Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo
Abstract
1. Introduction
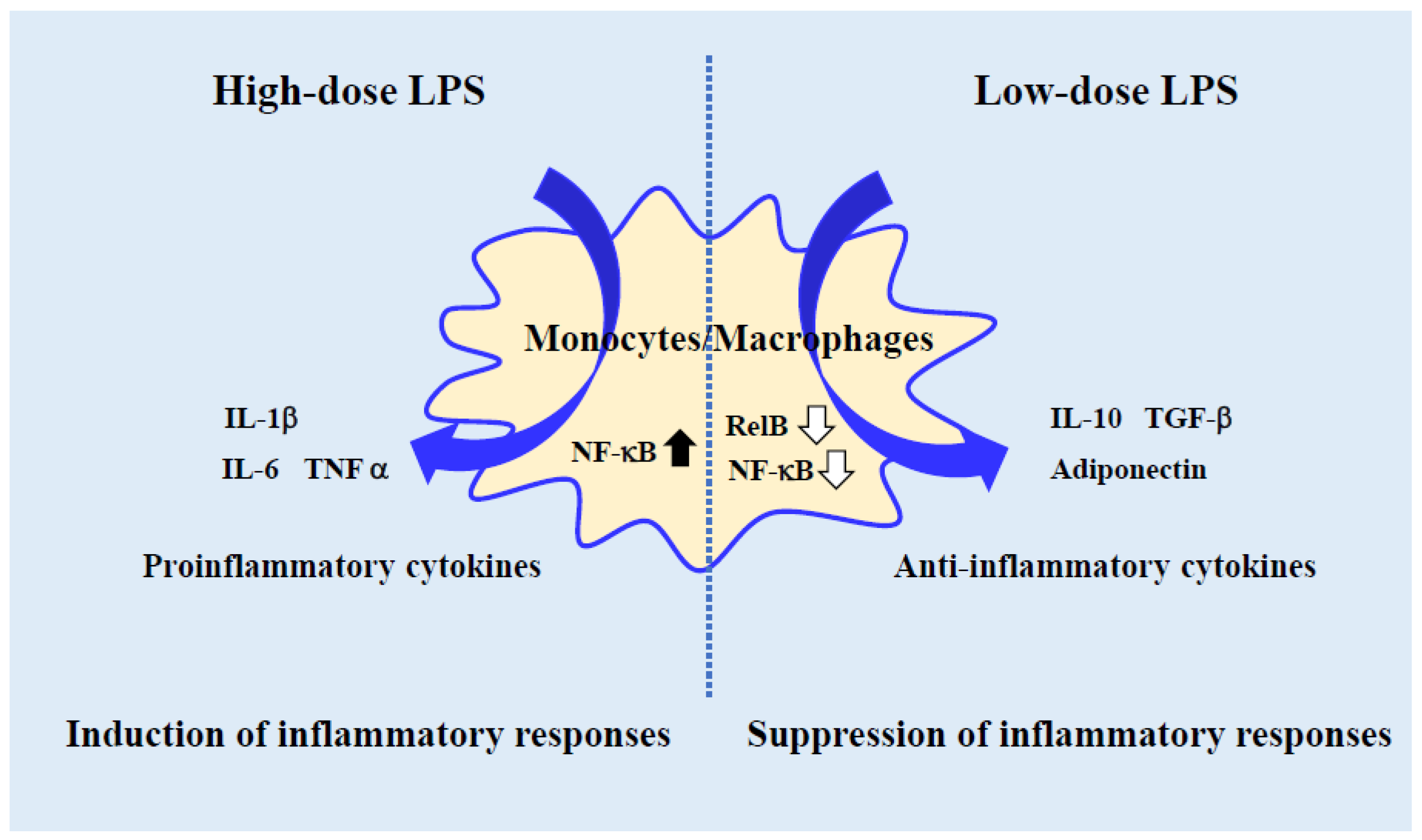
2. Usefulness of Low-Dose LPS-Activated Monocyte-Lineage Cells as an In Vitro Experimental Model
3. Regulation of Proinflammatory Cytokine Levels in Adipocytes and Vascular Endothelial Cells Using Low-Dose LPS-Activated Monocyte-Lineage Cells
4. Regulation of Factors Involved in Lifestyle-Related Diseases by Low-Dose LPS
5. Therapeutic Efficacy of Low-Dose LPS-Activated Cardiomyocytes in Myocardial Ischemia-Reperfusion Injury
6. Beneficial Effects of Low-Dose LPS Treatment on Neurons Cells in Spinal Cord Injury
7. Limitations and Development of In Vitro Experiments Using Low-Dose LPS to Reflect Oral LPS Administration
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar] [CrossRef]
- Kawai, T.; Adachi, O.; Ogawa, T.; Takeda, K.; Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 1999, 11, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Inagawa, H.; Nishizawa, T.; Tsukioka, D.; Suda, T.; Chiba, Y.; Okutomi, T.; Morikawa, A.; Soma, G.I.; Mizuno, D.I. Homeostasis as regulated by activated macrophage. II. LPS of plant origin other than wheat flour and their concomitant bacteria. Chem. Pharm. Bull. 1992, 40, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Yoshioka, N.; Nishizawa, T.; Inagawa, H.; Kohchi, C.; Soma, G.I. Utility and safety of LPS-based flour extract as a macrophage activator. Anticancer Res. 2009, 29, 859–864. [Google Scholar] [PubMed]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Yamaguchi, T.; Nagai, S.; Soma, G. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J. Biosci. Bioeng. 2006, 102, 485–496. [Google Scholar] [CrossRef]
- Braun-Fahrländer, C.; Riedler, J.; Herz, U.; Eder, W.; Waser, M.; Grize, L.; Maisch, S.; Carr, D.; Gerlach, F.; Bufe, A.; et al. Environmental Exposure to Endotoxin and Its Relation to Asthma in School-Age Children. N. Engl. J. Med. 2002, 347, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Gibson, A.M.; Bass, S.; Rydyznski, C.; Khurana Hershey, G.K. Exacerbation of Allergen-Induced Eczema in TLR4- and TRIF-Deficient Mice. J. Immunol. 2013, 191, 3519–3525. [Google Scholar] [CrossRef]
- Chen, L.; Guo, S.; Ranzer, M.J.; DiPietro, L.A. Toll-like receptor 4 plays an essential role in early skin wound healing. J. Investig. Dermatol. 2013, 133, 258–267. [Google Scholar] [CrossRef]
- Engelhardt, R.; Mackensen, A.; Galanos, C.; Andreesen, R. Biological response to intravenously administered endotoxin in patients with advanced cancer. J. Biol. Response Mod. 1990, 9, 480–491. [Google Scholar] [PubMed]
- Inagawa, H.; Kohchi, C.; Soma, G.I. Oral administration of lipopolysaccharides for the prevention of various diseases: Benefit and usefulness. Anticancer Res. 2011, 31, 2431–2436. [Google Scholar]
