Emerging Role of Decoy Receptor-2 as a Cancer Risk Predictor in Oral Potentially Malignant Disorders
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
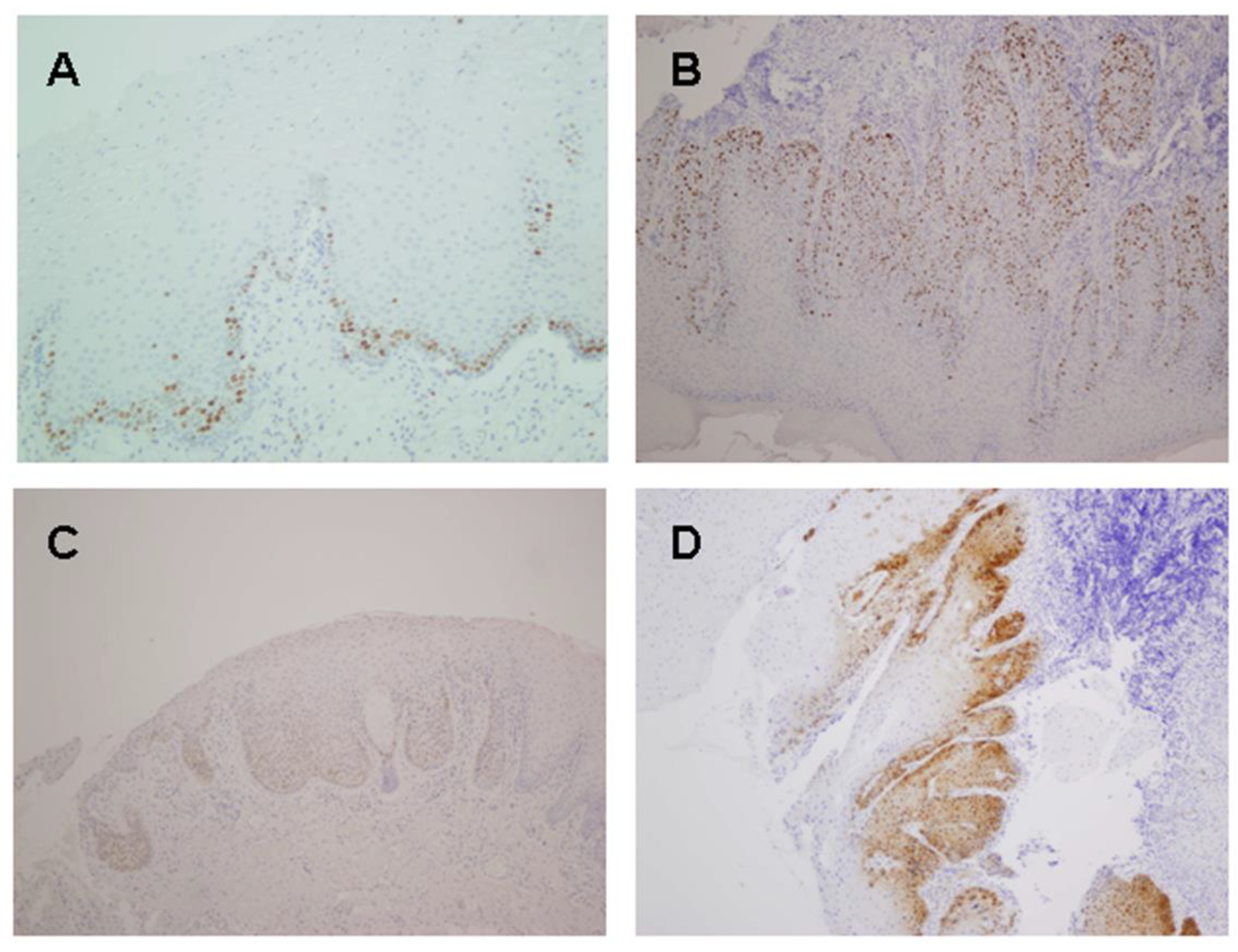
2.2. Ki67, p16, DcR2 and DEC1 Expression in Oral Tumorigenesis
2.3. Ki67, p16, DcR2 and DEC1 Expression in Oral Cancer
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Specimens
4.2. Immunohistochemistry
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, A.K.; Chan, J.K.; Grandis, J.R.; Takata, T.; Slootweg, P.J. Oral potentially malignant disorders and oral epitelial dysplaisa. In WHO Classification of Head and Neck Tumours; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 112–118. [Google Scholar]
- de Vicente, J.C.; Rodrigo, J.P.; Rodriguez-Santamarta, T.; Lequerica-Fernandez, P.; Allonca, E.; Garcia-Pedrero, J.M. Podoplanin expression in oral leukoplakia: Tumorigenic role. Oral. Oncol. 2013, 49, 598–603. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral. Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Bernardes de Jesus, B.; Blasco, M.A. Assessing cell and organ senescence biomarkers. Circ. Res. 2012, 111, 97–109. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer 2006, 6, 472–476. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes. Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Angiero, F.; Berenzi, A.; Benetti, A.; Rossi, E.; Del Sordo, R.; Sidoni, A.; Stefani, M.; Dessy, E. Expression of p16, p53 and Ki-67 proteins in the progression of epithelial dysplasia of the oral cavity. Anticancer. Res. 2008, 28, 2535–2539. [Google Scholar]
- Bascones-Martinez, A.; Lopez-Duran, M.; Cano-Sanchez, J.; Sanchez-Verde, L.; Diez-Rodriguez, A.; Aguirre-Echebarria, P.; Alvarez-Fernandez, E.; Gonzalez-Moles, M.A.; Bascones-Ilundain, J.; Muzio, L.L.; et al. Differences in the expression of five senescence markers in oral cancer, oral leukoplakia and control samples in humans. Oncol. Lett. 2012, 3, 1319–1325. [Google Scholar] [CrossRef]
- Kannan, S.; Chandran, G.J.; Pillai, K.R.; Mathew, B.; Sujathan, K.; Nalinakumary, K.R.; Nair, M.K. Expression of p53 in leukoplakia and squamous cell carcinoma of the oral mucosa: Correlation with expression of Ki67. Clin. Mol. Pathol. 1996, 49, M170–M175. [Google Scholar] [CrossRef]
- Nasser, W.; Flechtenmacher, C.; Holzinger, D.; Hofele, C.; Bosch, F.X. Aberrant expression of p53, p16INK4a and Ki-67 as basic biomarker for malignant progression of oral leukoplakias. J. Oral. Pathol. Med. 2011, 40, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rendon, A.; Roy, S.; Craig, G.T.; Speight, P.M. Expression of Mcm2, geminin and Ki67 in normal oral mucosa, oral epithelial dysplasias and their corresponding squamous-cell carcinomas. Br. J. Cancer 2009, 100, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, G.; Li, B.; Samaranayake, L.P. Expression of p16 and CDK4 in oral premalignant lesions and