Assessment of the Immunoprotective Efficacy of Recombinant 14-3-3 Protein and Dense Granule Protein 10 (GRA10) as Candidate Antigens for Rabbit Vaccines against Eimeria intestinalis
Abstract
:1. Introduction
2. Results
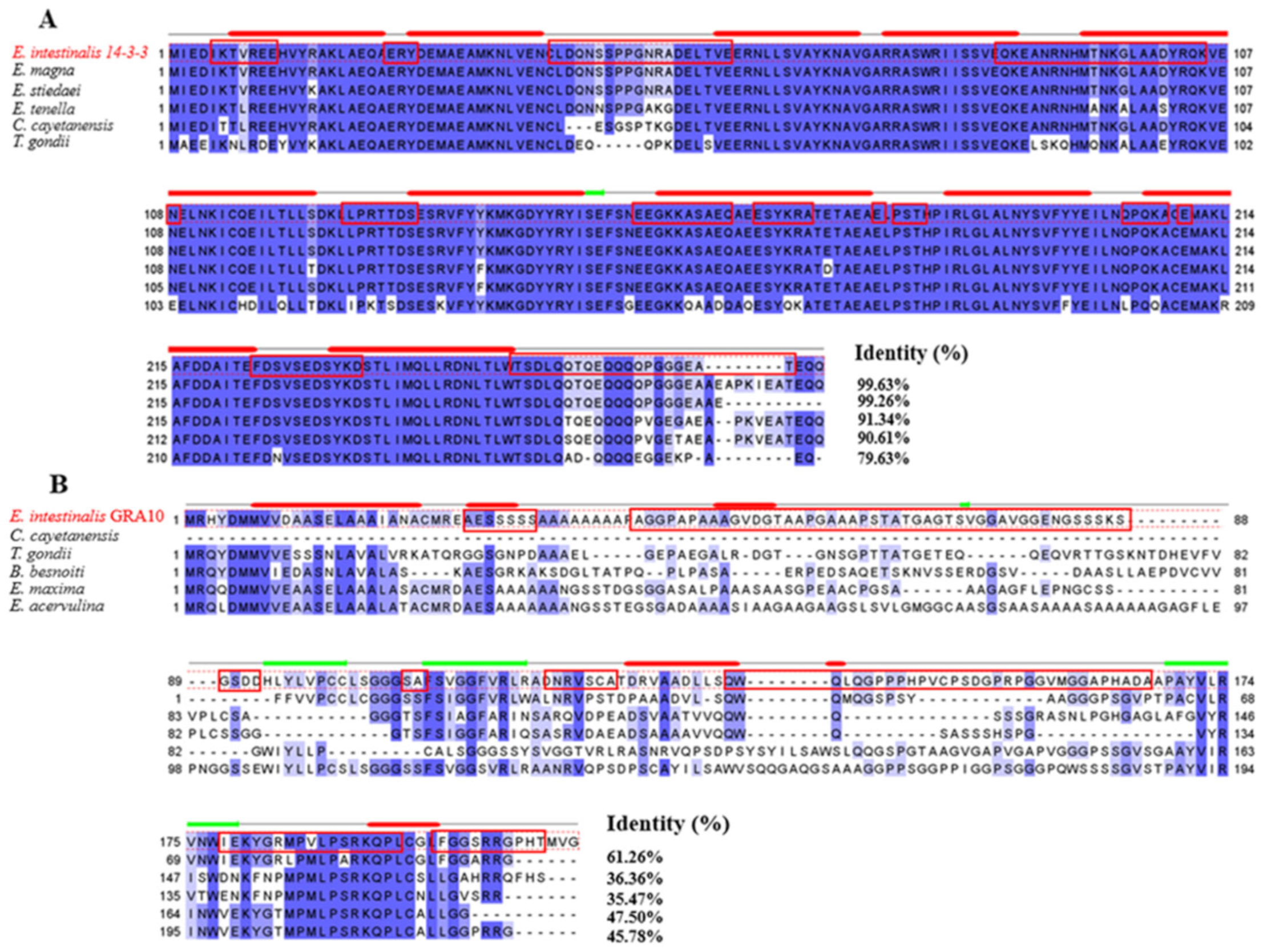
2.1. Gene Cloning and Bioinformatic Analysis
2.2. Expression of rEi-14-3-3 and rEi-GRA10
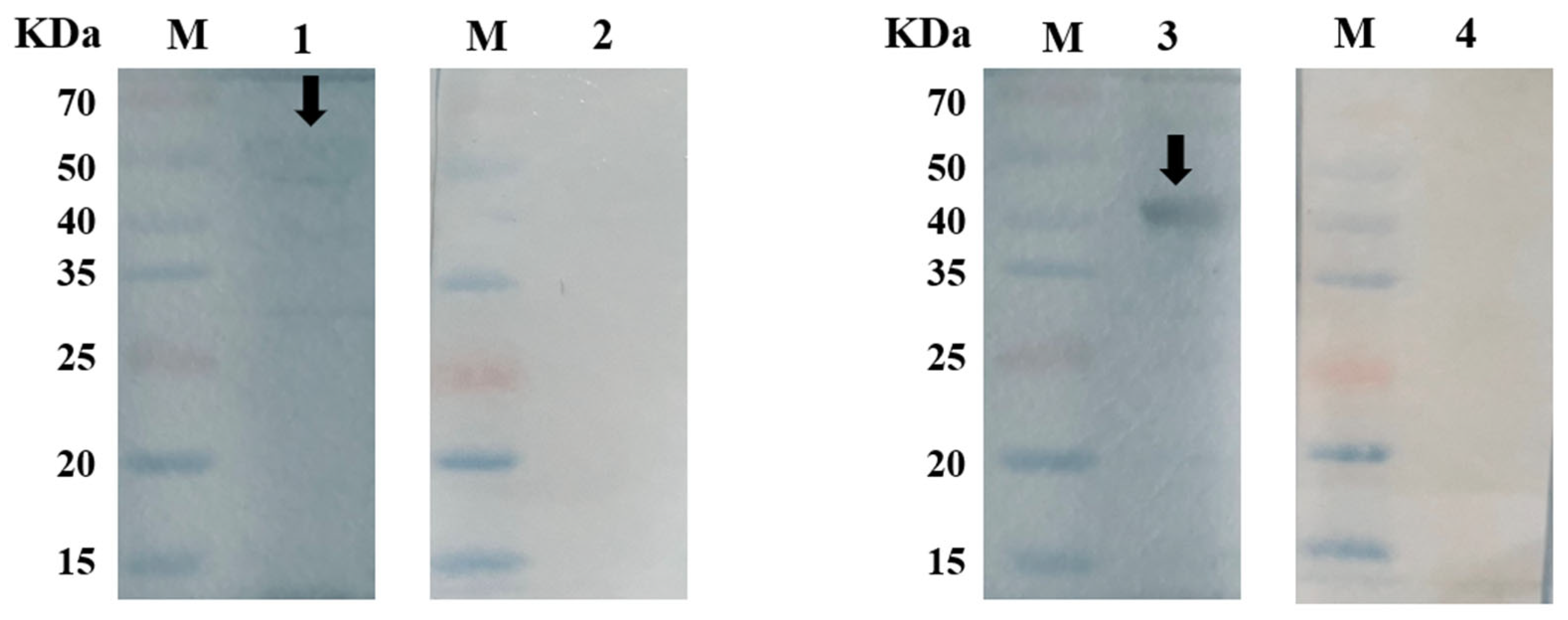
2.3. Western Blot Analyses
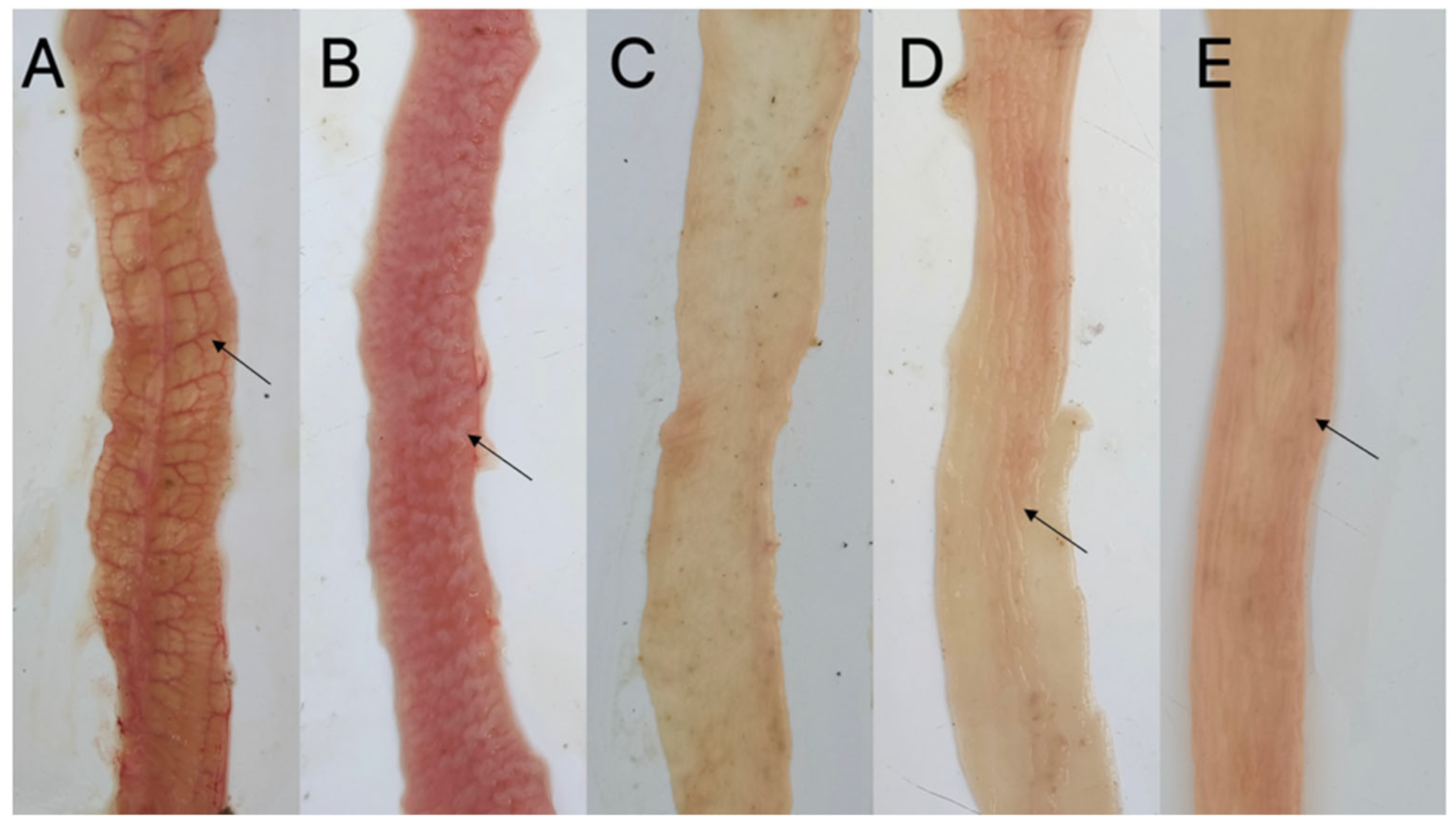
2.4. Evaluation of the Protective Effect of rEi-14-3-3 and rEi-GRA10
2.5. Calculation of ACI Value
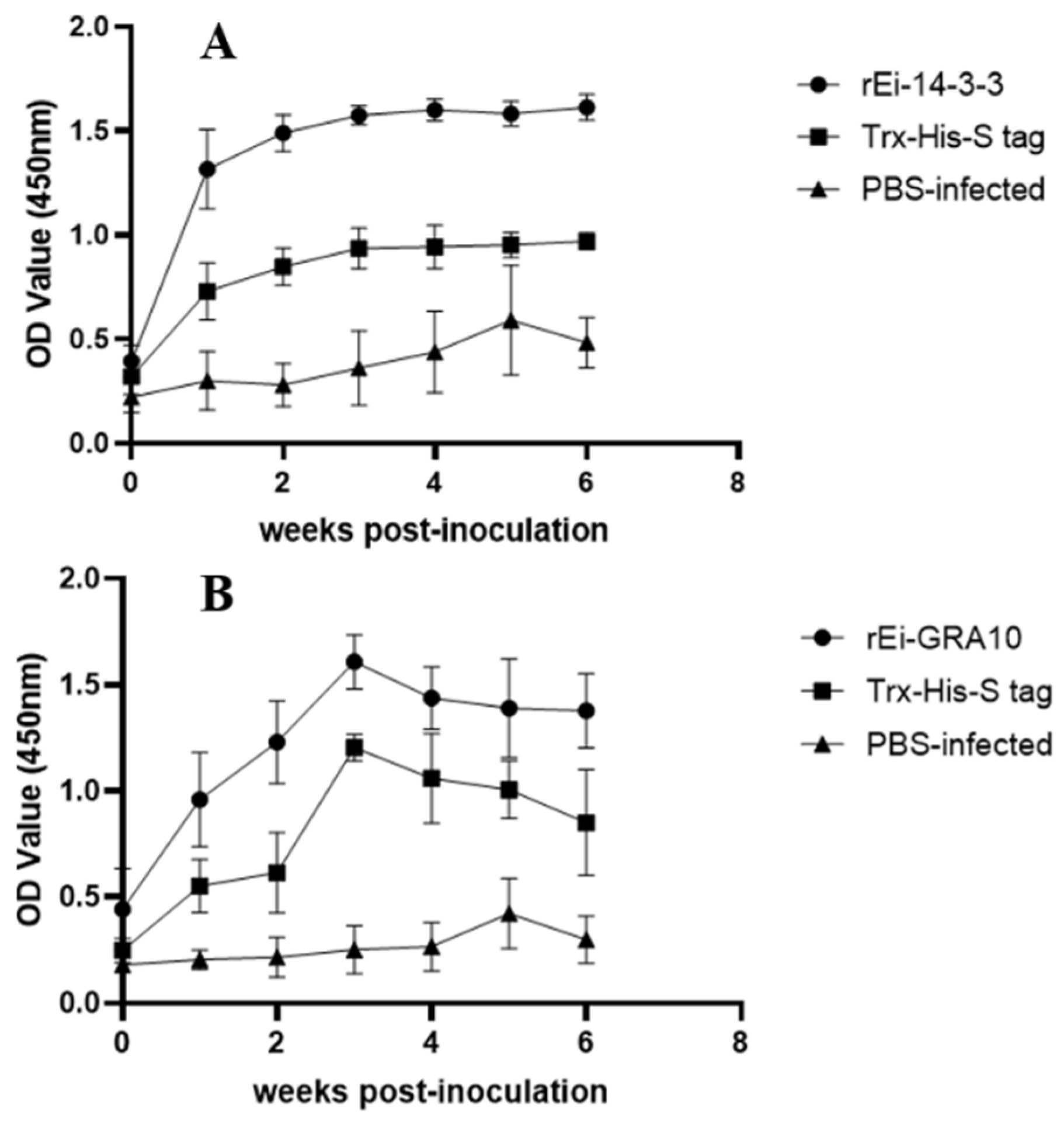
2.6. Specific Antibody Levels Detection
2.7. Cytokine Detection
3. Discussion
4. Materials and Methods
4.1. Animals, Parasites and Sera
4.2. Synthesis of cDNA
4.3. Bioinformatic Analysis
4.4. Cloning of the Ei-14-3-3 and Ei-GRA10 Genes
4.5. Expression and Purification of Recombinant Proteins
4.6. Western Blotting
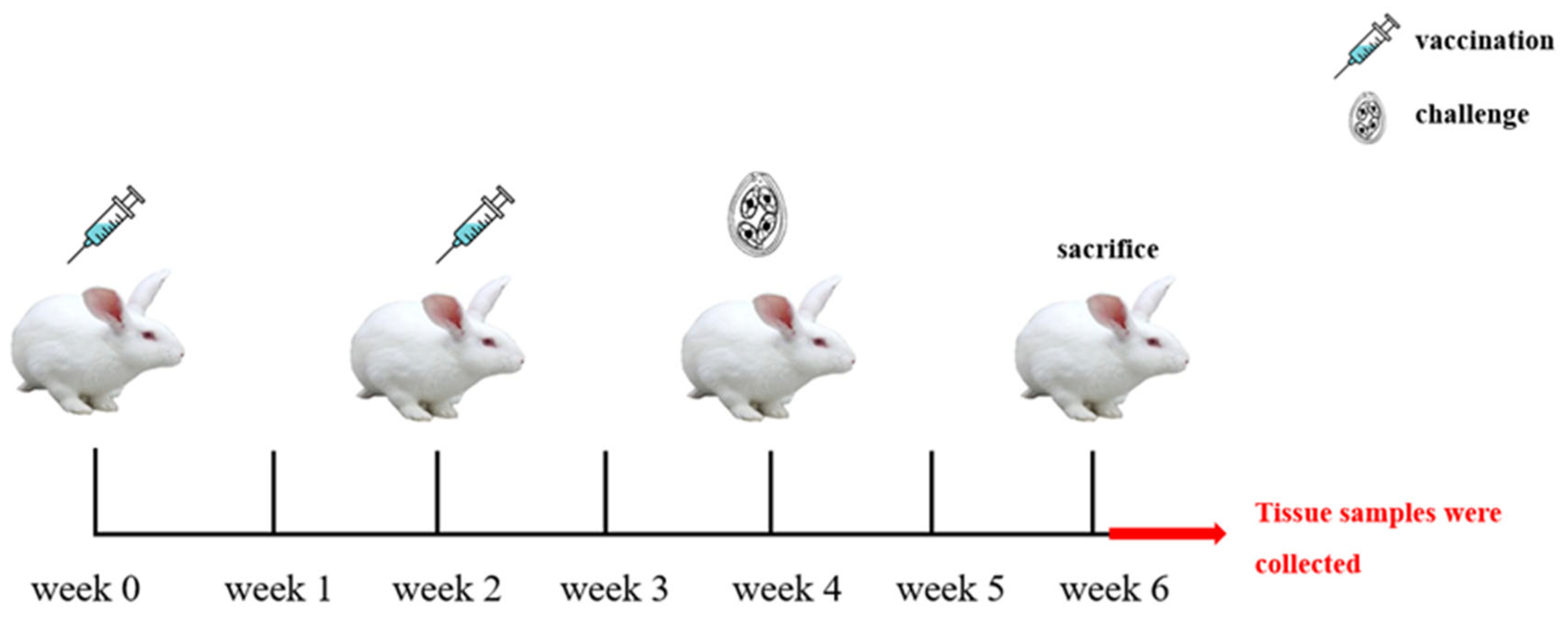
4.7. Immunization Procedure and Experimental Grouping
4.8. Evaluation of the Protective Effect
4.9. Specific IgG Antibody and Cytokine Level Detection
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, W.; Wang, T.; Suo, X.; Cui, P.; Gu, X.; Han, L.; Xue, B. Advances in coccidiosis of domestic rabbits. Chin. Vet. Sci. 2010, 40, 1200–1205. [Google Scholar] [CrossRef]
- Pakandl, M.; Hlásková, L.; Poplstein, M.; Chromá, V.; Vodicka, T.; Salát, J.; Mucksová, J. Dependence of the immune response to coccidiosis on the age of rabbit suckling. Parasitol. Res. 2008, 103, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Kvicerová, J.; Pakandl, M.; Hypsa, V. Phylogenetic relationships among Eimeria spp. (Apicomplexa, Eimeriidae) infecting rabbits: Evolutionary significance of biological and morphological features. Parasitology 2008, 135, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Licois, D. Comments on the article of Ming-Hsien Li and Hong-Kean Ooi “Fecal occult blood manifestation of intestinal Eimeria spp. infection in rabbit” [Vet. Parasitol. 