- Phipps, K.R.; Sulaiman, C.; Simon, R.; Holalagoudar, S.; Kohchi, C.; Nakata, Y. Subchronic (90-day) toxicity assessment of Somacy-FP100, a lipopolysaccharide-containing fermented wheat flour extract from Pantoea agglomerans. J. Appl. Toxicol. 2020, 40, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Inagawa, H.; Nishizawa, T.; Okutomi, T.; Morikawa, A.; Soma, G.I.; Mizuno, D.I. Homeostasis as regulated by activated macrophage. V. Suppression of diabetes mellitus in non-obese diabetic mice by LPSw (a lipopolysaccharide from wheat flour). Chem. Pharm. Bull. 1992, 40, 1004–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, K.; Yamashita, M.; Oda, M.; Tjendana Tjhin, V.; Inagawa, H.; Soma, G.I. Oral Administration of Lipopolysaccharide Enhances Insulin Signaling-Related Factors in the KK/Ay Mouse Model of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 4619. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Inagawa, H.; Kohchi, C.; Kazumura, K.; Tsuchiya, H.; Miwa, T.; Okazaki, K.; Soma, G.I. Oral administration of Pantoea agglomerans-derived lipopolysaccharide prevents development of atherosclerosis in high-fat diet-fed apoE-deficient mice via ameliorating hyperlipidemia, pro-inflammatory mediators and oxidative responses. PLoS ONE 2018, 213, e0195008. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Inagawa, H.; Kohchi, C.; Kazumura, K.; Tsuchiya, H.; Miwa, T.; Okazaki, K.; Soma, G.I. Oral administration of Pantoea agglomerans-derived lipopolysaccharide prevents metabolic dysfunction and Alzheimer’s disease-related memory loss in senescence-accelerated prone 8 (SAMP8) mice fed a high-fat diet. PLoS ONE 2018, 13, e0198493. [Google Scholar] [CrossRef]
- Mizobuchi, H. Oral route lipopolysaccharide as a potential dementia preventive agent inducing neuroprotective microglia. Front. Immunol. 2023, 14, 1110583. [Google Scholar] [CrossRef]
- Inagawa, H.; Kobayashi, Y.; Kohchi, C.; Zhang, R.; Shibasaki, Y.; Soma, G. Primed activation of macrophages by oral administration of lipopolysaccharide derived from Pantoea agglomerans. Vivo 2016, 30, 205–211. [Google Scholar]
- Honda, T.; Inagawa, H.; Yamamoto, I. Expression of chemotaxis- and angiogenesis-related factors in human monocytes following interaction with colon cancer cells is suppressed by low-dose lipopolysaccharide. Anticancer Res. 2014, 34, 4609–4613. [Google Scholar]
- Honda, T.; Inagawa, H. Molecular response of human monocytes following interaction with colon cancer cells by pretreatment with low-dose lipopolysaccharide. Anticancer Res. 2015, 35, 4473–4478. [Google Scholar]
- Honda, T.; Inagawa, H. Regulation of plasminogen activator inhibitor-1 in adipocytes by macrophages activated by low-dose lipopolysaccharide. Anticancer Res. 2021, 41, 4071–4076. [Google Scholar] [CrossRef]