oral squamous cell carcinomas: A semi-quantitative immunohistochemical study. J. Oral. Pathol. Med. 1999, 28, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Kaur, J.; Kumar, A.; Chakravarti, N.; Mathur, M.; Bahadur, S.; Shukla, N.K.; Deo, S.V.; Ralhan, R. Alterations of rb pathway components are frequent events in patients with oral epithelial dysplasia and predict clinical outcome in patients with squamous cell carcinoma. Oncology 2005, 68, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009, 285, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, S.; Luo, Q.; Huang, X.; Zhou, Y.; Li, Z. Hypermethylation of DcR1, DcR2, DR4, DR5 gene promoters and clinical significance in tongue carcinoma. Am. J. Otolaryngol. 2019, 40, 102258. [Google Scholar] [CrossRef]
- Walczak, H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008698. [Google Scholar] [CrossRef]
- Campo-Trapero, J.; Cano-Sanchez, J.; Palacios-Sanchez, B.; Llamas-Martinez, S.; Lo Muzio, L.; Bascones-Martinez, A. Cellular senescence in oral cancer and precancer and treatment implications: A review. Acta Oncol. 2008, 47, 1464–1474. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Baucum, D.C.; Wu, J.; Lou, Y.; Bouquot, J.; Muller, S.; Zacharias, W. Repression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but not its receptors during oral cancer progression. BMC Cancer 2007, 7, 108. [Google Scholar] [CrossRef]
- Yoldas, B.; Ozer, C.; Ozen, O.; Canpolat, T.; Dogan, I.; Griffith, T.S.; Sanlioglu, S.; Ozluoglu, L.N. Clinical significance of TRAIL and TRAIL receptors in patients with head and neck cancer. Head. Neck 2011, 33, 1278–1284. [Google Scholar] [CrossRef]
- Bhawal, U.K.; Sato, F.; Arakawa, Y.; Fujimoto, K.; Kawamoto, T.; Tanimoto, K.; Ito, Y.; Sasahira, T.; Sakurai, T.; Kobayashi, M.; et al. Basic helix-loop-helix transcription factor DEC1 negatively regulates cyclin D1. J. Pathol. 2011, 224, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Liao, S.Y.; Lerman, M.I.; Ivanov, S.; Stanbridge, E.J. STRA13 expression and subcellular localisation in normal and tumour tissues: Implications for use as a diagnostic and differentiation marker. J. Med. Genet. 2005, 42, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Turley, H.; Wykoff, C.C.; Troup, S.; Watson, P.H.; Gatter, K.C.; Harris, A.L. The hypoxia-regulated transcription factor DEC1 (Stra13, SHARP-2) and its expression in human tissues and tumours. J. Pathol. 2004, 203, 808–813. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Lin, L.; Liu, Q.; Zhu, T.; Xia, K.; Su, T. The correlation between the expression of differentiated embryo-chondrocyte expressed gene l and oral squamous cell carcinoma. Eur. J. Med. Res. 2014, 19, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, T.; Chen, W.; Xiong, H.; Wang, C.; Yang, L.; Hu, X.; Xia, K.; Wang, Z.; Su, T. DEC1 is a potential marker of early metastasis in Oral squamous cell carcinoma. Tissue Cell 2023, 82, 102094. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Xiong, H.; Hu, X.; Hu, Y.; Wang, C.; Yang, L.; Huang, D.; Xia, K.; Su, T. DEC1: A potential biomarker of malignant transformation in oral leukoplakia. Braz. Oral. Res. 2020, 34, e052. [Google Scholar] [CrossRef]