161 (2009) 327–329]. Vet. Parasitol. 2009, 164, 363–364, author reply 365–366. [Google Scholar] [CrossRef]
- Licois, D.; Coudert, P.; Boivin, M.; Drouet-Viard, F.; Provôt, F. Selection and characterization of a precocious line of Eimeria intestinalis, an intestinal rabbit coccidium. Parasitol. Res. 1990, 76, 192–198. [Google Scholar] [CrossRef]
- Michal, P. Coccidia of rabbit: A review. Folia Parasitol. 2009, 56, 153–166. [Google Scholar]
- Pakandl, M.; Sewald, B.; Drouet-Viard, F. Invasion of the intestinal tract by sporozoites of Eimeria coecicola and Eimeria intestinalis in naive and immune rabbits. Parasitol. Res. 2006, 98, 310–316. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, J.; Hu, X.; Yang, S.; Zhong, S.; Yang, T.; Zhou, Y.; Zhao, G.; Jiang, Y.; Li, Y. Alterations in the jejunal microbiota and fecal metabolite profiles of rabbits infected with Eimeria intestinalis. Parasites Vectors 2022, 15, 231. [Google Scholar] [CrossRef]
- Coudert, P.; Licois, D.; Provôt, F.; Drouet-Viard, F. Eimeria sp. from the rabbit (Oryctolagus cuniculus): Pathogenicity and immunogenicity of Eimeria intestinalis. Parasitol Res 1993, 79, 186–190. [Google Scholar] [CrossRef]
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O.; Nduka, F.O.; Abah, A.E. Populations of Eimeria tenella express resistance to commonly used anticoccidial drugs in southern Nigeria. Int. J. Vet. Sci. Med. 2018, 6, 192–200. [Google Scholar] [CrossRef]
- Gu, X.; Liu, H.; Li, C.; Fang, S.; Cui, P.; Liao, Q.; Zhang, S.; Wang, S.; Duan, C.; Yu, F.; et al. Selection and characterization of a precocious line of Eimeria media. Parasitol. Res. 2019, 118, 3033–3041. [Google Scholar] [CrossRef]
