Surface Functionalisation of Self-Assembled Quantum Dot Microlasers with a DNA Aptamer
Abstract
:1. Introduction
2. Results and Discussion
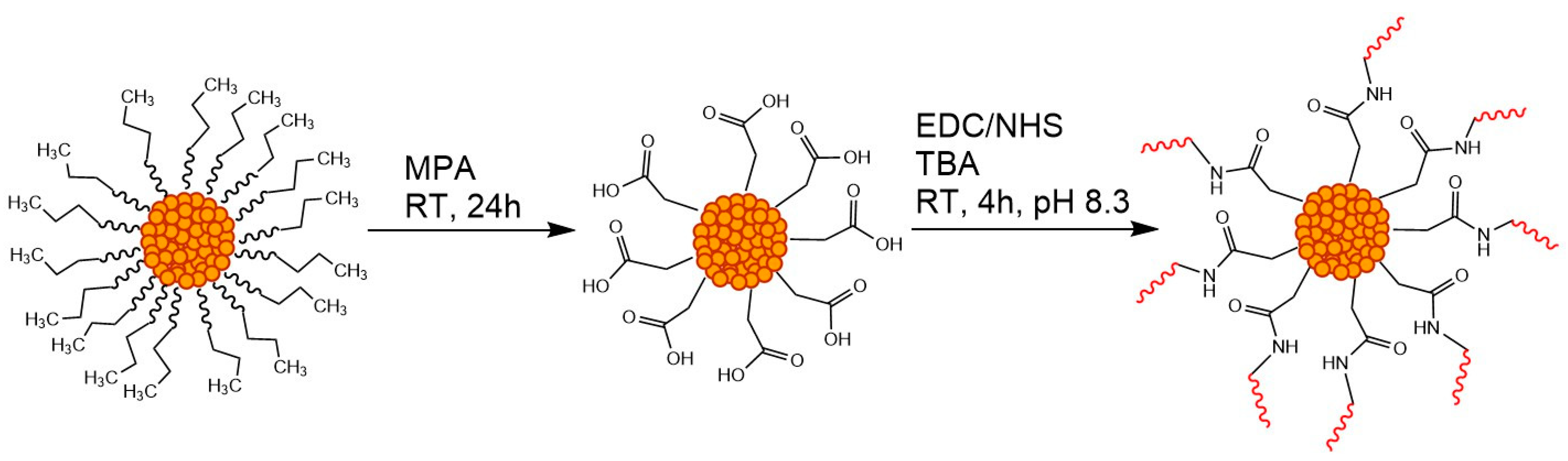
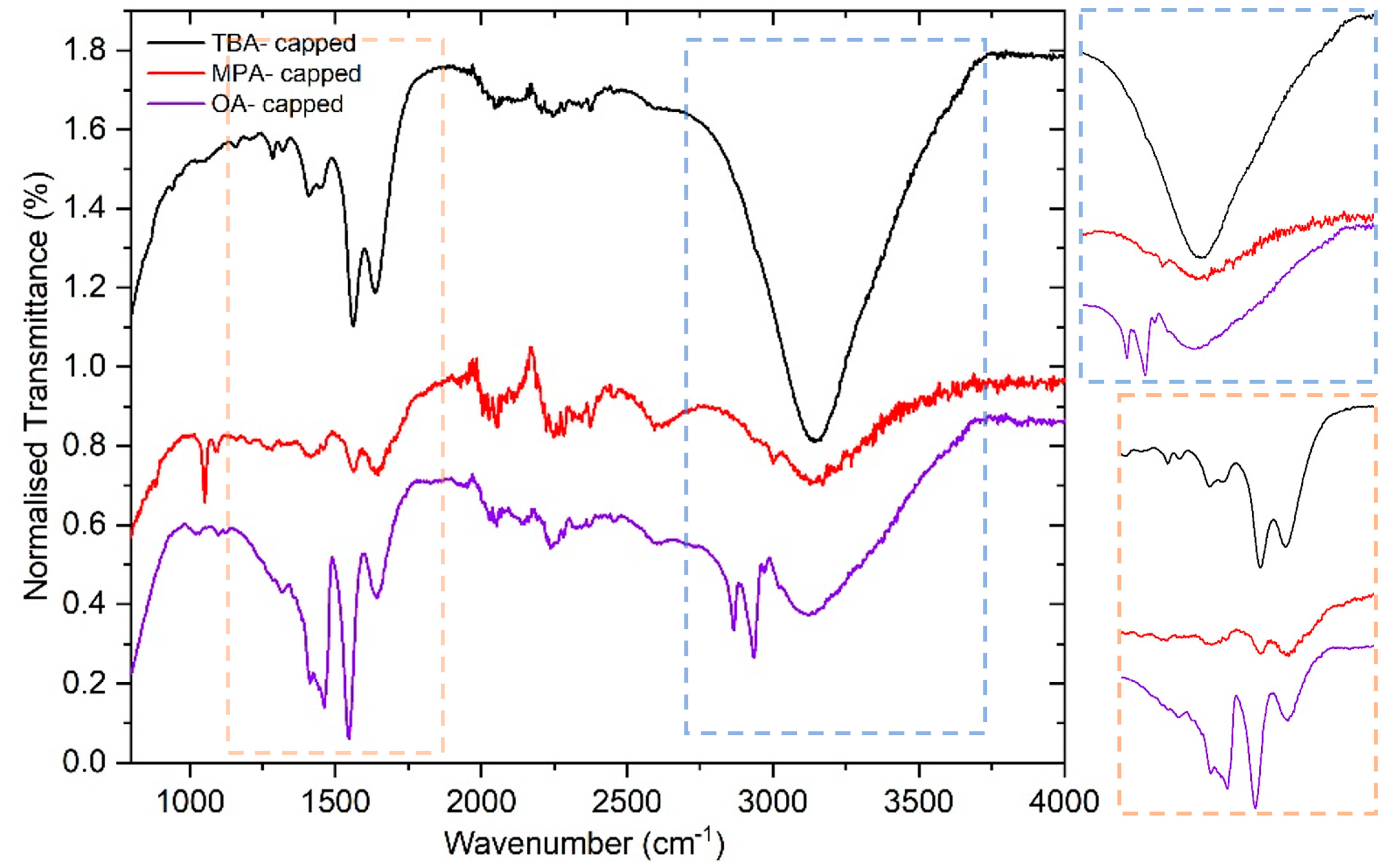
2.1. Synthesis and Characterisation
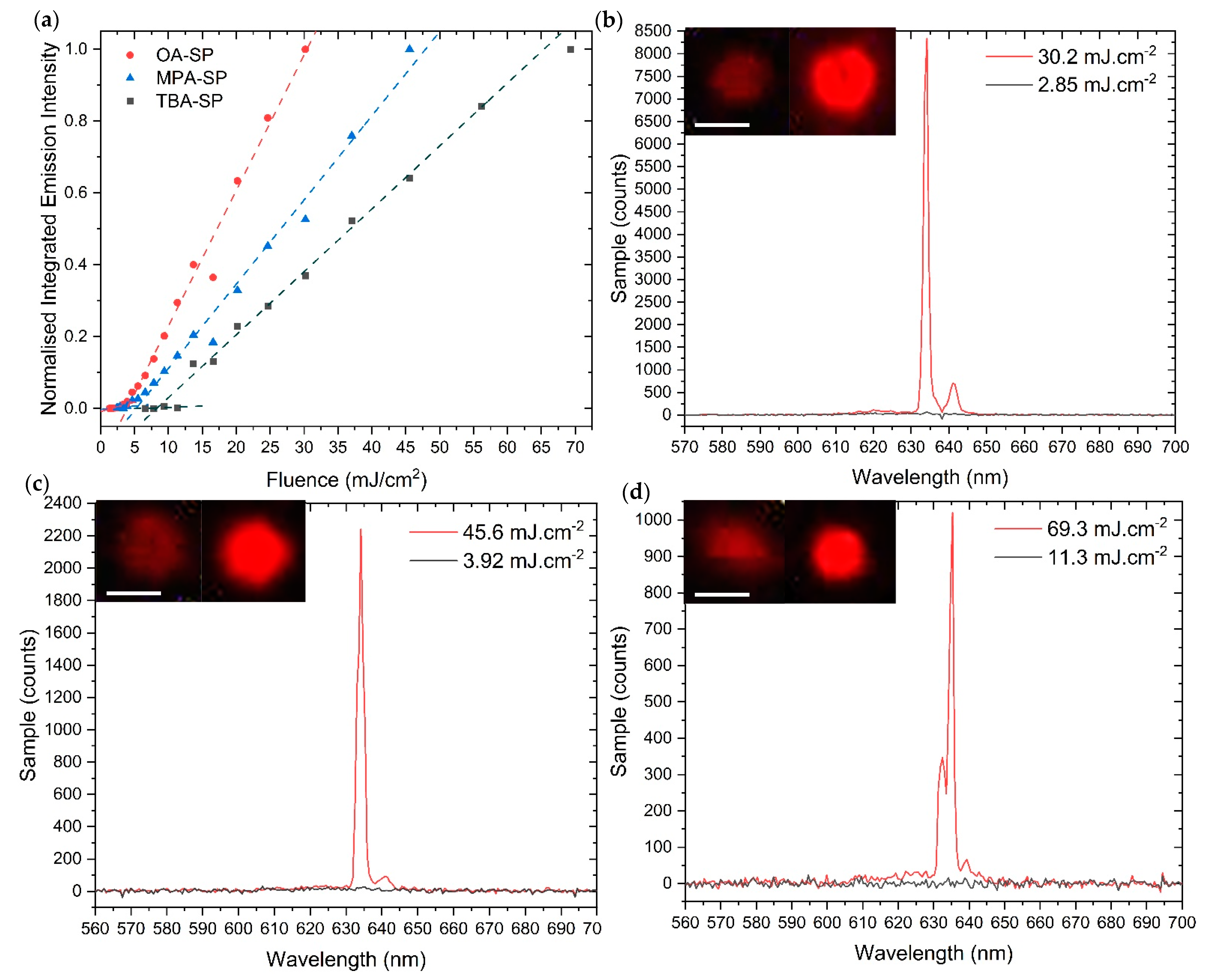
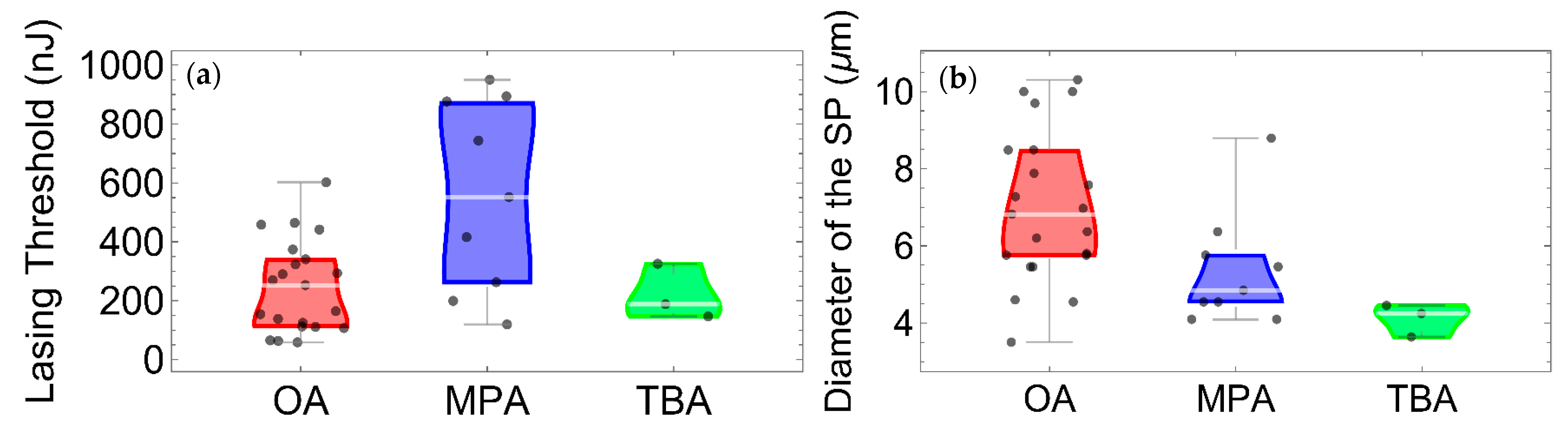
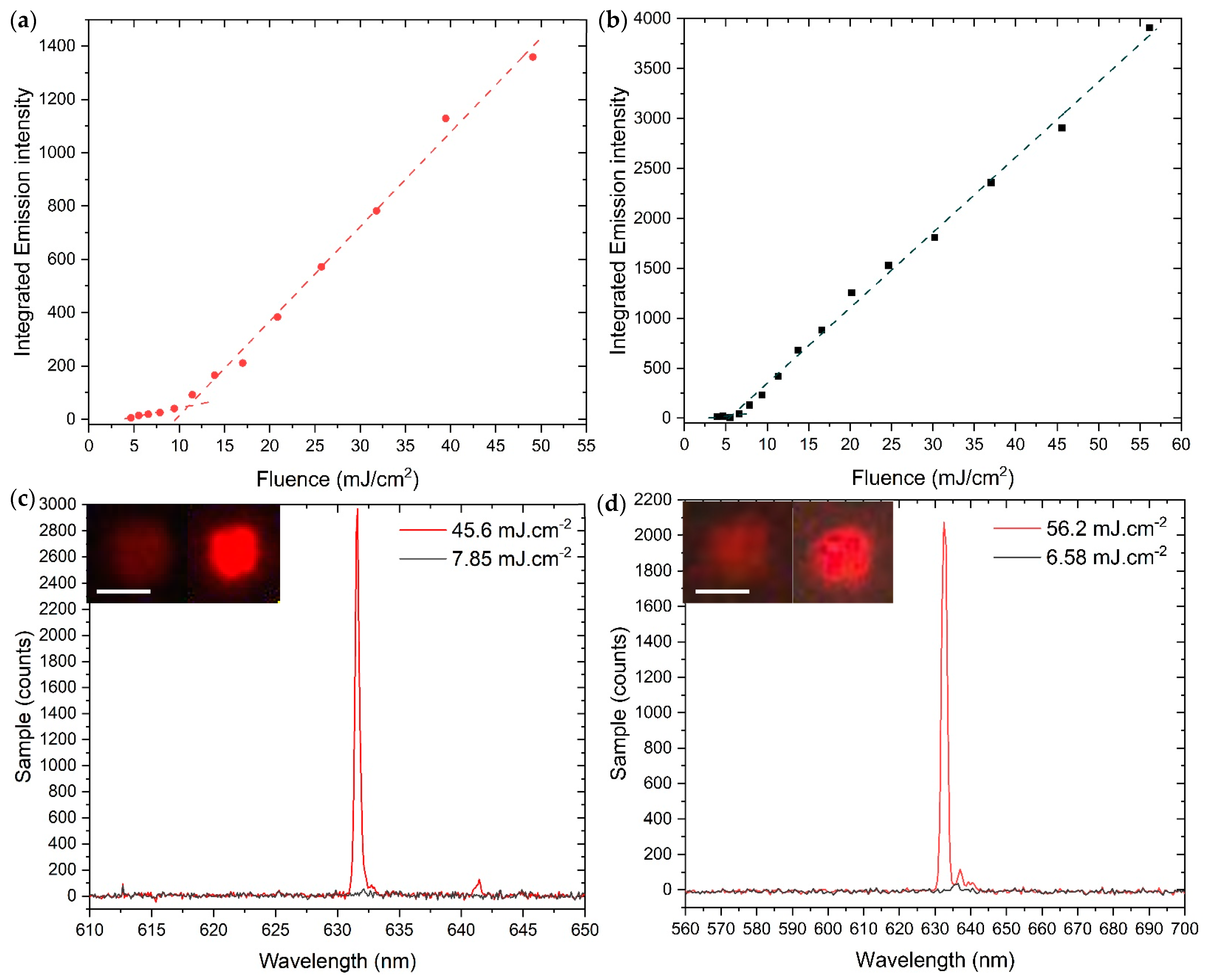
2.2. Optical Measurements
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CdSe1−xSx/ZnS Supraparticles
3.3. Surface Functionalisation of SPs
3.4. Optical Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.Y.; Voznyy, O.; Zhitomirsky, D.; Sargent, E.H. 25th Anniversary Article: Colloidal Quantum Dot Materials and Devices: A Quarter-Century of Advances. Adv. Mater. 2013, 25, 4986–5010. [Google Scholar] [CrossRef]
- Moreels, I.; Rainaó, G.; Gomes, R.; Hens, Z.; Stöferle, T.; Mahrt, R.F. Nearly Temperature-Independent Threshold for Amplified Spontaneous Emission in Colloidal CdSe/CdS Quantum Dot-in-Rods. Adv. Mater. 2012, 24, OP231–OP235. [Google Scholar] [CrossRef]
- Montanarella, F.; Urbonas, D.; Chadwick, L.; Moerman, P.G.; Baesjou, P.J.; Mahrt, R.F.; Van Blaaderen, A.; Stöferle, T.; Vanmaekelbergh, D. Lasing Supraparticles Self-Assembled from Nanocrystals. ACS Nano 2018, 12, 12788–12794. [Google Scholar] [CrossRef]
- Plunkett, A.; Eldridge, C.; Schneider, G.A.; Domènech, B. Controlling the Large-Scale Fabrication of Supraparticles. J. Phys. Chem. B 2020, 124, 11263–11272. [Google Scholar] [CrossRef]
- Alves, P.U.; Laurand, N.; Dawson, M.D. Multicolor Laser Oscillation in a Single Self-Assembled Colloidal Quantum Dot Microsphere. In Proceedings of the 2020 IEEE Photonics Conference, IPC 2020, Virtual, 29 September–1 October 2020; pp. 5–6. [Google Scholar]