- Bhawal, U.K.; Ito, Y.; Tanimoto, K.; Sato, F.; Fujimoto, K.; Kawamoto, T.; Sasahira, T.; Hamada, N.; Kuniyasu, H.; Arakawa, H.; et al. IL-1beta-mediated up-regulation of DEC1 in human gingiva cells via the Akt pathway. J. Cell Biochem. 2012, 113, 3246–3253. [Google Scholar] [CrossRef]
- Qian, Y.; Jung, Y.S.; Chen, X. Differentiated embryo-chondrocyte expressed gene 1 regulates p53-dependent cell survival versus cell death through macrophage inhibitory cytokine-1. Proc. Natl. Acad. Sci. USA 2012, 109, 11300–11305. [Google Scholar] [CrossRef]
- Anees, M.; Horak, P.; El-Gazzar, A.; Susani, M.; Heinze, G.; Perco, P.; Loda, M.; Lis, R.; Krainer, M.; Oh, W.K. Recurrence-free survival in prostate cancer is related to increased stromal TRAIL expression. Cancer 2011, 117, 1172–1182. [Google Scholar] [CrossRef]
- Gregorczyk-Maga, I.; Celejewska-Wojcik, N.; Gosiewska-Pawlica, D.; Darczuk, D.; Kesek, B.; Maga, M.; Wojcik, K. Exposure to air pollution and oxidative stress markers in patients with potentially malignant oral disorders. J. Physiol. Pharmacol. 2019, 70, 115–120. [Google Scholar] [CrossRef]
- Katakwar, P.; Metgud, R.; Naik, S.; Mittal, R. Oxidative stress marker in oral cancer: A review. J. Cancer Res. Ther. 2016, 12, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Di Giacomo, V.; Quaglietta, A.M.; Iacone, A.; Angelucci, D.; Tatasciore, U.; Rana, R.A.; Cataldi, A.; Zauli, G.; Di Pietro, R. TRAIL promotes a pro-survival signal in erythropoietin-deprived human erythroblasts through the activation of an NF-kB/IkBalpha pathway. J. Biol. Regul. Homeost. Agents 2011, 25, 375–386. [Google Scholar] [PubMed]
- Mahalingam, D.; Szegezdi, E.; Keane, M.; de Jong, S.; Samali, A. TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat. Rev. 2009, 35, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Shukla, Y. Death receptors: Targets for cancer therapy. Exp. Cell Res. 2010, 316, 887–899. [Google Scholar] [CrossRef]
- Micheau, O.; Shirley, S.; Dufour, F. Death receptors as targets in cancer. Br. J. Pharmacol. 2013, 169, 1723–1744. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Goruppi, S.; Dotto, G.P. Mesenchymal stroma: Primary determinant and therapeutic target for epithelial cancer. Trends Cell Biol. 2013, 23, 593–602. [Google Scholar] [CrossRef][Green Version]
- Alspach, E.; Fu, Y.; Stewart, S.A. Senescence and the pro-tumorigenic stroma. Crit. Rev. Oncog. 2013, 18, 549–558. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Parrinello, S.; Coppe, J.P.; Krtolica, A.; Campisi, J. Stromal-epithelial interactions in aging and cancer: Senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 2005, 118, 485–496. [Google Scholar] [CrossRef]
