Meta-Analysis of COVID-19 Metabolomics Identifies Variations in Robustness of Biomarkers
Abstract
:1. Introduction
2. Results
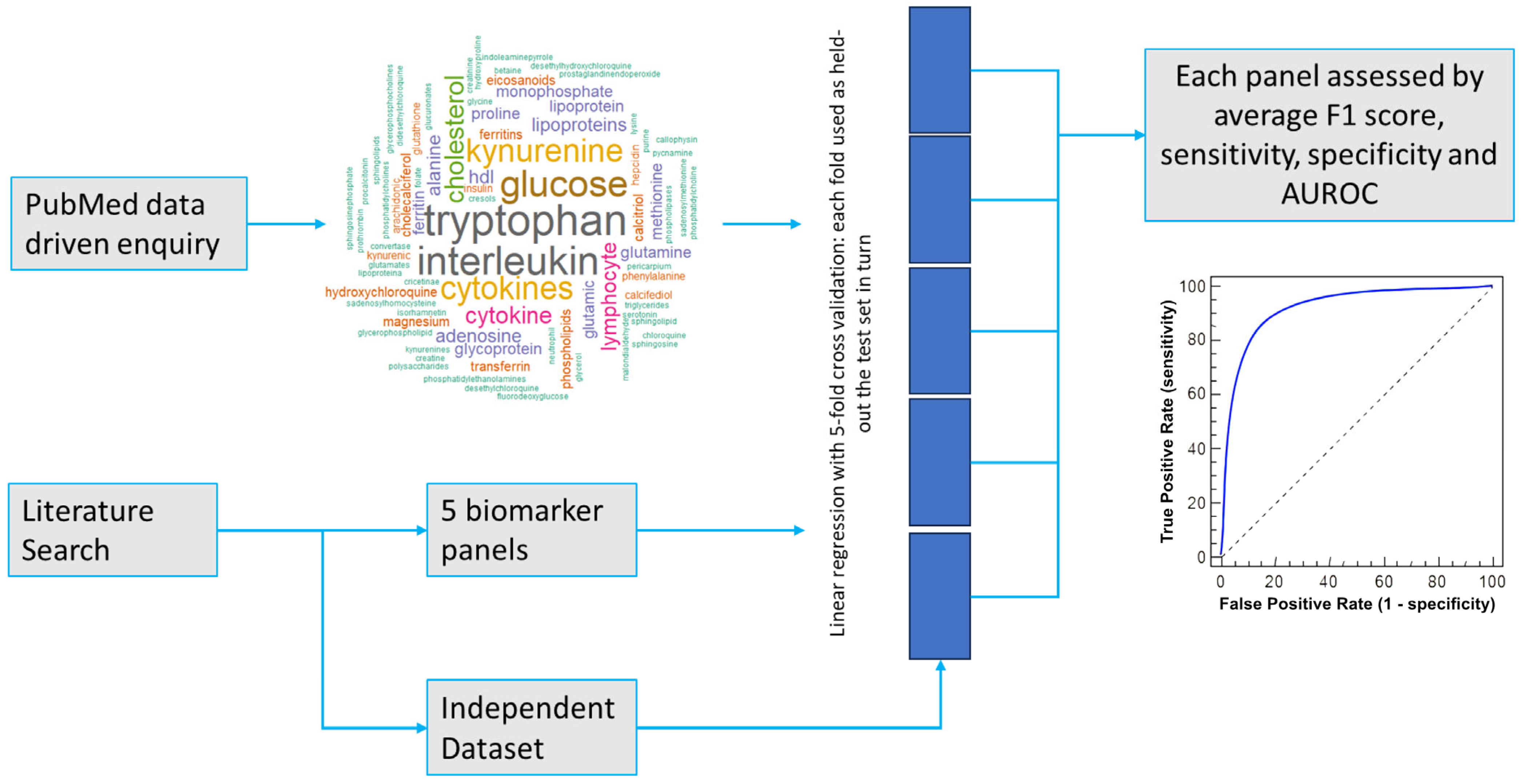
2.1. Biomarker and Panel Selection
2.2. Testing Performance Metrics for Proposed Biomarker Panels in the Independent Dataset
2.3. Variations in Performance by Fold
3. Discussion
4. Materials and Methods
4.1. Dataset and Biomarker Identification
4.2. Pre-Processing
4.3. Machine Learning Methodology: Supervised Binary Classification
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 Pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic Impact of COVID-19 Pandemic on Healthcare Facilities and Systems: International Perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Struwe, W.; Emmott, E.; Bailey, M.; Sharon, M.; Sinz, A.; Corrales, F.J.; Thalassinos, K.; Braybrook, J.; Mills, C.; Barran, P. The COVID-19 MS Coalition—Accelerating Diagnostics, Prognostics, and Treatment. Lancet 2020, 395, 1761–1762. [Google Scholar] [CrossRef]
- WHO. Target Product Profiles for Priority Diagnostics to Support Response to the COVID-19 Pandemic v. 1.0; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Hidayat, S.N.; Julian, T.; Dharmawan, A.B.; Puspita, M.; Chandra, L.; Rohman, A.; Julia, M.; Rianjanu, A.; Nurputra, D.K.; Triyana, K.; et al. Hybrid Learning Method Based on Feature Clustering and Scoring for Enhanced COVID-19 Breath Analysis by an Electronic Nose. Artif. Intell. Med. 2022, 129, 102323. [Google Scholar] [CrossRef] [PubMed]
- Spick, M.; Longman, K.; Frampas, C.; Lewis, H.; Costa, C.; Walters, D.D.; Stewart, A.; Wilde, M.; Greener, D.; Evetts, G.; et al. Changes to the Sebum Lipidome upon COVID-19 Infection Observed via Rapid Sampling from the Skin. EClinicalMedicine 2021, 33, 100786. [Google Scholar] [CrossRef]
- Bi, X.; Liu, W.; Ding, X.; Liang, S.; Zheng, Y.; Zhu, X.; Quan, S.; Yi, X.; Xiang, N.; Du, J.; et al. Proteomic and Metabolomic Profiling of Urine Uncovers Immune Responses in Patients with COVID-19. Cell Rep. 2022, 38, 110271. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Samadinia, H.; Sheini, A.; Aboonajmi, J.; Sharghi, H.; Hashemi, P.; Khoshsafar, H.; Ghanei, M.; Bagheri, H. A Colorimetric Electronic Tongue for Point-of-Care Detection of COVID-19 Using Salivary Metabolites. Talanta 2022, 246, 123537. [Google Scholar] [CrossRef]
- Bourgin, M.; Durand, S.; Kroemer, G. Diagnostic, Prognostic and Mechanistic Biomarkers of COVID-19 Identified by Mass Spectrometric Metabolomics. Metabolites 2023, 13, 342. [Google Scholar] [CrossRef]
