Exosomes in the Diagnosis and Treatment of Renal Cell Cancer
Abstract
:1. Introduction
2. Methods
3. Composition, Secretion, and Detection of Exosomes
3.1. Composition of Exosomes
3.2. Secretion of Exosomes
3.3. Detection of Exosomes
4. The Contribution of Exosomes to RCC
4.1. TME
4.2. Angiogenesis
4.3. Immune Escape
4.4. Cancer Cell Invasion and Metastasis
5. The Role of Exosomes in the Diagnosis of RCC
5.1. mRNA
5.2. miRNA
5.3. lncRNAs and circRNAs
6. Exosomes in the Treatment of RCC
6.1. Tumour Drug Resistance
6.2. Tumour Vaccines
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Gill, A.J.; Argani, P.; Chen, Y.B.; Egevad, L.; Kristiansen, G.; Grignon, D.J.; Hes, O. Report from the International Society of Urological Pathology (ISUP) Consultation Conference on Molecular Pathology of Urogenital Cancers: III: Molecular Pathology of Kidney Cancer. Am. J. Surg. Pathol. 2020, 44, e47–e65. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.I.; Leow, J.J.; Gelpi-Hammerschmidt, F.; Wang, Y.; Del Giudice, F.; De, S.; Chou, E.P.; Song, K.H.; Almario, L.; Chang, S.L. Racial Disparities in Postoperative Complications After Radical Nephrectomy: A Population-based Analysis. Urology 2015, 85, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.R.; Finelli, A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat. Rev. Urol. 2014, 11, 517–525. [Google Scholar] [CrossRef]
- Koul, H.; Huh, J.S.; Rove, K.O.; Crompton, L.; Koul, S.; Meacham, R.B.; Kim, F.J. Molecular aspects of renal cell carcinoma: A review. Am. J. Cancer Res. 2011, 1, 240–254. [Google Scholar]
- Kumbla, R.A.; Figlin, R.A.; Posadas, E.M. Recent Advances in the Medical Treatment of Recurrent or Metastatic Renal Cell Cancer. Drugs 2017, 77, 17–28. [Google Scholar] [CrossRef]
- Savaliya, M.; Surati, D.; Surati, R.; Padmani, S.; Boussios, S. Posterior Reversible Encephalopathy Syndrome after Pazopanib Therapy. Diseases 2023, 11, 76. [Google Scholar] [CrossRef]
- Pal, K.; Madamsetty, V.S.; Dutta, S.K.; Wang, E.; Angom, R.S.; Mukhopadhyay, D. Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer. NPJ Precis. Oncol. 2019, 3, 31. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A.; ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Vader, P.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Emerging targets for cancer therapy. Trends Mol. Med. 2014, 20, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Zhou, J.; Liu, X.; Wang, L.; Hu, X.; Mao, Y.; Gan, R.; Chen, Z. Extracellular vesicles in renal cell carcinoma: Challenges and opportunities coexist. Front. Immunol. 2023, 14, 1212101. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-García, F.; Gómez-de Frutos, M.D.; Rodríguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci. Rep. 2017, 7, 44433. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Xu, L.; Gimple, R.C.; Lau, W.B.; Lau, B.; Fei, F.; Shen, Q.; Liao, X.; Li, Y.; Wang, W.; He, Y.; et al. The present and future of the mass spectrometry-based investigation of the exosome landscape. Mass. Spectrom. Rev. 2020, 39, 745–762. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Muluhngwi, P.; Valdes, R., Jr.; Fernandez-Botran, R.; Burton, E.; Williams, B.; Linder, M.W. Cell-free DNA diagnostics: Current and emerging applications in oncology. Pharmacogenomics 2019, 20, 357–380. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, E.; Moschetta, M.; Shah, S.; Parker, J.S.; Ozturk, M.A.; Pappas-Gogos, G.; Sheriff, M.; Rassy, E.; Boussios, S. Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 9926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yao, X.; Liu, X.; He, X.; Li, L.; Liu, X.; Yan, Z.; Wu, J.; Fu, B.M. Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. J. Extracell. Vesicles 2019, 8, 1629865. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, M.; Vilaça, R.; Lopes, C. Exosomes: Innocent Bystanders or Critical Culprits in Neurodegenerative Diseases. Front. Cell Dev. Biol. 2021, 9, 635104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Y.; Chen, X.; Wu, J.; Gu, T.; Tang, X. Host derived exosomes-pathogens interactions: Potential functions of exosomes in pathogen infection. Biomed. Pharmacother. 2018, 108, 1451–1459. [Google Scholar] [CrossRef]