- Li, C.; Tao, G.; Gu, X.; Cui, Y.; Wang, Y.; Suo, J.; Lv, Y.; Yu, F.; Mamoun, C.B.; Suo, X.; et al. Selection and identification of a precocious line of Eimeria intestinalis with enlarged oocysts and deletion of one generation of schizogony. Parasitol. Res. 2019, 118, 969–976. [Google Scholar] [CrossRef]
- Fang, S.; Gu, X.; El-Ashram, S.; Li, X.; Yu, X.; Guo, B.; Li, H.; Liu, N.; Liu, X.; Cui, P.; et al. Immune protection provided by a precocious line trivalent vaccine against rabbit Eimeria. Vet. Parasitol. 2019, 275, 108927. [Google Scholar] [CrossRef]
- Peek, H.W.; Landman, W.J. Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef]
- Zai, X.; Yin, Y.; Guo, F.; Yang, Q.; Li, R.; Li, Y.; Zhang, J.; Xu, J.; Chen, W. Screening of potential vaccine candidates against pathogenic Brucella spp. using compositive reverse vaccinology. Vet. Res. 2021, 52, 75. [Google Scholar] [CrossRef]
- Rawal, K.; Sinha, R.; Abbasi, B.A.; Chaudhary, A.; Nath, S.K.; Kumari, P.; Preeti, P.; Saraf, D.; Singh, S.; Mishra, K.; et al. Identification of vaccine targets in pathogens and design of a vaccine using computational approaches. Sci. Rep. 2021, 11, 17626. [Google Scholar] [CrossRef]
- Xiao, J.; He, W.; Xiong, C.; Hao, G.; Pu, J.; Chen, H.; Xu, L.; Zhu, Y.; Ren, Y.; Yang, G. Protective efficacy of recombinant proteins AMA1 and IMP1 in rabbits infected with Eimeria intestinalis. Vet. Parasitol. 2023, 320, 109985. [Google Scholar] [CrossRef]
- Siles-Lucas Mdel, M.; Gottstein, B. The 14-3-3 protein: A key molecule in parasites as in other organisms. Trends Parasitol. 2003, 19, 575–581. [Google Scholar] [CrossRef]
- Weidner, J.M.; Kanatani, S.; Uchtenhagen, H.; Varas-Godoy, M.; Schulte, T.; Engelberg, K.; Gubbels, M.J.; Sun, H.S.; Harrison, R.E.; Achour, A.; et al. Migratory activation of parasitized dendritic cells by the protozoan Toxoplasma gondii 14-3-3 protein. Cell. Microbiol. 2016, 18, 1537–1550. [Google Scholar] [CrossRef]