- Alves, P.U.; Jevtics, D.; Strain, M.J.; Dawson, M.D.; Laurand, N. Enhancing Self-Assembled Colloidal Quantum Dot Microsphere Lasers. In Proceedings of the 2021 IEEE Photonics Conference (IPC), Virtual, 18–21 October 2021; IEEE: New York, NY, USA, 2021; pp. 1–2. [Google Scholar]
- Kim, K.H.; Dannenberg, P.H.; Yan, H.; Cho, S.; Yun, S.H. Compact Quantum-Dot Microbeads with Sub-Nanometer Emission Linewidth. Adv. Funct. Mater. 2021, 31, 2103413. [Google Scholar] [CrossRef]
- Beier, H.T.; Coté, G.L.; Meissner, K.E. Modeling Whispering Gallery Modes in Quantum Dot Embedded Polystyrene Microspheres. J. Opt. Soc. Am. B 2010, 27, 536. [Google Scholar] [CrossRef]
- Francois, A.; Himmelhaus, M. Whispering Gallery Mode Biosensor Operated in the Stimulated Emission Regime. Appl. Phys. Lett. 2009, 94, 031101. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent Advances on Fluorescent Biomarkers of Near-Infrared Quantum Dots for In Vitro and In Vivo Imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Aldana, J.; Lavelle, N.; Wang, Y.; Peng, X. Size-Dependent Dissociation PH of Thiolate Ligands from Cadmium Chalcogenide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 2496–2504. [Google Scholar] [CrossRef]
- Elimelech, O.; Aviv, O.; Oded, M.; Banin, U. A Tale of Tails: Thermodynamics of CdSe Nanocrystal Surface Ligand Exchange. Nano Lett. 2020, 20, 6396–6403. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Chen, Y.; Cordero, J.M.; Wang, H.; Franke, D.; Achorn, O.B.; Freyria, F.S.; Coropceanu, I.; Wei, H.; Chen, O.; Mooney, D.J.; et al. A Ligand System for the Flexible Functionalization of Quantum Dots via Click Chemistry. Angew. Chem. Int. Ed. 2018, 57, 4652–4656. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Ma, Y.; Wu, G.; Du, M.; Tian, S.; Huang, S.; Ji, X.; He, Z. Novel Method of Clickable Quantum Dot Construction for Bioorthogonal Labeling. Anal. Chem. 2021, 93, 777–783. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mosleh, N. Modified CdS Quantum Dots as Selective Turn-on Fluorescent Nanosensor for Detection and Determination of Methamphetamine. J. Mater. Sci. Mater. Electron. 2019, 30, 21170–21176. [Google Scholar] [CrossRef]
- Iyer, A.; Chandra, A.; Swaminathan, R. Hydrolytic Enzymes Conjugated to Quantum Dots Mostly Retain Whole Catalytic Activity. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 2935–2943. [Google Scholar] [CrossRef]
- Marino, E.; Bharti, H.; Xu, J.; Kagan, C.R.; Murray, C.B. Nanocrystal Superparticles with Whispering-Gallery Modes Tunable through Chemical and Optical Triggers. Nano Lett. 2022, 22, 4765–4773. [Google Scholar] [CrossRef]
- Marino, E.; van Dongen, S.W.; Neuhaus, S.J.; Li, W.; Keller, A.W.; Kagan, C.R.; Kodger, T.E.; Murray, C.B. Monodisperse Nanocrystal Superparticles through a Source–Sink Emulsion System. Chem. Mater. 2022, 34, 2779–2789. [Google Scholar] [CrossRef]
- Pasquardini, L.; Berneschi, S.; Barucci, A.; Cosi, F.; Dallapiccola, R.; Insinna, M.; Lunelli, L.; Conti, G.N.; Pederzolli, C.; Salvadori, S.; et al. Whispering Gallery Mode Aptasensors for Detection of Blood Proteins. J. Biophotonics 2013, 6, 178–187. [Google Scholar] [CrossRef]
- Conti, G.N.; Berneschi, S.; Soria, S. Aptasensors Based on Whispering Gallery Mode Resonators. Biosensors 2016, 6, 28. [Google Scholar] [CrossRef]