- Baharlooi, H.; Azimi, M.; Salehi, Z.; Izad, M. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases. Int. J. Stem. Cells 2020, 13, 13–23. [Google Scholar] [CrossRef]
- O’Leary, V.B.; Ovsepian, S.V.; Carrascosa, L.G.; Buske, F.A.; Radulovic, V.; Niyazi, M.; Moertl, S.; Trau, M.; Atkinson, M.J.; Anastasov, N. PARTICLE, a Triplex-Forming Long ncRNA, Regulates Locus-Specific Methylation in Response to Low-Dose Irradiation. Cell Rep. 2015, 11, 474–485. [Google Scholar] [CrossRef]
- O’Leary, V.B.; Smida, J.; Matjanovski, M.; Brockhaus, C.; Winkler, K.; Moertl, S.; Ovsepian, S.V.; Atkinson, M.J. The circRNA interactome-innovative hallmarks of the intra- and extracellular radiation response. Oncotarget 2017, 8, 78397–78409. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X.; et al. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018, 46, D106–D112. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Henrickson, L.; Vijayan, M.M. Plasma exosomes are enriched in Hsp70 and modulated by stress and cortisol in rainbow trout. J. Endocrinol. 2017, 232, 237–246. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Kajimoto, T.; Okada, T.; Miya, S.; Zhang, L.; Nakamura, S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 2013, 4, 2712. [Google Scholar] [CrossRef]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Marbán, E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu. Rev. Physiol. 2016, 78, 67–83. [Google Scholar] [CrossRef]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Gibbings, D.J.; Ciaudo, C.; Erhardt, M.; Voinnet, O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 2009, 11, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamamoto, Y.; Sato, T.A.; Ochiya, T. Extracellular vesicles as trans-genomic agents: Emerging roles in disease and evolution. Cancer Sci. 2017, 108, 824–830. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Hinger, S.A.; Cha, D.J.; Franklin, J.L.; Higginbotham, J.N.; Dou, Y.; Ping, J.; Shu, L.; Prasad, N.; Levy, S.; Zhang, B.; et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell Rep. 2018, 25, 715–725.e4. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.P.; et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef]
- Bai, H.; Lei, K.; Huang, F.; Jiang, Z.; Zhou, X. Exo-circRNAs: A new paradigm for anticancer therapy. Mol. Cancer 2019, 18, 56. [Google Scholar] [CrossRef]
- Conlan, R.S.; Pisano, S.; Oliveira, M.I.; Ferrari, M.; Mendes Pinto, I. Exosomes as Reconfigurable Therapeutic Systems. Trends Mol. Med. 2017, 23, 636–650. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Jin, F.; Bian, Z.; Li, L.; Liang, H.; Li, M.; Shi, L.; Pan, C.; Zhu, D.; et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat. Commun. 2017, 8, 14041. [Google Scholar] [CrossRef] [PubMed]
- Ovsepian, S.V.; Dolly, J.O. Dendritic SNAREs add a new twist to the old neuron theory. Proc. Natl. Acad. Sci. USA 2011, 108, 19113–19120. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; O’Brien, K.; Kelleher, F.C.; Corcoran, C.; Germano, S.; Radomski, M.W.; Crown, J.; O’Driscoll, L. Isolation of exosomes for subsequent mRNA, MicroRNA, and protein profiling. Methods Mol. Biol. 2011, 784, 181–195. [Google Scholar] [PubMed]