- Bruzzone, C.; Conde, R.; Embade, N.; Mato, J.M.; Millet, O. Metabolomics as a Powerful Tool for Diagnostic, Pronostic and Drug Intervention Analysis in COVID-19. Front. Mol. Biosci. 2023, 10, 1111482. [Google Scholar] [CrossRef]
- Páez-Franco, J.C.; Torres-Ruiz, J.; Sosa-Hernández, V.A.; Cervantes-Díaz, R.; Romero-Ramírez, S.; Pérez-Fragoso, A.; Meza-Sánchez, D.E.; Germán-Acacio, J.M.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; et al. Metabolomics Analysis Reveals a Modified Amino Acid Metabolism That Correlates with Altered Oxygen Homeostasis in COVID-19 Patients. Sci. Rep. 2021, 11, 6350. [Google Scholar] [CrossRef]
- Philips, A.M.; Khan, N. Amino Acid Sensing Pathway: A Major Check Point in the Pathogenesis of Obesity and COVID-19. Obes. Rev. 2021, 22, e13221. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Melchior, D.; Obled, C. Modifications of Protein and Amino Acid Metabolism during Inflammation and Immune System Activation. Livest. Prod. Sci. 2004, 87, 37–45. [Google Scholar] [CrossRef]
- Wu, D.; Shu, T.; Yang, X.; Song, J.-X.; Zhang, M.; Yao, C.; Liu, W.; Huang, M.; Yu, Y.; Yang, Q.; et al. Plasma Metabolomic and Lipidomic Alterations Associated with COVID-19. Natl. Sci. Rev. 2020, 7, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Chen, Z.; Nie, P.; Ge, H.; Shen, L.; Yang, F.; Qu, X.L.; Ying, X.Y.; Zhou, Y.; Wang, W.; et al. Lipid Profile Features and Their Associations with Disease Severity and Mortality in Patients with COVID-19. Front. Cardiovasc. Med. 2020, 7, 584987. [Google Scholar] [CrossRef] [PubMed]
- Hensen, T.; Fässler, D.; O’Mahony, L.; Albrich, W.C.; Barda, B.; Garzoni, C.; Kleger, G.-R.; Pietsch, U.; Suh, N.; Hertel, J.; et al. The Effects of Hospitalisation on the Serum Metabolome in COVID-19 Patients. Metabolites 2023, 13, 951. [Google Scholar] [CrossRef]
- Roccaforte, V.; Daves, M.; Lippi, G.; Spreafico, M.; Bonato, C. Altered Lipid Profile in Patients with COVID-19 Infection. J. Lab. Precis. Med. 2021, 6. [Google Scholar] [CrossRef]
- Castañé, H.; Iftimie, S.; Baiges-Gaya, G.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Garrido, P.; Castro, A.; Camps, J.; Joven, J. Machine Learning and Semi-Targeted Lipidomics Identify Distinct Serum Lipid Signatures in Hospitalized COVID-19-Positive and COVID-19-Negative Patients. Metabolism 2022, 131, 155197. [Google Scholar] [CrossRef]
- Lewis, H.-M.; Liu, Y.; Frampas, C.F.; Longman, K.; Spick, M.; Stewart, A.; Sinclair, E.; Kasar, N.; Greener, D.; Whetton, A.D.; et al. Metabolomics Markers of COVID-19 Are Dependent on Collection Wave. Metabolites 2022, 12, 713. [Google Scholar] [CrossRef]