- Ganten, T.M.; Sykora, J.; Koschny, R.; Batke, E.; Aulmann, S.; Mansmann, U.; Stremmel, W.; Sinn, H.P.; Walczak, H. Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J. Mol. Med. 2009, 87, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, W.; Hu, Y.; Chen, W.; Wang, C.; Yang, L.; Mao, T.; Xia, K.; Min, A.; Xiong, H.; et al. Overexpression of DEC1 in the epithelium of OSF promotes mesenchymal transition via activating FAK/Akt signal axis. J. Oral. Pathol. Med. 2022, 51, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, L.; Wang, Z.; Hu, X.; Xiong, H.; Zhang, T.; Chen, W.; Xia, K.; Su, T. Differentiated embryo chondrocyte 1, induced by hypoxia-inducible factor 1alpha, promotes cell migration in oral squamous cell carcinoma cell lines. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2022, 133, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; El-Naggar, A.K.; Papadimitrakopoulou, V.; Ren, H.; Fan, Y.H.; Feng, L.; Lee, J.J.; Kim, E.; Hong, W.K.; Lippman, S.M.; et al. Podoplanin: A novel marker for oral cancer risk in patients with oral premalignancy. J. Clin. Oncol. 2008, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | No. Cases (%) | Progression to OSCC [No. Cases (%)] | p |
|---|---|---|---|
| Histopathological diagnosis | |||
| Without dysplasia | 47 (78) | 5 (11) | <0.0001 |
| Mild–moderate dysplasia | 7 (12) | 5 (71) | |
| Severe dysplasia | 6 (10) | 5 (83) | |
| Ki67% expression (% of positive epithelial cells) | |||
| Mild (score 0) | 32 (53) | 2 (6) | <0.0001 |
| Moderate (score 1) | 27 (45) | 12 (44) | |
| Strong (score 2) | 1 (2) | 1 (100) | |
| Ki67 expression (epithelial distribution) | |||
| Restricted to basal third (score 0) | 38 (63) | 3 (8) | <0.0001 * |
| Above basal third (score 1) | 22 (37) | 12 (55) | |
| p16 expression (% of positive epithelial cells) | |||
| Negative (score 0) | 52 (87) | 13 (25) | 0.68 * |
| Positive (score 1) | 8 (13) | 2 (25) | |
| Epithelial DcR2 expression (% of positive cells) | |||
| Mild (score 0) | 51 (85) | 8 (16) | <0.0001 |
| Moderate (score 1) | 8 (13) | 6 (75) | |
| Strong (score 2) | 1 (2) | 1 (100) | |
| Stromal DcR2 expression (% of positive cells) | |||
| Mild (score 0) | 35 (58) | 5 (14) | 0.039 |
| Moderate (score 1) | 24 (40) | 10 (42) | |
| Strong (score 2) | 1 (2) | 0 (0) | |
| Nuclear DEC1 expression (epithelial distribution) | |||
| No expression (score 0) | 7 (12) | 1 (14) | 0.001 |
| Restricted to basal layer (score 1) | 31 (55) | 3 (10) | |
| Suprabasal layer (score 2) | 19 (33) | 11 (58) | |
| Cytoplasmic DEC1 expression (epithelial distribution) | |||
| No expression (score 0) | 30 (53) | 4 (13) | <0.0001 |
| Restricted to basal layer (score 1) | 19 (33) | 3 (16) | |
| Suprabasal layer (score 2) | 8 (14) | 8 (100) | |
