 110.9 *** |
| Subcutaneous Fat | 504.27 ± 115.1 | 666.18 ± 143.3 ** |
| Brown Fat | 112.50 ± 28.6 | 115.55 ± 51.1 |
| PVAT | 16.26 ± 5.1 | 21.53 ± 2.2 * |
| Gastrocnemius | 505.90 ± 12.7 | 491.43 ± 11.8 |
| Soleus | 39.50 ± 1.8 | 45.56 ± 1.4 * |
| Liver | 2435.83 ± 59.3 | 2354.85 ± 51.2 |
| Heart | 432.24 ± 19.7 | 387.08 ± 10.6 * |
| Gene | L12 (Basal) | L3 (Gene Expression/18S) |
|---|---|---|
| COX2 | 100 ± 20.7 | 80.30 ± 15 |
| GRS | 100 ± 18.9 | 121.95 ± 7 |
| GPX | 100 ± 7.7 | 115.45 ± 1.4 |
| TNFα | 100 ± 17.5 | 80.68 ± 24.1 |
| SOD1 | 100 ± 9.6 | 105.13 ± 0.8 |
| iNOS | 100 ± 44.3 | 45.21 ± 13.8 |
| IL-1β | 100 ± 20.1 | 126.32 ± 32.3 |
| IL-6 | 100 ± 13.9 | 79.14 ± 3.5 |
| IL-10 | 100 ± 19.4 | 42.86 ± 18.7 * |
| NOX1 | 100 ± 34.2 | 156.31 ± 22.1 |
| NOX4 | 100 ± 30.9 | 94.14 ± 6.5 |
| Gene | L12 (Basal) | L3 (Gene Expression/18S) |
|---|---|---|
| COX2 | 100 ± 10.7 | 114.82 ± 24.6 |
| GRS | 100 ± 18.1 | 149.72 ± 43.2 |
| GPX | 100 ± 12.4 | 174.25 ± 70.2 |
| TNFα | 100 ± 27.6 | 175.17 ± 27.3 * |
| SOD1 | 100 ± 30.6 | 131.69 ± 34.1 |
| iNOS | 100 ± 29.5 | 24.4 ± 10.6 * |
| IL-1β | 100 ± 25.1 | 185.93 ± 56.9 |
| IL-6 | 100 ± 17.5 | 191.78 ± 14.7 * |
| IL-10 | 100 ± 33.9 | 84.52 ± 15.8 |
| NOX1 | 100 ± 56 | 118.88 ± 66.5 |
| NOX4 | 100 ± 21.3 | 89.84 ± 11.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejera-Muñoz, A.; Guerra-Menéndez, L.; Amor, S.; González-Hedström, D.; García-Villalón, Á.L.; Granado, M. Postnatal Overfeeding during Lactation Induces Endothelial Dysfunction and Cardiac Insulin Resistance in Adult Rats. Int. J. Mol. Sci. 2023, 24, 14443. https://doi.org/10.3390/ijms241914443
Tejera-Muñoz A, Guerra-Menéndez L, Amor S, González-Hedström D, García-Villalón ÁL, Granado M. Postnatal Overfeeding during Lactation Induces Endothelial Dysfunction and Cardiac Insulin Resistance in Adult Rats. International Journal of Molecular Sciences. 2023; 24(19):14443. https://doi.org/10.3390/ijms241914443
Chicago/Turabian StyleTejera-Muñoz, Antonio, Lucía Guerra-Menéndez, Sara Amor, Daniel González-Hedström, Ángel Luis García-Villalón, and Miriam Granado. 2023. "Postnatal Overfeeding during Lactation Induces Endothelial Dysfunction and Cardiac Insulin Resistance in Adult Rats" International Journal of Molecular Sciences 24, no. 19: 14443. https://doi.org/10.3390/ijms241914443
APA StyleTejera-Muñoz, A., Guerra-Menéndez, L., Amor, S., González-Hedström, D., García-Villalón, Á. L., & Granado, M. (2023). Postnatal Overfeeding during Lactation Induces Endothelial Dysfunction and Cardiac Insulin Resistance in Adult Rats. International Journal of Molecular Sciences, 24(19), 14443. https://doi.org/10.3390/ijms241914443