- Spick, M.; Lewis, H.M.; Wilde, M.J.; Hopley, C.; Huggett, J.; Bailey, M.J. Systematic Review with Meta-Analysis of Diagnostic Test Accuracy for COVID-19 by Mass Spectrometry. Metabolism 2022, 126, 154922. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Howard, A.G.; Durazo-Arvizu, R.; Eckfeldt, J.H.; Gellman, M.D.; Kim, R.S.; Liu, K.; Mendez, A.J.; Penedo, F.J.; Talavera, G.A.; et al. Analytical and Biological Variability in Biomarker Measurement in the Hispanic Community Health Study/Study of Latinos. Clin. Chim. Acta 2016, 463, 129–137. [Google Scholar] [CrossRef]
- Broadhurst, D.I.; Kell, D.B. Statistical Strategies for Avoiding False Discoveries in Metabolomics and Related Experiments. Metabolomics 2007, 2, 171–196. [Google Scholar] [CrossRef]
- Lamb, L.E.; Janicki, J.J.; Bartolone, S.N.; Peters, K.M.; Chancellor, M.B. Development of an Interstitial Cystitis Risk Score for Bladder Permeability. PLoS ONE 2017, 12, e0185686. [Google Scholar] [CrossRef] [PubMed]
- Sieberts, S.K.; Schaff, J.; Duda, M.; Pataki, B.Á.; Sun, M.; Snyder, P.; Daneault, J.-F.; Parisi, F.; Costante, G.; Rubin, U.; et al. Crowdsourcing Digital Health Measures to Predict Parkinson’s Disease Severity: The Parkinson’s Disease Digital Biomarker DREAM Challenge. NPJ Digit. Med. 2021, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Sieberts, S.K.; Zhu, F.; García-García, J.; Stahl, E.; Pratap, A.; Pandey, G.; Pappas, D.; Aguilar, D.; Anton, B.; Bonet, J.; et al. Crowdsourced Assessment of Common Genetic Contribution to Predicting Anti-TNF Treatment Response in Rheumatoid Arthritis. Nat. Commun. 2016, 7, 12460. [Google Scholar] [CrossRef]
- Uhteg, K.; Jarrett, J.; Richards, M.; Howard, C.; Morehead, E.; Geahr, M.; Gluck, L.; Hanlon, A.; Ellis, B.; Kaur, H.; et al. Comparing the Analytical Performance of Three SARS-CoV-2 Molecular Diagnostic Assays. J. Clin. Virol. 2020, 127, 104384. [Google Scholar] [CrossRef]
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-Acute Sequelae of COVID-19: A Metabolic Perspective. eLife 2022, 11, e78200. [Google Scholar] [CrossRef]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [CrossRef]
- Tomo, S.; Banerjee, M.; Karli, S.; Purohit, P.; Mitra, P.; Sharma, P.; Garg, M.K.; Kumar, B. Assessment of DHEAS, Cortisol, and DHEAS/Cortisol Ratio in Patients with COVID-19: A Pilot Study. Hormones 2022, 21, 515–518. [Google Scholar] [CrossRef]
- Fraser, D.D.; Slessarev, M.; Martin, C.M.; Daley, M.; Patel, M.A.; Miller, M.R.; Patterson, E.K.; O’Gorman, D.B.; Gill, S.E.; Wishart, D.S.; et al. Metabolomics Profiling of Critically Ill Coronavirus Disease 2019 Patients: Identification of Diagnostic and Prognostic Biomarkers. Crit. Care Explor. 2020, 2, e0272. [Google Scholar] [CrossRef]
- Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Algon, A.A.A.; Al-Hakeim, H.K.; Maes, M. The Tryptophan Catabolite or Kynurenine Pathway in COVID-19 and Critical COVID-19: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2022, 22, 615. [Google Scholar] [CrossRef]
- Khodadoust, M.M. Inferring a Causal Relationship between Ceramide Levels and COVID-19 Respiratory Distress. Sci. Rep. 2021, 11, 20866. [Google Scholar] [CrossRef] [PubMed]
- Caterino, M.; Costanzo, M.; Fedele, R.; Cevenini, A.; Gelzo, M.; Di Minno, A.; Andolfo, I.; Capasso, M.; Russo, R.; Annunziata, A.; et al. The Serum Metabolome of Moderate and Severe COVID-19 Patients Reflects Possible Liver Alterations Involving Carbon and Nitrogen Metabolism. Int. J. Mol. Sci. 2021, 22, 9548. [Google Scholar] [CrossRef] [PubMed]
- Kaluarachchi, M.; Boulangé, C.L.; Karaman, I.; Lindon, J.C.; Ebbels, T.M.D.; Elliott, P.; Tracy, R.P.; Olson, N.C. A Comparison of Human Serum and Plasma Metabolites Using Untargeted 1H NMR Spectroscopy and UPLC-MS. Metabolomics 2018, 14, 32. [Google Scholar] [CrossRef]
- Dettmer, K.; Almstetter, M.F.; Appel, I.J.; Nürnberger, N.; Schlamberger, G.; Gronwald, W.; Meyer, H.H.D.; Oefner, P.J. Comparison of Serum versus Plasma Collection in Gas Chromatography--Mass Spectrometry-Based Metabolomics. Electrophoresis 2010, 31, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Spick, M.; Campbell, A.; Baricevic-Jones, I.; von Gerichten, J.; Lewis, H.-M.; Frampas, C.F.; Longman, K.; Stewart, A.; Dunn-Walters, D.; Skene, D.J.; et al. Multi-Omics Reveals Mechanisms of Partial Modulation of COVID-19 Dysregulation by Glucocorticoid Treatment. Int. J. Mol. Sci. 2022, 23, 12079. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.; Elliott, J.; Bodinier, B.; Barclay, W.; Ward, H.; Cooke, G.; Donnelly, C.A.; Chadeau-Hyam, M.; Elliott, P. Variant-Specific Symptoms of COVID-19 among 1,542,510 People in England. Nat. Commun. 2022, 13, 6856. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef]
- Kornhuber, J.; Hoertel, N.; Gulbins, E. The Acid Sphingomyelinase/Ceramide System in COVID-19. Mol. Psychiatry 2022, 27, 307–314. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pöhlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of Acid Sphingomyelinase by Ambroxol Prevents SARS-CoV-2 Entry into Epithelial Cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Chen, H.; De, P.; Hu, Y.; Hwang, B.-H. Wisdom of Crowds: The Value of Stock Opinions Transmitted Through Social Media. Rev. Financ. Stud. 2014, 27, 1367–1403. [Google Scholar] [CrossRef]
- Golub, B.; Jackson, M.O. Naïve Learning in Social Networks and the Wisdom of Crowds. Am. Econ. J. Microecon. 2010, 2, 112–149. [Google Scholar] [CrossRef]