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.B.; Peiris, H.N.; Mitchell, M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- He, Y.D.; Tao, W.; He, T.; Wang, B.Y.; Tang, X.M.; Zhang, L.M.; Wu, Z.Q.; Deng, W.M.; Zhang, L.X.; Shao, C.K.; et al. A urine extracellular vesicle circRNA classifier for detection of high-grade prostate cancer in patients with prostate-specific antigen 2-10 ng/mL at initial biopsy. Mol. Cancer 2021, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Su, H.; Li, J.; Lyon, C.; Tang, W.; Wan, M.; Hu, T.Y. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020, 3, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles 2019, 9, 1697028. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Fairey, A.S.; Paproski, R.J.; Pink, D.; Sosnowski, D.L.; Vasquez, C.; Donnelly, B.; Hyndman, M.E.; Aprikian, A.G.; Beatty, P.; Lewis, J.D. Clinical analysis of the extracellular vesicle-fingerprint score blood test to refine the prediction of clinically significant prostate cancer and avoid prostate biopsy. J. Clin. Oncol. 2020, 38, 5530. [Google Scholar] [CrossRef]
- Ferguson, S.; Weissleder, R.; Modeling, E.V. Kinetics for Use in Early Cancer Detection. Adv. Biosyst. 2020, 4, e1900305. [Google Scholar] [CrossRef]
- Park, J.; Park, J.S.; Huang, C.H.; Jo, A.; Cook, K.; Wang, R.; Lin, H.Y.; Van Deun, J.; Li, H.; Min, J.; et al. An integrated magneto-electrochemical device for the rapid profiling of tumour extracellular vesicles from blood plasma. Nat. Biomed. Eng. 2021, 5, 678–689. [Google Scholar] [CrossRef]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, H.; Li, N.; Wang, H.; Ma, L.; Chen, S.; Liu, J.; Zheng, Y.; Zhang, Y. Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression. Cell Commun. Signal. 2020, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Ren, Y.; Liu, J.; Qu, J. Promising Epigenetic Biomarkers Associated with Cancer-Associated-Fibroblasts for Progression of Kidney Renal Clear Cell Carcinoma. Front. Genet. 2021, 12, 736156. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Zhao, D.; Wang, J.; Bai, Y.; Peng, Q.; Wang, H.; Fang, R.; Chen, G.; Wang, Z.; et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: Role of remote MiR-19b-3p. Mol. Cancer 2019, 18, 86. [Google Scholar] [CrossRef]
- Song, S.; Long, M.; Yu, G.; Cheng, Y.; Yang, Q.; Liu, J.; Wang, Y.; Sheng, J.; Wang, L.; Wang, Z.; et al. Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5. J. Cell. Mol. Med. 2019, 23, 6755–6765. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, W.; Cao, X.; Li, S.; Xiong, T.; Zhang, X.; Wu, X.; Cheng, L.; Wei, Y.; Gao, B. Delivery of miR-224-5p by Exosomes from Cancer-Associated Fibroblasts Potentiates Progression of Clear Cell Renal Cell Carcinoma. Comput. Math. Methods Med. 2021, 2021, 5517747. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, X.; Chen, X.; Diao, W.; Kan, Y.; Cao, W.; Chen, W.; Jiang, B.; Qin, H.; Gao, J.; et al. Cancer-associated fibroblasts promote the stemness and progression of renal cell carcinoma via exosomal miR-181d-5p. Cell Death. Discov. 2022, 8, 439. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Guan, J.; Zheng, Z.; Hao, J.; Sheng, Z.; Wang, M.; Xu, T.; Guo, G.; Yao, L. Exosomal Circsafb2 Reshaping Tumor Environment to Promote Renal Cell Carcinoma Progression by Mediating M2 Macrophage Polarization. Front. Oncol. 2022, 12, 808888. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jayaprakash, P.; Dan, J.; Wise, P.; Jang, G.B.; Liang, C.; Chen, M.; Woodley, D.T.; Fabbri, M.; Li, W. PRAS40 Connects Microenvironmental Stress Signaling to Exosome-Mediated Secretion. Mol. Cell. Biol. 2017, 37, e00171-17. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Chen, C.; Wu, Z.; Bai, P.; Li, S.; Chen, B.; Liu, R.; Zhang, K.; Li, W.; et al. Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma. J. Cell. Biochem. 2019, 120, 1492–1502. [Google Scholar] [CrossRef]