| Protein Expression | Histological Grade of Epithelial Dysplasia | p | |||
|---|---|---|---|---|---|
| Absent | Mild | Moderate | Severe | ||
| Ki67 expression (% of positive epithelial cells) | |||||
| Mild (score 0) | 30 (63.8) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0.001 |
| Moderate (score 1) | 17 (36.2) | 3 (60.0) | 3 (100.0) | 4 (80.0) | |
| Strong (score 2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | |
| Ki67 expression (epithelial distribution) | |||||
| Restricted to basal third (score 0) | 35 (74.5) | 2 (40.0) | 1 (33.3) | 0 (0.0) | 0.001 |
| Above basal third (score 1) | 12 (25.5) | 3 (60.0) | 2 (66.7) | 5 (100.0) | |
| p16 epithelial expression | |||||
| Negative (score 0) | 41 (87.2) | 5 (100) | 3 (100) | 3 (60) | 0.28 |
| Positive (score 1) | 6 (12.8) | 0 (0) | 0 (0) | 2 (40) | |
| DcR2 expression (% of positive epithelial cells) | |||||
| Mild (score 0) | 44 (93.6) | 3 (60.0) | 2 (66.7) | 2 (40.0) | 0.003 |
| Moderate (score 1) | 2 (4.3) | 2 (40.0) | 1 (33.3) | 3 (60.0) | |
| Strong (score 2) | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Nuclear DEC1 expression (epithelial distribution) | |||||
| No expression (score 0) | 6 (13.6) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0.045 |
| Restricted to basal layer (score 1) | 27 (61.4) | 3 (60.0) | 0 (0.0) | 1 (20.0) | |
| Suprabasal layer (score 2) | 11 (25.0) | 1 (20.0) | 3 (100.0) | 4 (80.0) | |
| Cytoplasmic DEC1 expression (epithelial distribution) | |||||
| No expression (score 0) | 26 (59.1) | 1 (20.0) | 1 (33.3) | 2 (40.0) | 0.005 |
| Restricted to basal layer (score 1) | 16 (36.4) | 2 (40.0) | 0 (0.0) | 1 (20.0) | |
| Suprabasal layer (score 2) | 2 (4.5) | 2 (40.0) | 2 (66.7) | 2 (40.0) | |
| Variables | Mean Time [Months] to Progression (95% CI) | HR (95% CI) | p |
|---|---|---|---|
| Dysplasia No dysplasia Mild–moderate Severe | 189.26 (164.21–214.31) 92.60 (21.71–163.49) 89.33 (64.04–114.62) | Reference 4.69 (1.33–16.56) 4.76 (1.34–16.82) | 0.008 |
| Ki67 expression Mild Moderate Strong | 244.57 (204.32–284.82) 128.35 (92.63–164.07) 52.00 (52.00–52.00) | Reference 4.91 (1.09–22.14) 28.11 (2.22–355.10) | 0.005 |
| Ki67 epithelial distribution Basal Suprabasal | 235.01 (193.26–276.77) 123.18 (86.48–159.88) | 4.02 (1.12–14.41) | 0.021 |
| p16 expression Negative Positive | 171.56 (131.52–211.60) 106.00 (73.66–138.33) | 1.37 (0.30–6.22) | 0.68 |
| Epithelial DcR2 expression Mild Moderate Strong | 197.29 (155.74–238.85) 70.88 (48.87–92.89) 57.00 (57.00–57.00) | Reference 6.47 (2.05–20.39) 13.32 (1.40–126.10) | <0.0001 |
| Stromal DcR2 expression Mild Moderate Strong | 200.90 (143.97–257.82) 130.48 (91.06–169.90) 57.00 (57.00–57.00) | Reference 2.46 (0.83–7.25) 0.00 (0.00–0.00) | 0.12 |
| Nuclear DEC1 expression Negative Basal Suprabasal | 64.50 (51.76–77.23) 234.48 (191.24–277.73) 120.04 (83.46–156.63) | Reference 0.44 (0.04–4.40) 1.68 (0.21–13.44) | 0.08 |