- The DREAM5 Consortium; Marbach, D.; Costello, J.C.; Küffner, R.; Vega, N.M.; Prill, R.J.; Camacho, D.M.; Allison, K.R.; Kellis, M.; Collins, J.J.; et al. Wisdom of Crowds for Robust Gene Network Inference. Nat. Methods 2012, 9, 796–804. [Google Scholar] [CrossRef]
- Kao, A.B.; Berdahl, A.M.; Hartnett, A.T.; Lutz, M.J.; Bak-Coleman, J.B.; Ioannou, C.C.; Giam, X.; Couzin, I.D. Counteracting Estimation Bias and Social Influence to Improve the Wisdom of Crowds. J. R. Soc. Interface 2018, 15, 20180130. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Panagiotou, O.A. Comparison of Effect Sizes Associated with Biomarkers Reported in Highly Cited Individual Articles and in Subsequent Meta-Analyses. JAMA 2011, 305, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Danlos, F.-X.; Grajeda-Iglesias, C.; Durand, S.; Sauvat, A.; Roumier, M.; Cantin, D.; Colomba, E.; Rohmer, J.; Pommeret, F.; Baciarello, G.; et al. Metabolomic Analyses of COVID-19 Patients Unravel Stage-Dependent and Prognostic Biomarkers. Cell Death Dis. 2021, 12, 258. [Google Scholar] [CrossRef]
- Sindelar, M.; Stancliffe, E.; Schwaiger-Haber, M.; Anbukumar, D.S.; Adkins-Travis, K.; Goss, C.W.; O’Halloran, J.A.; Mudd, P.A.; Liu, W.-C.; Albrecht, R.A.; et al. Longitudinal Metabolomics of Human Plasma Reveals Prognostic Markers of COVID-19 Disease Severity. Cell Rep. Med. 2021, 2, 100369. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Yu, Y.; Wang, Y.; Huang, Q.; Qian, F.; Sun, L.; Song, Z.; Chen, Z.; Feng, J.; et al. Blood Molecular Markers Associated with COVID-19 Immunopathology and Multi-organ Damage. EMBO J. 2020, 39, e105896. [Google Scholar] [CrossRef] [PubMed]
- Ballout, R.A.; Kong, H.; Sampson, M.; Otvos, J.D.; Cox, A.L.; Agbor-Enoh, S.; Remaley, A.T. The NIH Lipo-COVID Study: A Pilot NMR Investigation of Lipoprotein Subfractions and Other Metabolites in Patients with Severe COVID-19. Biomedicines 2021, 9, 1090. [Google Scholar] [CrossRef]
- Delafiori, J.; Navarro, L.C.; Siciliano, R.F.; De Melo, G.C.; Busanello, E.N.B.; Nicolau, J.C.; Sales, G.M.; De Oliveira, A.N.; Val, F.F.A.; De Oliveira, D.N.; et al. COVID-19 Automated Diagnosis and Risk Assessment through Metabolomics and Machine Learning. Anal. Chem. 2021, 93, 2471–2479. [Google Scholar] [CrossRef]
- Mazzucco, S.; Agostini, F.; Mangogna, A.; Cattin, L.; Biolo, G. Prolonged Inactivity Up-Regulates Cholesteryl Ester Transfer Protein Independently of Body Fat Changes in Humans. J. Clin. Endocrinol. Metab. 2010, 95, 2508–2512. [Google Scholar] [CrossRef]
- easyPubMed. Available online: https://cran.r-project.org/web/packages/easyPubMed/ (accessed on 5 June 2023).