- Jingushi, K.; Uemura, M.; Ohnishi, N.; Nakata, W.; Fujita, K.; Naito, T.; Fujii, R.; Saichi, N.; Nonomura, N.; Tsujikawa, K.; et al. Extracellular vesicles isolated from human renal cell carcinoma tissues disrupt vascular endothelial cell morphology via azurocidin. Int. J. Cancer 2018, 142, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y.; Shen, H. Role of hypoxia-induced exosomes in tumor biology. Mol. Cancer 2018, 17, 120. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Hou, Y.; Fan, L.; Li, H. Oncogenic miR-27a delivered by exosomes binds to SFRP1 and promotes angiogenesis in renal clear cell carcinoma. Mol. Ther. Nucleic Acids 2020, 24, 92–103. [Google Scholar] [CrossRef]
- Xuan, Z.; Chen, C.; Tang, W.; Ye, S.; Zheng, J.; Zhao, Y.; Shi, Z.; Zhang, L.; Sun, H.; Shao, C. TKI-Resistant Renal Cancer Secretes Low-Level Exosomal miR-549a to Induce Vascular Permeability and Angiogenesis to Promote Tumor Metastasis. Front. Cell Dev. Biol. 2021, 9, 689947. [Google Scholar]
- Qu, L.; Ding, J.; Chen, C.; Wu, Z.J.; Liu, B.; Gao, Y.; Chen, W.; Liu, F.; Sun, W.; Li, X.F.; et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell 2016, 29, 653–668. [Google Scholar] [CrossRef]
- Li, Y.L.; Wu, L.W.; Zeng, L.H.; Zhang, Z.Y.; Wang, W.; Zhang, C.; Lin, N.M. ApoC1 promotes the metastasis of clear cell renal cell carcinoma via activation of STAT3. Oncogene 2020, 39, 6203–6217. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Kawakami, K.; Fujita, Y.; Sugaya, M.; Kameyama, K.; Mizutani, K.; Deguchi, T.; Ito, M. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem. Biophys. Res. Commun. 2017, 492, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Pomatto, M.A.C.; Grange, C.; Deregibus, M.C.; Camussi, G. Extracellular vesicles in onco-nephrology. Exp. Mol. Med. 2019, 51, 1–8. [Google Scholar] [CrossRef]
- Yang, C.; Robbins, P.D. The roles of tumor-derived exosomes in cancer pathogenesis. Clin. Dev. Immunol. 2011, 2011, 842849. [Google Scholar] [CrossRef]
- Elsharkawi, F.; Elsabah, M.; Shabayek, M.; Khaled, H. Urine and Serum Exosomes as Novel Biomarkers in Detection of Bladder Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 2219–2224. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Tritta, S.; Deregibus, M.C.; Battaglia, A.; Gontero, P.; Frea, B.; Camussi, G. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer 2015, 15, 1009. [Google Scholar] [CrossRef]
- González, A.; Rebmann, V.; LeMaoult, J.; Horn, P.A.; Carosella, E.D.; Alegre, E. The immunosuppressive molecule HLA-G and its clinical implications. Crit. Rev. Clin. Lab. Sci. 2012, 49, 63–84. [Google Scholar] [CrossRef]
- Li, B.L.; Lin, A.; Zhang, X.J.; Zhang, X.; Zhang, J.G.; Wang, Q.; Zhou, W.J.; Chen, H.X.; Wang, T.J.; Yan, W.H. Characterization of HLA-G expression in renal cell carcinoma. Tissue Antigens 2009, 74, 213–221. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Q.; Zhen, Q.; Zhao, Y.; Liu, N.; Li, T.; Hao, Y.; Zhang, Y.; Luo, C.; Wu, X. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget 2017, 8, 37783–37795. [Google Scholar] [CrossRef]
- Diao, J.; Yang, X.; Song, X.; Chen, S.; He, Y.; Wang, Q.; Chen, G.; Luo, C.; Wu, X.; Zhang, Y. Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med. Oncol. 2015, 32, 453. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [PubMed]
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the Wide Spectrum of Melanoma Biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. Hematol. 2021, 158, 103208. [Google Scholar] [CrossRef]
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef]
- Huang, M.; Yang, J.; Wang, T.; Song, J.; Xia, J.; Wu, L.; Wang, W.; Wu, Q.; Zhu, Z.; Song, Y.; et al. Homogeneous, Low-volume, Efficient, and Sensitive Quantitation of Circulating Exosomal PD-L1 for Cancer Diagnosis and Immunotherapy Response Prediction. Angew. Chem. Int. Ed. Engl. 2020, 59, 4800–4805. [Google Scholar] [CrossRef]