- Liang, S.; Zhao, Q.; Ye, Y.; Zhu, S.; Dong, H.; Yu, Y.; Huang, B.; Han, H. Characteristics analyses of Eimeria tenella 14-3-3 protein and verification of its interaction with calcium-dependent protein kinase 4. Eur. J. Protistol. 2022, 85, 125895. [Google Scholar] [CrossRef]
- Zhao, N.; Gong, P.; Cheng, B.; Li, J.; Yang, Z.; Li, H.; Yang, J.; Zhang, G.; Zhang, X. Eimeria tenella: 14-3-3 protein interacts with telomerase. Parasitol. Res. 2014, 113, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, T.; Wang, J.; Zheng, W.; Zhu, X. Research progress on biological functions of dense granule protein of Toxoplasma gondii. Acta Vet. Zootech. Sin. 2022, 53, 3345–3357. [Google Scholar]
- Blader, I.J.; Coleman, B.I.; Chen, C.T.; Gubbels, M.J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef]
- Meng, M.; Zhou, A.; Lu, G.; Wang, L.; Zhao, G.; Han, Y.; Zhou, H.; Cong, H.; Zhao, Q.; Zhu, X.Q.; et al. DNA prime and peptide boost immunization protocol encoding the Toxoplasma gondii GRA4 induces strong protective immunity in BALB/c mice. BMC Infect. Dis. 2013, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Qu, D.; Li, C.; Song, X.; Zhao, Q.; Li, X.A.; Yang, Y.; Liu, Q.; He, S.; Zhou, H. Enhancement of protective immune responses induced by Toxoplasma gondii dense granule antigen 7 (GRA7) against toxoplasmosis in mice using a prime-boost vaccination strategy. Vaccine 2012, 30, 5631–5636. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Sharif, M.; Sarvi, S.; Hejazi, S.H.; Aghayan, S.; Pagheh, A.S.; Dodangeh, S.; Daryani, A. A systematic review on the role of GRA proteins of Toxoplasma gondii in host immunization. J. Microbiol. Methods 2019, 165, 105696. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, Y.; Bai, X.; Chen, H.; Pu, J.; Zheng, R.; Xiao, J.; Yang, G. Preliminary observation on the immunoprotective effects of recombinant surface antigens SAG13 and SAG14 of Eimeria stiedae in rabbits. Acta Vet. Zootech. Sin. 2022, 53, 883–893. [Google Scholar]