- Choi, J.H.; Chen, K.H.; Strano, M.S. Aptamer-Capped Nanocrystal Quantum Dots: A New Method for Label-Free Protein Detection. J. Am. Chem. Soc. 2006, 128, 15584–15585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, B.; Xiang, Y.; Zhang, Y.; Chai, Y.; Yuan, R. Aptamer/Quantum Dot-Based Simultaneous Electrochemical Detection of Multiple Small Molecules. Anal. Chim. Acta 2011, 688, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Fang, X.; Bao, J.; Han, M.; Dai, Z. Electrochemiluminescence Biosensor Based on CdSe Quantum Dots for the Detection of Thrombin. Electrochim. Acta 2012, 65, 1–6. [Google Scholar] [CrossRef]
- Levy, M.; Cater, S.F.; Ellington, A.D. Quantum-Dot Aptamer Beacons for the Detection of Proteins. ChemBioChem 2005, 6, 2163–2166. [Google Scholar] [CrossRef]
- Liu, J.; Jung, H.L.; Lu, Y. Quantum Dot Encoding of Aptamer-Linked Nanostructures for One-Pot Simultaneous Detection of Multiple Analytes. Anal. Chem. 2007, 79, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Ma, X.; Xia, Y.; Wang, H.; Wang, Z.; Zhang, Q. Multiplexed Fluorescence Resonance Energy Transfer Aptasensor between Upconversion Nanoparticles and Graphene Oxide for the Simultaneous Determination of Mycotoxins. Anal. Chem. 2012, 84, 6263–6270. [Google Scholar] [CrossRef]
- Wang, C.; Lim, C.Y.; Choi, E.; Park, Y.; Park, J. Highly Sensitive User Friendly Thrombin Detection Using Emission Light Guidance from Quantum Dots-Aptamer Beacons in 3-Dimensional Photonic Crystal. Sens. Actuators B Chem. 2016, 223, 372–378. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Su, L.; Gao, X.; Tang, Y.; Ma, T.; Zhu, L.; Li, J. Novel Hybrid Probe Based on Double Recognition of Aptamer-Molecularly Imprinted Polymer Grafted on Upconversion Nanoparticles for Enrofloxacin Sensing. Biosens. Bioelectron. 2017, 87, 203–208. [Google Scholar] [CrossRef]