- Pang, Y.; Shi, J.; Yang, X.; Wang, C.; Sun, Z.; Xiao, R. Personalized detection of circling exosomal PD-L1 based on Fe3O4@TiO2 isolation and SERS immunoassay. Biosens. Bioelectron. 2020, 148, 111800. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Yan, J. Exosomal PD-L1: Roles in Tumor Progression and Immunotherapy. Trends Cancer 2020, 6, 550–558. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef]
- Gyukity-Sebestyén, E.; Harmati, M.; Dobra, G.; Németh, I.B.; Mihály, J.; Zvara, Á.; Hunyadi-Gulyás, É.; Katona, R.; Nagy, I.; Horváth, P.; et al. Melanoma-Derived Exosomes Induce PD-1 Overexpression and Tumor Progression via Mesenchymal Stem Cell Oncogenic Reprogramming. Front. Immunol. 2019, 10, 2459. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, H.; Sun, W.; Chen, L.; Jin, S.; Xu, Q.; Liu, Y.; Yu, L.; Zeng, S. miR-224-5p Contained in Urinary Extracellular Vesicles Regulates PD-L1 Expression by Inhibiting Cyclin D1 in Renal Cell Carcinoma Cells. Cancers 2021, 13, 618. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.G.; Aliyuda, F.; Taiwo, D.; Adekeye, K.; Agada, G.; Sanchez, E.; Ghose, A.; Rassy, E.; Boussios, S. From Biology to Diagnosis and Treatment: The Ariadne’s Thread in Cancer of Unknown Primary. Int. J. Mol. Sci. 2023, 24, 5588. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- McAtee, C.O.; Booth, C.; Elowsky, C.; Zhao, L.; Payne, J.; Fangman, T.; Caplan, S.; Henry, M.D.; Simpson, M.A. Prostate tumor cell exosomes containing hyaluronidase Hyal1 stimulate prostate stromal cell motility by engagement of FAK-mediated integrin signaling. Matrix. Biol. 2019, 78–79, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Corrò, C.; Moch, H. Biomarker discovery for renal cancer stem cells. J. Pathol. Clin. Res. 2018, 4, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Dekel, B.; Azzarone, B.; Camussi, G. Human renal cancer stem cells. Cancer Lett. 2013, 338, 141–146. [Google Scholar] [CrossRef]
- Bussolati, B.; Bruno, S.; Grange, C.; Ferrando, U.; Camussi, G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008, 22, 3696–3705. [Google Scholar] [CrossRef]
- Zhong, Y.; Guan, K.; Guo, S.; Zhou, C.; Wang, D.; Ma, W.; Zhang, Y.; Li, C.; Zhang, S. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett. 2010, 299, 150–160. [Google Scholar] [CrossRef]
- Lindoso, R.S.; Collino, F.; Camussi, G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget 2015, 6, 7959–7969. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Singh, A.; Fedele, C.; Lu, H.; Nevalainen, M.T.; Keen, J.H.; Languino, L.R. Exosome-mediated Transfer of αvβ3 Integrin from Tumorigenic to Nontumorigenic Cells Promotes a Migratory Phenotype. Mol. Cancer Res. 2016, 14, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Lin, F.F.; Li, G.P.; Zeng, F.C. Exosomal microRNA-15a from ACHN cells aggravates clear cell renal cell carcinoma via the BTG2/PI3K/AKT axis. Kaohsiung. J. Med. Sci. 2021, 37, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Ma, J.; Zhang, J.; Chen, Y.; Liu, H.; Huang, Y.; Zheng, J.; Xu, Y.; Xue, W.; Zhai, W. Hypoxia-induced lncHILAR promotes renal cancer metastasis via ceRNA for the miR-613/206/1-1-3p/Jagged-1/Notch/CXCR4 signaling pathway. Mol. Ther. 2021, 29, 2979–2994. [Google Scholar] [CrossRef] [PubMed]
- Greef, B.; Eisen, T. Medical treatment of renal cancer: New horizons. Br. J. Cancer 2016, 115, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Shingarev, R.; Jaimes, E.A. Renal cell carcinoma: New insights and challenges for a clinician scientist. Am. J. Physiol. Renal. Physiol. 2017, 313, F145–F154. [Google Scholar] [CrossRef]
- Barata, P.C.; Rini, B.I. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer. J. Clin. 2017, 67, 507–524. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef]