- Xiao, J.; Zheng, R.; Bai, X.; Pu, J.; Chen, H.; Gu, X.; Xie, Y.; He, R.; Xu, J.; Jing, B.; et al. Preliminary evaluation of the protective effects of recombinant AMA1 and IMP1 against Eimeria stiedae infection in rabbits. Parasites Vectors 2022, 15, 400. [Google Scholar] [CrossRef]
- Pu, J.; Xiao, J.; Bai, X.; Chen, H.; Zheng, R.; Gu, X.; Xie, Y.; He, R.; Xu, J.; Jing, B.; et al. Prokaryotic Expression of Eimeria magna SAG10 and SAG11 Genes and the Preliminary Evaluation of the Effect of the Recombinant Protein on Immune Protection in Rabbits. Int. J. Mol. Sci. 2022, 23, 10942. [Google Scholar] [CrossRef]
- Chen, H.; Pu, J.; Xiao, J.; Bai, X.; Zheng, R.; Gu, X.; Xie, Y.; He, R.; Xu, J.; Jing, B.; et al. Evaluation of the immune protective effects of rEmMIC2 and rEmMIC3 from Eimeria magna in rabbits. Parasitol. Res. 2023, 122, 661–669. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, H.; Zheng, R.; Pu, J.; Gu, X.; Xie, Y.; He, R.; Xu, J.; Jing, B.; Peng, X.; et al. Recombinant GMA56 and ROP17 of Eimeria magna conferred protection against infection by homologous species. Front. Immunol. 2022, 13, 1037949. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L.; Li, L.; Tian, D.; Li, W.; Xu, L.; Yan, R.; Li, X.; Song, X. Protective immunity induced by Eimeria common antigen 14-3-3 against Eimeria tenella, Eimeria acervulina and Eimeria maxima. BMC Vet. Res. 2018, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, X.; Yin, H.; Wang, T.; Li, Y.; Zhou, C.; Zhou, H.; He, S.; Cong, H. Chitosan Microsphere Used as an Effective System to Deliver a Linked Antigenic Peptides Vaccine Protect Mice Against Acute and Chronic Toxoplasmosis. Front. Cell. Infect. Microbiol. 2018, 8, 163. [Google Scholar] [CrossRef]