- Marino, E.; Marino, E.; Keller, A.W.; An, D.; Van Dongen, S.; Van Dongen, S.; Kodger, T.E.; MacArthur, K.E.; Heggen, M.; Kagan, C.R.; et al. Favoring the Growth of High-Quality, Three-Dimensional Supercrystals of Nanocrystals. J. Phys. Chem. C 2020, 124, 11256–11264. [Google Scholar] [CrossRef]
- Wang, J.; Kang, E.; Sultan, U.; Merle, B.; Inayat, A.; Graczykowski, B.; Fytas, G.; Vogel, N. Influence of Surfactant-Mediated Interparticle Contacts on the Mechanical Stability of Supraparticles. J. Phys. Chem. C 2021, 125, 23445–23456. [Google Scholar] [CrossRef]
- Wang, Y.; Ta, V.D.; Leck, K.S.; Tan, B.H.I.; Wang, Z.; He, T.; Ohl, C.D.; Demir, H.V.; Sun, H. Robust Whispering-Gallery-Mode Microbubble Lasers from Colloidal Quantum Dots. Nano Lett. 2017, 17, 2640–2646. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, L.; Zhao, S.; Liu, X.; Yang, Y.; Du, J.; Li, Q.; Yu, S. Green-Emissive Carbon Quantum Dots with High Fluorescence Quantum Yield: Preparation and Cell Imaging. Front. Mater. Sci. 2021, 15, 253–265. [Google Scholar] [CrossRef]
- Vo, N.T.; Ngo, H.D.; Do Thi, N.P.; Nguyen Thi, K.P.; Duong, A.P.; Lam, V. Stability Investigation of Ligand-Exchanged CdSe/ZnS-Y (Y =3-Mercaptopropionic Acid or Mercaptosuccinic Acid) through Zeta Potential Measurements. J. Nanomater. 2016, 2016, 8564648. [Google Scholar] [CrossRef]
- Marcel, J.E. Fischer Amine Coupling Through EDC/NHS: A Practical Approach. In Surface Plasmon Resonance: Methods and Protocols; Mol, N.J., Fischer, M.J.E., Eds.; Methods in Molecular Biology; Humana: Totowa, NJ, USA, 2010; Volume 627, pp. 55–73. ISBN 978-1-60761-669-6. [Google Scholar]