- Shah, S.; Rachmat, R.; Enyioma, S.; Ghose, A.; Revythis, A.; Boussios, S. BRCA Mutations in Prostate Cancer: Assessment, Implications and Treatment Considerations. Int. J. Mol. Sci. 2021, 22, 12628. [Google Scholar] [CrossRef]
- De Palma, G.; Sallustio, F.; Curci, C.; Galleggiante, V.; Rutigliano, M.; Serino, G.; Ditonno, P.; Battaglia, M.; Schena, F.P. The Three-Gene Signature in Urinary Extracellular Vesicles from Patients with Clear Cell Renal Cell Carcinoma. J. Cancer 2016, 7, 1960–1967. [Google Scholar] [CrossRef]
- Zhang, W.; Ni, M.; Su, Y.; Wang, H.; Zhu, S.; Zhao, A.; Li, G. MicroRNAs in Serum Exosomes as Potential Biomarkers in Clear-cell Renal Cell Carcinoma. Eur. Urol. Focus 2018, 4, 412–419. [Google Scholar] [CrossRef]
- Xiao, C.T.; Lai, W.J.; Zhu, W.A.; Wang, H. MicroRNA Derived from Circulating Exosomes as Noninvasive Biomarkers for Diagnosing Renal Cell Carcinoma. Onco Targets Ther. 2020, 13, 10765–10774. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Hirata, H.; Ueno, K.; Mori, J.; Oka, S.; Shimizu, K.; Kawai, Y.; Inoue, R.; Yamamoto, Y.; Matsumoto, H.; et al. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget 2017, 8, 109877–109888. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Kawakami, K.; Fujita, Y.; Matsuda, Y.; Arai, T.; Suzui, N.; Miyazaki, T.; Koie, T.; Mizutani, K.; Ito, M. Serum Exosomal Gamma-Glutamyltransferase Activity Increased in Patients with Renal Cell Carcinoma with Advanced Clinicopathological Features. Oncology 2020, 98, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Tatarano, S.; Tamai, M.; Tsuruda, M.; Iizasa, S.; Arima, J.; Kawakami, I.; Fukumoto, W.; Kawahara, I.; Li, G.; et al. Exosomal microRNA-1 and MYO15A as a target for therapy and diagnosis in renal cell carcinoma. Biochem. Biophys. Res. Commun. 2022, 630, 71–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Ishihara, M.; Sharrow, A.C.; Flora, K.; He, Y.; Wu, L. The miRNA-21-5p Payload in Exosomes from M2 Macrophages Drives Tumor Cell Aggression via PTEN/Akt Signaling in Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3005. [Google Scholar] [CrossRef]
- Li, D.; Lin, F.; Li, G.; Zeng, F. Exosomes derived from mesenchymal stem cells curbs the progression of clear cell renal cell carcinoma through T-cell immune response. Cytotechnology 2021, 73, 593–604. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Qi, P.; Zhou, X.Y.; Du, X. Circulating long non-coding RNAs in cancer: Current status and future perspectives. Mol. Cancer 2016, 15, 39. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Zhou, F. Biological functions and clinical applications of exosomal long non-coding RNAs in cancer. J. Cell. Mol. Med. 2020, 24, 11656–11666. [Google Scholar] [CrossRef]
- Xiao, H.; Shi, J. Exosomal circular RNA_400068 promotes the development of renal cell carcinoma via the miR-210-5p/SOCS1 axis. Mol. Med. Rep. 2020, 22, 4810–4820. [Google Scholar] [CrossRef] [PubMed]
- Crentsil, V.C.; Liu, H.; Sellitti, D.F. Comparison of exosomal microRNAs secreted by 786-O clear cell renal carcinoma cells and HK-2 proximal tubule-derived cells in culture identifies microRNA-205 as a potential biomarker of clear cell renal carcinoma. Oncol. Lett. 2018, 16, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, R.; Kadomatsu, T.; Baba, M.; Hara, C.; Itoh, H.; Miyata, K.; Endo, M.; Morinaga, J.; Terada, K.; Araki, K.; et al. MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci. 2019, 110, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Butz, H.; Nofech-Mozes, R.; Ding, Q.; Khella, H.W.Z.; Szabó, P.M.; Jewett, M.; Finelli, A.; Lee, J.; Ordon, M.; Stewart, R.; et al. Exosomal MicroRNAs Are Diagnostic Biomarkers and Can Mediate Cell-Cell Communication in Renal Cell Carcinoma. Eur. Urol. Focus 2016, 2, 210–218. [Google Scholar] [CrossRef]
- Du, M.; Giridhar, K.V.; Tian, Y.; Tschannen, M.R.; Zhu, J.; Huang, C.C.; Kilari, D.; Kohli, M.; Wang, L. Plasma exosomal miRNAs-based prognosis in metastatic kidney cancer. Oncotarget 2017, 8, 63703–63714. [Google Scholar] [CrossRef]