- wordcloud2. Available online: https://cran.r-project.org/web/packages/wordcloud2/ (accessed on 16 June 2023).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]

| Study | Participants (Positive/Negative) | Sampling Matrix | Identified Biomarkers of COVID-19-Positive Status |
|---|---|---|---|
| 1. Tomo et al. (2022) [29] | 76/79 | Sera | DHEAS, cortisol, DHEAS/cortisol ratio |
| 2. Fraser et al. (2020) [30] | 10/10 | Plasma | Arginine, kynurenine, arginine/kynurenine ratio |
| 3. Almulla et al. (2022) [31] | 329/475 * | n/a | Kynurenine, tryptophan, kynurenine/tryptophan ratio |
| 4. Khodadoust et al. (2021) [32] | 60/36 | Plasma | Cer (d18:0/24:1), Cer (d18:1/24:1), Cer (d18:1/20:0), Cer (d18:1/22:0) |
| 5. Caterino et al. (2021) [33] | 52/9 | Sera | Lactic acid, glutamate, aspartate, phenylalanine, β-alanine, ornithine, arachidonic acid, choline, and hypoxanthine |
| 6. Crowdsourced Panel | n/a | n/a | Tryptophan, kynurenine, alanine, glutamine, proline |
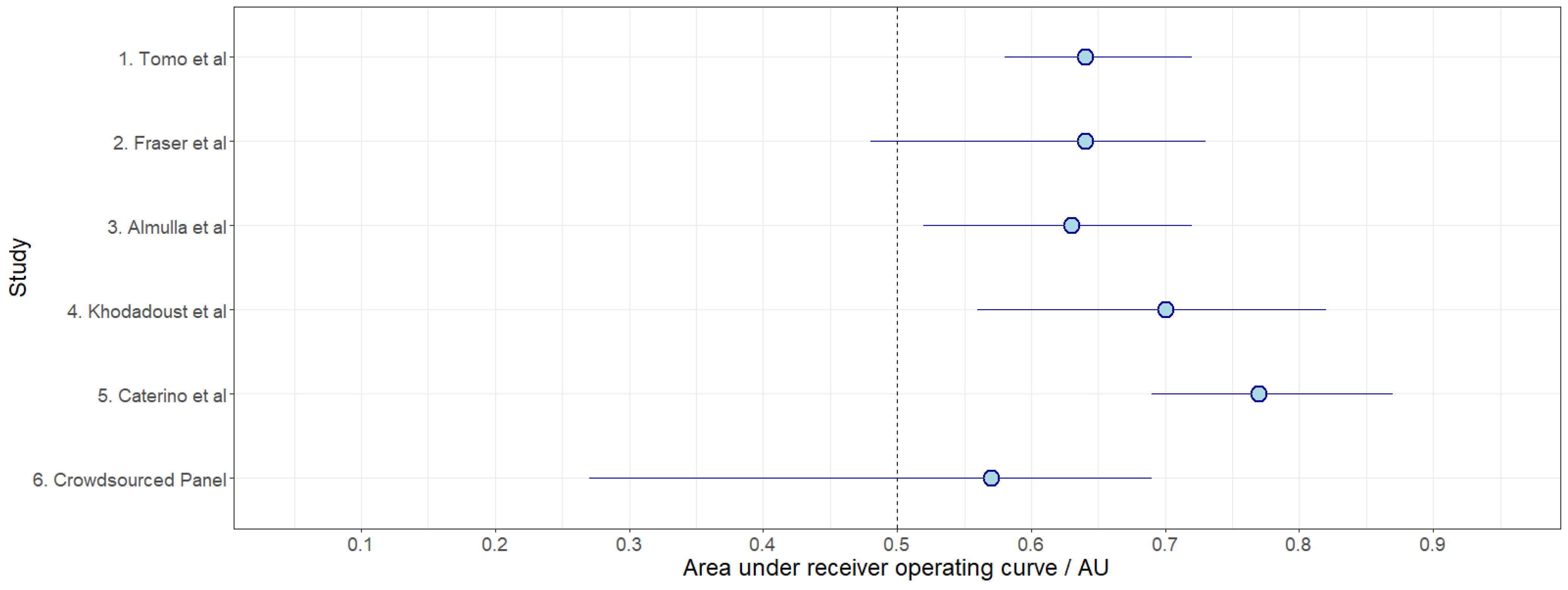
| Panel | Avg. F1 Score | Avg. Sensitivity | Avg. Specificity | Avg. Youden Index | Avg. AUROC |
|---|---|---|---|---|---|
| 1. Tomo et al. (2022) [29] | 0.69 | 0.62 | 0.68 | 0.30 | 0.64 |
| 2. Fraser et al. (2020) [30] | 0.70 | 0.64 | 0.62 | 0.25 | 0.64 |
| 3. Almulla et al. (2022) [31] | 0.61 | 0.53 | 0.59 | 0.12 | 0.63 |
| 4. Khodadoust et al. (2021) [32] | 0.70 | 0.63 | 0.67 | 0.30 | 0.70 |