- Reynolds, T.; François, A.; Riesen, N.; Turvey, M.E.; Nicholls, S.J.; Hoffmann, P.; Monro, T.M. Using Whispering Gallery Mode Micro Lasers for Biosensing within Undiluted Serum. In Proceedings of the SPIE BioPhotonics Australasia, Adelaide, Australia, 16–19 October 2016; Hutchinson, M.R., Goldys, E.M., Eds.; SPIE: Bellingham, WA, USA, 2016; Volume 10013, p. 100132X. [Google Scholar]
- Tan, K.X.; Ujan, S.; Danquah, M.K.; Lau, S.Y. Design and Characterization of a Multi-Layered Polymeric Drug Delivery Vehicle. Can. J. Chem. Eng. 2019, 97, 1243–1252. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, B.H.; Kim, S.H.; Gu, M.B. Aptamer-Aptamer Linkage Based Aptasensor for Highly Enhanced Detection of Small Molecules. Biotechnol. J. 2016, 11, 843–849. [Google Scholar] [CrossRef]
- Singh, N.; Prajapati, S.; Prateek; Gupta, R.K. Investigation of Ag Doping and Ligand Engineering on Green Synthesized CdS Quantum Dots for Tuning Their Optical Properties. Nanofabrication 2022, 7, 89–103. [Google Scholar] [CrossRef]
- Bel Haj Mohamed, N.; Haouari, M.; Zaaboub, Z.; Hassen, F.; Maaref, H.; Ben Ouada, H. Effect of Surface on the Optical Structure and Thermal Properties of Organically Capped CdS Nanoparticles. J. Phys. Chem. Solids 2014, 75, 936–944. [Google Scholar] [CrossRef]
- Bharathi, M.V.; Roy, N.; Moharana, P.; Ghosh, K.; Paira, P. Green Synthesis of Highly Luminescent Biotin-Conjugated CdSe Quantum Dots for Bioimaging Applications. New J. Chem. 2020, 44, 16891–16899. [Google Scholar] [CrossRef]
- Li, P.; Luo, C.; Chen, X.; Huang, C. An Off-on Fluorescence Aptasensor for Trace Thrombin Detection Based on FRET between CdS QDs and AuNPs. RSC Adv. 2022, 12, 35763–35769. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, R.X.; Chen, X.; He, X.X.; Han, A.L.; Fang, G.Z.; Liu, J.F.; Wang, S. Study of Efficiency of Coupling Peptides with Gold Nanoparticles. Chin. J. Anal. Chem. 2017, 45, 662–667. [Google Scholar] [CrossRef]