- Yu, G.; Liang, W.; Yang, Q.; Wang, J.; Wang, Y.; Zhang, T.; Zhang, X.; Fan, H.; Zhao, P.; Cao, L.; et al. Immune Protective Evaluation Elicited by DNA Vaccination With Neospora caninum Dense Granules Proteins in Mice. Front. Vet. Sci. 2021, 8, 638067. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, X.; Zhang, T.; Liu, J.; Liu, Q. A Novel Rhoptry Protein as Candidate Vaccine against Eimeria tenella Infection. Vaccines 2020, 8, 452. [Google Scholar] [CrossRef]
- Karakus, U.; Sahin, D.; Mittl, P.R.E.; Mooij, P.; Koopman, G.; Boyman, O. Receptor-gated IL-2 delivery by an anti-human IL-2 antibody activates regulatory T cells in three different species. Sci. Transl. Med. 2020, 12, eabb9283. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005, 49, 1–8. [Google Scholar] [CrossRef]
- Lillehoj, H.S. Role of T lymphocytes and cytokines in coccidiosis. Int. J. Parasitol. 1998, 28, 1071–1081. [Google Scholar] [CrossRef]
- Hirai, T.; Ramos, T.L.; Lin, P.Y.; Simonetta, F.; Su, L.L.; Picton, L.K.; Baker, J.; Lin, J.X.; Li, P.; Seo, K.; et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J. Clin. Investig. 2021, 131, e139991. [Google Scholar] [CrossRef]
- Isobe, T.; Lillehoj, H.S. Dexamethasone suppresses T cell-mediated immunity and enhances disease susceptibility to Eimeria mivati infection. Vet. Immunol. Immunopathol. 1993, 39, 431–446. [Google Scholar] [CrossRef]
- Ma, C.; Li, G.; Chen, W.; Jia, Z.; Yang, X.; Pan, X.; Ma, D. Eimeria tenella: IMP1 protein delivered by Lactococcus lactis induces immune responses against homologous challenge in chickens. Vet. Parasitol. 2021, 289, 109320. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lillehoj, H.S.; Lee, S.H.; Dalloul, R.A.; Lillehoj, E.P. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006, 114, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, N.F.; Baron, R.R. Coccidiosis: Evaluation of coccidiostats by mortality, weight gains, and fecal scores. Exp. Parasitol. 1970, 28, 25–29. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- McManus, E.C.; Campbell, W.C.; Cuckler, A.C. Development of resistance to quinoline coccidiostats under field and laboratory conditions. J. Parasitol. 1968, 54, 1190–1193. [Google Scholar] [CrossRef]


| Groups | Average Body Weight Gain (g) | Relative Body Weight Gain Rate (%) | Oocyst Shedding Per Rabbit (×104/g) | Oocyst Decrease Ratio (%) | Mean Lesion Score | Survival Rate (%) | Anticoccidial Index (ACI) |
|---|---|---|---|---|---|---|---|
| Uninfected | 360.00 ± 39.44 a | 100 | 0 a | — | 0 a | 100 | — |
| PBS-infected | 140.00 ± 61.46 b | 38.89 | 21.26 ± 3.07 b | 0 | 2.3 b | 100 | — |
| Trx-His-S tag | 170.00 ± 42.16 b | 47.22 | 17.18 ± 1.68 b | 19.20 | 2.6 b | 100 | 81.22 |
| rEi-14-3-3 | 295.00 ± 55.03 c | 81.94 | 2.95 ± 2.15 c | 86.13 | 1.27 c | 100 | 168.24 |
| rEi-GRA10 | 265.00 ± 66.87 c | 73.61 | 3.22 ± 1.80 c | 84.87 | 1.27 c | 100 | 159.91 |
| Genes | Primers | Primer Sequences |
|---|---|---|
| 14-3-3 | Upstream | 5′-CGGGATCCATGATTGAGGATATAAAGACCG-3′ (Bam HI site underlined) |
| Downstream | 5′-CCCTCGAGCTACTGCTGCTCAGTAGCCT-3′ (Xho I site underlined) | |
| GRA10 | Upstream | 5′-CGGGATCCATGCGACATTACGACATGA-3′ (Bam HI site underlined) |
| Downstream | 5′-CCAAGCTTTTACCCAACCATAGTGTGG-3′ (Hind III site underlined) |
| Groups | Number of Rabbits | Immunogen and Dosage | Challenge |
|---|---|---|---|
| Uninfected | 10 | 1 mL of Sterile PBS | — |
| PBS-infected | 10 | 1 mL of Sterile PBS | 7 × 104 sporulated oocysts |