| 5. Caterino et al. (2021) [33] | 0.76 | 0.72 | 0.64 | 0.36 | 0.77 |
| 6. Crowdsourced Panel | 0.64 | 0.57 | 0.61 | 0.18 | 0.57 |
| Control: median result from 5000 random 5-biomarker panels (independent dataset) | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onoja, A.; von Gerichten, J.; Lewis, H.-M.; Bailey, M.J.; Skene, D.J.; Geifman, N.; Spick, M. Meta-Analysis of COVID-19 Metabolomics Identifies Variations in Robustness of Biomarkers. Int. J. Mol. Sci. 2023, 24, 14371. https://doi.org/10.3390/ijms241814371
Onoja A, von Gerichten J, Lewis H-M, Bailey MJ, Skene DJ, Geifman N, Spick M. Meta-Analysis of COVID-19 Metabolomics Identifies Variations in Robustness of Biomarkers. International Journal of Molecular Sciences. 2023; 24(18):14371. https://doi.org/10.3390/ijms241814371
Chicago/Turabian StyleOnoja, Anthony, Johanna von Gerichten, Holly-May Lewis, Melanie J. Bailey, Debra J. Skene, Nophar Geifman, and Matt Spick. 2023. "Meta-Analysis of COVID-19 Metabolomics Identifies Variations in Robustness of Biomarkers" International Journal of Molecular Sciences 24, no. 18: 14371. https://doi.org/10.3390/ijms241814371
APA StyleOnoja, A., von Gerichten, J., Lewis, H.-M., Bailey, M. J., Skene, D. J., Geifman, N., & Spick, M. (2023). Meta-Analysis of COVID-19 Metabolomics Identifies Variations in Robustness of Biomarkers. International Journal of Molecular Sciences, 24(18), 14371. https://doi.org/10.3390/ijms241814371