- Eling, C.J.; Laurand, N.; Gunasekar, N.K.; Edwards, P.R.; Martin, R.W. Silica Coated Colloidal Semiconductor Quantum Dot Supracrystal Microlasers. In Proceedings of the 2022 IEEE 9th International Conference on Photonics, Virtual, 8–10 August 2022; pp. 2022–2023. [Google Scholar] [CrossRef]
- Chang, H.; Zhong, Y.; Dong, H.; Wang, Z.; Xie, W.; Pan, A.; Zhang, L. Ultrastable Low-Cost Colloidal Quantum Dot Microlasers of Operative Temperature up to 450 K. Light Sci. Appl. 2021, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Righini, G.C.; Soria, S. Biosensing by WGM Microspherical Resonators. Sensors 2016, 16, 905. [Google Scholar] [CrossRef]
- Wu, Y.; Leung, P.T. Lasing Threshold for Whispering-Gallery-Mode Microsphere Lasers. Phys. Rev. A-At. Mol. Opt. Phys. 1999, 60, 630–633. [Google Scholar] [CrossRef]
- Cai, L.; Pan, J.; Zhao, Y.; Wang, J.; Xiao, S. Whispering Gallery Mode Optical Microresonators: Structures and Sensing Applications. Phys. Status Solidi Appl. Mater. Sci. 2020, 217, 1900825. [Google Scholar] [CrossRef]
- Rahman, S.A.; Ariffin, N.; Yusof, N.A.; Abdullah, J.; Mohammad, F.; Zubir, Z.A.; Aziz, N.M.A.N.A. Thiolate-Capped CdSe/ZnS Core-Shell Quantum Dots for the Sensitive Detection of Glucose. Sensors 2017, 17, 1537. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.H.; Chi, C.W.; Friedrich, S.M.; Peck, K.; Wang, T.H.; Leong, K.W.; Chen, L.C. Signal-on Protein Detection via Dye Translocation between Aptamer and Quantum Dot. ACS Appl. Mater. Interfaces 2016, 8, 12048–12055. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, H.; Ding, C.; Gan, H.; Jiang, S.; Zhang, G.; Bi, J.; Yan, S.; Hou, H. A Novel Photoelectrochemical Aptamer Sensor Based on Cdte Quantum Dots Enhancement and Exonuclease I-Assisted Signal Amplification for Listeria Monocytogenes Detection. Foods 2021, 10, 2896. [Google Scholar] [CrossRef]