| Trx-His-S-infected | 10 | 100 μg Trx-His-S tag + 1 mg Quil-A dilution in 1 mL of PBS | 7 × 104 sporulated oocysts |
| rEi-14-3-3 | 10 | 100 μg rEi-14-3-3 + 1 mg Quil-A dilution in 1 mL of PBS | 7 × 104 sporulated oocysts |
| rEi-GRA10 | 10 | 100 μg rEi-GRA10 + 1 mg Quil-A dilution in 1 mL of PBS | 7 × 104 sporulated oocysts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, C.; He, W.; Xiao, J.; Hao, G.; Pu, J.; Chen, H.; Xu, L.; Zhu, Y.; Yang, G. Assessment of the Immunoprotective Efficacy of Recombinant 14-3-3 Protein and Dense Granule Protein 10 (GRA10) as Candidate Antigens for Rabbit Vaccines against Eimeria intestinalis. Int. J. Mol. Sci. 2023, 24, 14418. https://doi.org/10.3390/ijms241914418
Xiong C, He W, Xiao J, Hao G, Pu J, Chen H, Xu L, Zhu Y, Yang G. Assessment of the Immunoprotective Efficacy of Recombinant 14-3-3 Protein and Dense Granule Protein 10 (GRA10) as Candidate Antigens for Rabbit Vaccines against Eimeria intestinalis. International Journal of Molecular Sciences. 2023; 24(19):14418. https://doi.org/10.3390/ijms241914418
Chicago/Turabian StyleXiong, Changming, Wei He, Jie Xiao, Ge Hao, Jiayan Pu, Hao Chen, Liwen Xu, Yuhua Zhu, and Guangyou Yang. 2023. "Assessment of the Immunoprotective Efficacy of Recombinant 14-3-3 Protein and Dense Granule Protein 10 (GRA10) as Candidate Antigens for Rabbit Vaccines against Eimeria intestinalis" International Journal of Molecular Sciences 24, no. 19: 14418. https://doi.org/10.3390/ijms241914418
APA StyleXiong, C., He, W., Xiao, J., Hao, G., Pu, J., Chen, H., Xu, L., Zhu, Y., & Yang, G. (2023). Assessment of the Immunoprotective Efficacy of Recombinant 14-3-3 Protein and Dense Granule Protein 10 (GRA10) as Candidate Antigens for Rabbit Vaccines against Eimeria intestinalis. International Journal of Molecular Sciences, 24(19), 14418. https://doi.org/10.3390/ijms241914418