- Honda, T.; Inagawa, H. Suppression of inflammatory cytokine genes expression in vascular endothelial cells by super-low dose lipopolysaccharide-activated macrophages. Anticancer Res. 2022, 42, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Desnues, B.; Mege, J.L. Macrophage polarization in bacterial infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Tagliabue, A.; Mantovani, A.; Kilgallen, M.; Herberman, R.B.; McCoy, J.L. Natural cytotoxicity of mouse monocytes and macrophages. J. Immunol. 1979, 122, 2363–2370. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003, 112, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Nishikori, M. Classical and Alternative NF-κB Activation Pathways and Their Roles in Lymphoid Malignancies. J. Clin. Exp. Hematopathol. 2005, 45, 15–24. [Google Scholar] [CrossRef]
- Maitra, U.; Gan, L.; Chang, S.; Li, L. Low-dose endotoxin induces inflammation by selectively removing nuclear receptors and activating CCAAT/enhancer-binding protein δ. J. Immunol. 2011, 186, 4467–4473. [Google Scholar] [CrossRef]
- Deng, H.; Maitra, U.; Morris, M.; Li, L. Molecular Mechanism Responsible for the Priming of Macrophage Activation. J. Biol. Chem. 2013, 288, 3897–3906. [Google Scholar] [CrossRef]
- Morris, M.C.; Gilliam, E.A.; Button, J.; Li, L. Dynamic modulation of innate immune response by varying dosages of lipopolysaccharide (LPS) in human monocytic cells. J. Biol. Chem. 2014, 289, 21584–21590. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Yamamoto, K.; Saito, H. A pathological role of increased expression of plasminogen activator inhibitor-1 in human or animal disorders. Int. J. Hematol. 1998, 68, 371–385. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Libby, P. Adiponectin: A key adipocytokine in metabolic syndrome. Clin. Sci. 2006, 110, 267–278. [Google Scholar] [CrossRef]
- Nader, N.D.; Asgeri, M.; Davari-Farid, S.; Pourafkari, L.; Ahmadpour, F.; Porhomayon, J.; Javadzadeghan, H.; Negargar, S.; Knight, P.R. The Effect of Lipopolysaccharide on Ischemic-Reperfusion Injury of Heart: A Double Hit Model of Myocardial Ischemia and Endotoxemia. J. Cardiovasc. Thorac. Res. 2015, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jiang, N.; Ji, Z.; Shi, G. The IRE1 signaling pathway is involved in the protective effect of low-dose LPS on myocardial ischemia-reperfusion injury. Life Sci. 2019, 231, 116569. [Google Scholar] [CrossRef]
- Li, W.C.; Jiang, R.; Jiang, D.M.; Zhu, F.C.; Su, B.; Qiao, B.; Qi, X.T. Lipopolysaccharide preconditioning attenuates apoptotic processes and improves neuropathologic changes after spinal cord injury in rats. Int. J. Neurosci. 2014, 124, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, G. Matrine protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via upregulation of miR-9. Pharm. Biol. 2020, 58, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Yao, S.P.; Zhang, J.; Liu, W.B.; Liu, J.; Geng, C.K. Low-dose lipopolysaccharide protects nerve cells against spinal cord injury via regulating the PI3K-AKT-Nrf2 signaling pathway. Biochem. Cell Biol. 2021, 99, 527–535. [Google Scholar] [CrossRef]