| Sample | Zeta Potential (mV) | σ (mV) | ΔpH |
|---|---|---|---|
| OA-SPs | −19.7 | 6.73 | −0.42 |
| MPA-SPs | −31.7 | 5.12 | 0.07 |
| TBA-SPs | −12.5 | 4.43 | −0.49 |
| Sum of Squares | Degrees of Freedom | Mean Square | F Ratio | p-Value | |
|---|---|---|---|---|---|
| Between Groups | 6.42 × 105 | 2 | 3.21 × 105 | 7.18 | 2.8 × 10−3 |
| Within Groups | 1.34 × 106 | 30 | 4.47 × 104 | ||
| Total | 1.98 × 106 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlton, B.K.; Downie, D.H.; Noman, I.; Alves, P.U.; Eling, C.J.; Laurand, N. Surface Functionalisation of Self-Assembled Quantum Dot Microlasers with a DNA Aptamer. Int. J. Mol. Sci. 2023, 24, 14416. https://doi.org/10.3390/ijms241914416
Charlton BK, Downie DH, Noman I, Alves PU, Eling CJ, Laurand N. Surface Functionalisation of Self-Assembled Quantum Dot Microlasers with a DNA Aptamer. International Journal of Molecular Sciences. 2023; 24(19):14416. https://doi.org/10.3390/ijms241914416
Chicago/Turabian StyleCharlton, Bethan K., Dillon H. Downie, Isaac Noman, Pedro Urbano Alves, Charlotte J. Eling, and Nicolas Laurand. 2023. "Surface Functionalisation of Self-Assembled Quantum Dot Microlasers with a DNA Aptamer" International Journal of Molecular Sciences 24, no. 19: 14416. https://doi.org/10.3390/ijms241914416
APA StyleCharlton, B. K., Downie, D. H., Noman, I., Alves, P. U., Eling, C. J., & Laurand, N. (2023). Surface Functionalisation of Self-Assembled Quantum Dot Microlasers with a DNA Aptamer. International Journal of Molecular Sciences, 24(19), 14416. https://doi.org/10.3390/ijms241914416