| Cytoplasmic DEC1 expression Negative Basal Suprabasal | 119.62 (93.79–145.46) 218.10 (162.93–273.26) 84.12 (49.62–118.62) | Reference 0.52 (0.09–2.93) 4.08 (1.20–13.84) | 0.002 |
| Variables | P | Hazard Ratio (HR) | 95% Confidence Interval |
|---|---|---|---|
| Dysplasia (no vs. yes) | 0.08 | 4.225 | 0.826–21.611 |
| Ki67 expression (Upper two thirds vs. basal third) | 0.02 | 4.14 | 1.19–14.39 |
| p16 expression | 0.425 | 0.47 | 0.07–2.98 |
| Epithelial DcR2 expression | 0.05 | ||
| 5–50% | 0.499 | 2.19 | 0.22–21.50 |
| >50% | 0.015 | 59.7 | 2.23–1595.1 |
| Stromal DcR2 expression | 0.62 | 1.35 | 0.39–4.60 |
| Nuclear DEC1 expression | 0.84 | 1.12 | 0.35–3.58 |
| Cytoplasmic DEC1 expression | 0.91 | 1.03 | 0.53–2.0 |
| Variables | No. Cases (%) |
|---|---|
| Ki67 expression (% of tumor-stained cells) | |
| 10% | 4 (26.7) |
| 10–50% | 2 (13.3) |
| >50% | 9 (60.0) |
| p16 expression (% of tumor-stained cells) | |
| <10% | 10 (66.7) |
| ≥10% | 5 (33.3) |
| Epithelial DcR2 (% of stained cells) | |
| <5% | 11 (73.3) |
| 5–50% | 2 (13.3) |
| >50% | 2 (13.3) |
| Stromal DcR2 (% of stained cells) | |
| <5% | 2 (13.3) |
| 5–50% | 6 (40.0) |
| >50% | 7 (46.7) |
| Nuclear DEC1 expression | |
| Negative immunostaining | 4 (26.7) |
| Positive immunostaining | 11 (73.3) |
| Cytoplasmic DEC1 expression | |
| Negative immunostaining | 3 (20) |
| Positive immunostaining | 12 (80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Villalaín, L.; Álvarez-Teijeiro, S.; Rodríguez-Santamarta, T.; Fernández del Valle, Á.; Allonca, E.; Rodrigo, J.P.; de Vicente, J.C.; García-Pedrero, J.M. Emerging Role of Decoy Receptor-2 as a Cancer Risk Predictor in Oral Potentially Malignant Disorders. Int. J. Mol. Sci. 2023, 24, 14382. https://doi.org/10.3390/ijms241814382
de Villalaín L, Álvarez-Teijeiro S, Rodríguez-Santamarta T, Fernández del Valle Á, Allonca E, Rodrigo JP, de Vicente JC, García-Pedrero JM. Emerging Role of Decoy Receptor-2 as a Cancer Risk Predictor in Oral Potentially Malignant Disorders. International Journal of Molecular Sciences. 2023; 24(18):14382. https://doi.org/10.3390/ijms241814382
Chicago/Turabian Stylede Villalaín, Lucas, Saúl Álvarez-Teijeiro, Tania Rodríguez-Santamarta, Álvaro Fernández del Valle, Eva Allonca, Juan P. Rodrigo, Juan Carlos de Vicente, and Juana M. García-Pedrero. 2023. "Emerging Role of Decoy Receptor-2 as a Cancer Risk Predictor in Oral Potentially Malignant Disorders" International Journal of Molecular Sciences 24, no. 18: 14382. https://doi.org/10.3390/ijms241814382
APA Stylede Villalaín, L., Álvarez-Teijeiro, S., Rodríguez-Santamarta, T., Fernández del Valle, Á., Allonca, E., Rodrigo, J. P., de Vicente, J. C., & García-Pedrero, J. M. (2023). Emerging Role of Decoy Receptor-2 as a Cancer Risk Predictor in Oral Potentially Malignant Disorders. International Journal of Molecular Sciences, 24(18), 14382. https://doi.org/10.3390/ijms241814382