- Mizobuchi, H.; Yamamoto, K.; Yamashita, M.; Nakata, Y.; Inagawa, H.; Kohchi, C.; Soma, G.I. Prevention of Diabetes-Associated Cognitive Dysfunction through Oral Administration of Lipopolysaccharide Derived from Pantoea agglomerans . Front. Immunol. 2021, 12, 650176. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamashita, M.; Inagawa, H.; Kohchi, C.; Soma, G. Anti-inflammatory and Insulin Signaling Phenotype Induced by Repeated Lipopolysaccharide Stimulation in 3T3-L1 Adipocytes. Anticancer Res. 2022, 42, 3983–3991. [Google Scholar] [CrossRef]
- Mizobuchi, H.; Yamamoto, K.; Yamashita, M.; Inagawa, H.; Kohchi, C.; Soma, G. A Novel Anti-inflammatory Phenotype Transformed by Repetitive Low-dose Lipopolysaccharide in Primary Peritoneal Tissue-resident Macrophages. Anticancer Res. 2020, 40, 4457–4464. [Google Scholar] [CrossRef]
- Mizobuchi, H.; Yamamoto, K.; Tsutsui, S.; Yamashita, M.; Nakata, Y.; Inagawa, H.; Kohchi, C.; Soma, G. A unique hybrid characteristic having both pro- and anti-inflammatory phenotype transformed by repetitive low-dose lipopolysaccharide in C8-B4 microglia. Sci. Rep. 2020, 10, 8945. [Google Scholar] [CrossRef]

| Adipocytes | LPS | - | 100 pg/mL | 10 ng/mL | 1 μg/mL | |
| Gene | ||||||
| IL6 | 1.00 | 0.64 | 0.74 | 1.33 | ||
| IL8 | 1.00 | 0.50 | 0.57 | 1.81 | ||
| SERPINE1 | 1.00 | 0.82 * | 0.77 | 1.06 | ||
| ADIPOQ | 1.00 | 1.71 * | 1.16 | 0.77 | ||
| HAoECs | LPS | - | 100 pg/mL | 10 ng/mL | 1 μg/mL | |
| Gene | ||||||
| IL1B | 1.00 | 0.76 * | 1.44 | 2.34 | ||
| IL8 | 1.00 | 0.57 * | 1.66 | 1.85 | ||
| TGFB1 | 1.00 | 1.14 | 2.18 | 2.32 | ||
| SERPINE1 | 1.00 | 0.84 * | 0.96 | 1.04 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, T.; Inagawa, H. Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo. Int. J. Mol. Sci. 2023, 24, 14387. https://doi.org/10.3390/ijms241814387
Honda T, Inagawa H. Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo. International Journal of Molecular Sciences. 2023; 24(18):14387. https://doi.org/10.3390/ijms241814387
Chicago/Turabian StyleHonda, Teruko, and Hiroyuki Inagawa. 2023. "Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo" International Journal of Molecular Sciences 24, no. 18: 14387. https://doi.org/10.3390/ijms241814387
APA StyleHonda, T., & Inagawa, H. (2023). Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo. International Journal of Molecular Sciences, 24(18), 14387. https://doi.org/10.3390/ijms241814387