- Raimondo, F.; Morosi, L.; Corbetta, S.; Chinello, C.; Brambilla, P.; Della Mina, P.; Villa, A.; Albo, G.; Battaglia, C.; Bosari, S.; et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. Biosyst. 2013, 9, 1220–1233. [Google Scholar] [CrossRef]
- Del Boccio, P.; Raimondo, F.; Pieragostino, D.; Morosi, L.; Cozzi, G.; Sacchetta, P.; Magni, F.; Pitto, M.; Urbani, A. A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: Application to RCC urinary exosomes. Electrophoresis 2012, 33, 689–696. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Brown, J.E.; Royle, K.L.; Gregory, W.; Ralph, C.; Maraveyas, A.; Din, O.; Eisen, T.; Nathan, P.; Powles, T.; Griffiths, R.; et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): An open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023, 24, 213–227. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.D.; Sun, M.; Zhang, X.; German, P.; Bai, S.; Ding, Z.; Tannir, N.; Wood, C.G.; Matin, S.F.; et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016, 35, 2687–2697. [Google Scholar] [CrossRef]
- Yin, J.; Sun, W.; Li, F.; Hong, J.; Li, X.; Zhou, Y.; Lu, Y.; Liu, M.; Zhang, X.; Chen, N.; et al. VARIDT 1.0: Variability of drug transporter database. Nucleic Acids Res. 2020, 48, D1042–D1050. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.; Lima, R.T.; Vasconcelos, M.H. Intercellular Transfer of Cancer Drug Resistance Traits by Extracellular Vesicles. Trends. Mol. Med. 2015, 21, 595–608. [Google Scholar]
- Duran, I.; Lambea, J.; Maroto, P.; González-Larriba, J.L.; Flores, L.; Granados-Principal, S.; Graupera, M.; Sáez, B.; Vivancos, A.; Casanovas, O. Resistance to Targeted Therapies in Renal Cancer: The Importance of Changing the Mechanism of Action. Target. Oncol. 2017, 12, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, M.; Yoshino, H.; Okamura, S.; Kuroshima, K.; Osako, Y.; Sakaguchi, T.; Sugita, S.; Tatarano, S.; Nakagawa, M.; Enokida, H. Oncogenic effects of RAB27B through exosome independent function in renal cell carcinoma including sunitinib-resistant. PLoS ONE 2020, 15, e0232545. [Google Scholar] [CrossRef]
- Yu, X.; Guo, G.; Li, X.; Zhang, C.; Huang, L.; Fang, D.; Song, Y.; Zhang, X.; Zhou, L. Retrospective Analysis of the Efficacy and Safety of Sorafenib in Chinese Patients With Metastatic Renal Cell Carcinoma and Prognostic Factors Related to Overall Survival. Medicine 2015, 94, e1361. [Google Scholar] [CrossRef]
- He, J.; He, J.; Min, L.; He, Y.; Guan, H.; Wang, J.; Peng, X. Extracellular vesicles transmitted miR-31-5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma. Int. J. Cancer 2020, 146, 1052–1063. [Google Scholar] [CrossRef]
- Datta, A.; Kim, H.; McGee, L.; Johnson, A.E.; Talwar, S.; Marugan, J.; Southall, N.; Hu, X.; Lal, M.; Mondal, D.; et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci. Rep. 2018, 8, 8161. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Kim, H.; Ahn, M.; Moustafa, A.A.; Zhou, H.; Barata, P.C.; Boulares, A.H.; Abdel-Mageed, A.B.; Krane, L.S. Combination of Tipifarnib and Sunitinib Overcomes Renal Cell Carcinoma Resistance to Tyrosine Kinase Inhibitors via Tumor-Derived Exosome and T Cell Modulation. Cancers 2022, 14, 903. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Kim, H.; Moustafa, A.A.; Datta, A.; Barata, P.C.; Boulares, A.H.; Abdel-Mageed, A.B.; Krane, L.S. Repurposing ketoconazole as an exosome directed adjunct to sunitinib in treating renal cell carcinoma. Sci. Rep. 2021, 11, 10200. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.H.; Baek, M.C. Dissecting exosome inhibitors: Therapeutic insights into small-molecule chemicals against cancer. Exp. Mol. Med. 2022, 54, 1833–1843. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, L.; Mi, Q.S.; Jiang, A. DC-Based Vaccines for Cancer Immunotherapy. Vaccines 2020, 8, 706. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Zöller, M.; Hadjati, J.; Ghods, R.; Ranaei Pirmardan, E.; Kiani, J.; Eini, L.; Bozorgmehr, M.; Madjd, Z. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. Front. Immunol. 2023, 14, 1212101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.H.; Luo, C.L.; Zhang, J.M.; He, B.C.; Chen, G. Interleukin-12-anchored exosomes increase cytotoxicity of T lymphocytes by reversing the JAK/STAT pathway impaired by tumor-derived exosomes. Int. J. Mol. Med. 2010, 25, 695–700. [Google Scholar]
- Zhang, Y.; Luo, C.L.; He, B.C.; Zhang, J.M.; Cheng, G.; Wu, X.H. Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: A novel vaccine for renal cell carcinoma. Int. J. Oncol. 2010, 36, 133–140. [Google Scholar]
- Gu, X.; Erb, U.; Büchler, M.W.; Zöller, M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int. J. Cancer 2015, 136, E74–E84. [Google Scholar] [CrossRef] [PubMed]
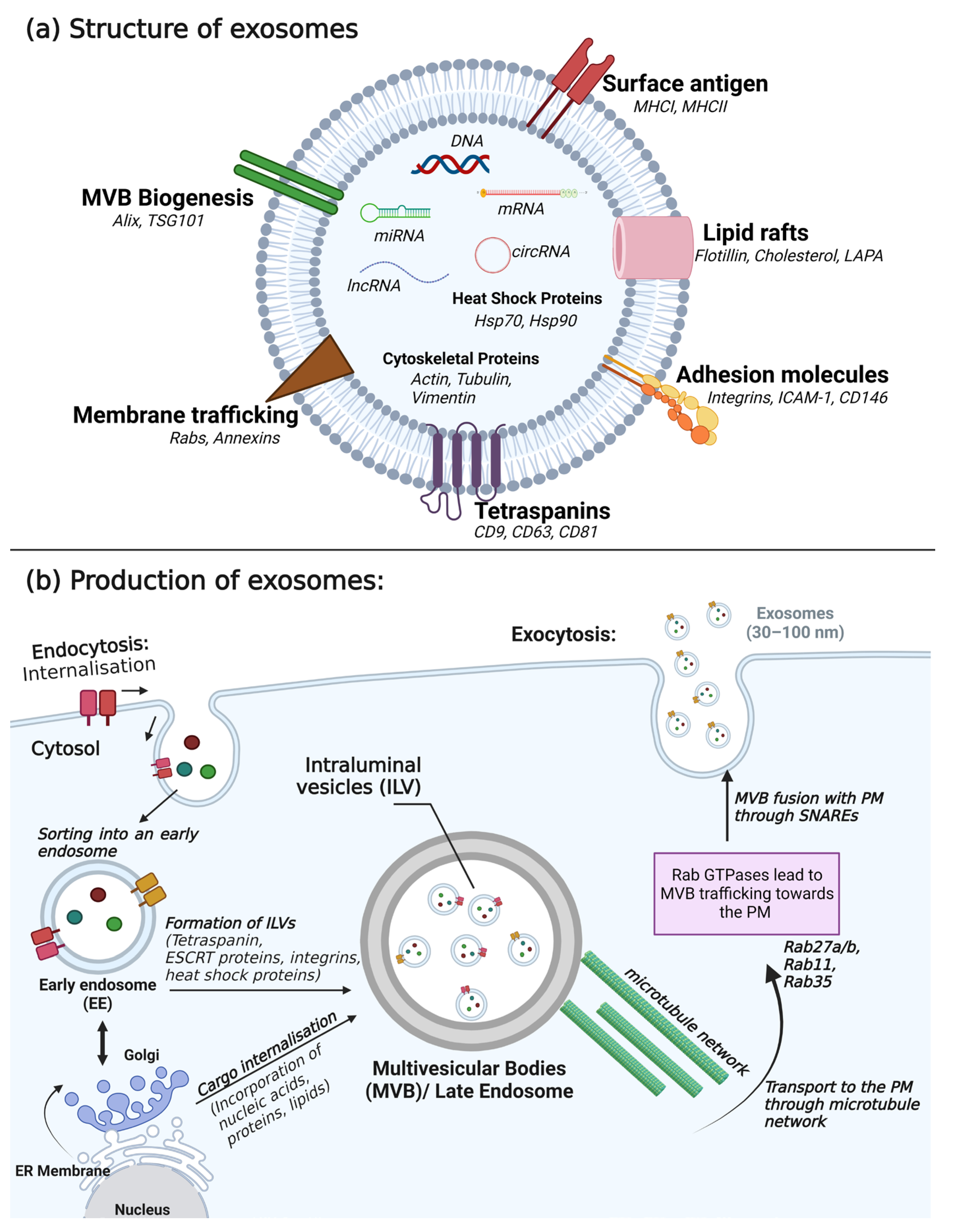

| Size | 30–100 nm | |
| Formation and release | Formed intracellularly within multivesicular bodies | |
| Isolation and detection | Ultracentrifugation, electron microscopy, western blotting, mass spectrometry, and nanoparticle tracking analysis | |
| Proteins | Type | Cargo |
| Tetraspanin | CD9, CD63, CD81 | |
| Heat shock protein | Hsp70, Hsp90 | |
| Membrane transport and fusion proteins | Rab, Annexins (I, II, IV, V, VI) | |
| Antigen presentation | MCH I, MCH II | |
| Adhesion molecules | Integrins, ICAM-1, CD146 | |
| Lipid raft | LAPA, Flotillin-1, Cholesterol | |
| Cytoskeletal proteins | ||
| Lipids | Cholesterol, ceramide, phosphatidylserine, phosphatidylinositol, phosphatidylcholine, sphingomyelin, ganglioside | |
| Nucleic acids | DNA, mRNA, miRNA, lncRNA, circRNA | |
| Type | EV Source | EV Cargoes | Analysis Method | Cohorts | Clinical Significance | Ref. |
|---|---|---|---|---|---|---|
| mRNA | Supernatants | VEGF, FGF2, ANGPT1, EFNA3, MMP2, and MMP9 | Microarray, qRT-PCR | Cancer stem cells | mRNAs implicated in tumor progression and metastasis through molecular characterization of VEGF, FGF2, ANGPT1, EFNA3, MMP2, and MMP9. | [76] |
| Urine | GSTA1, CEBPA, and PCBD1 genes | Microarray, qRT-PCR | 46 RCC patients (33 with ccRCC), 22 HS | Significantly lower in ccRCC patients compared with HS. Increased to normal level 1 month after nephrectomy. | [130] | |
| miRNA | Supernatants | miR-200c, miR-650 | Microarray, qRT-PCR | Cancer stem cells | miR-200c and miR-650 significantly upregulated in CD105+ microvesicles. | [76] |
| miR-100, miR-296 | miR-100 and miR-296 significantly downregulated. | |||||
| miR-29a, miR-650, miR-151 | miR-29a, miR-650, and miR-151 are associated with tumor invasion and metastasis. | |||||
| miR-549a | qRT-PCR | 786-O cell, 786-O-SR cell | Lower in TKI-resistant cells and exosomes. | [89] | ||
| miR-205 | qRT-PCR | HK-2 cell, 786-O cell | Significant differences in concentration between the two cell lines. | [142] | ||
| Urine/Supernatants | miR-204-5p | qRT-PCR | Mouse and human tRCC cell lines | Significantly increased in primary RCC cell lines established from transgenic mouse tumors and tumor tissue from 2 Xp11 tRCC patients. | [143] | |
| Urine | miR-126-3p | Microarray, qRT-PCR | 81 ccRCC patients, 33 HS | Differentiated ccRCC patients from HS | [144] | |
| miR-126-3p combined miR-449a | ||||||
| miR-126-3p combined miR-34b-5p | Differentiated patients with ccRCC and small renal masses (pT1a, ≤4 cm) from HS. | |||||
| miR-126-3p combined miR-486-5p | 24 benign renal tumor patients, 33 HS | Differentiated benign patients from HS. | ||||
| miR-30c-5p | RNA sequencing, qRT-PCR | 70 early-stage ccRCC patients, 30 HS | Significantly lower in early-stage ccRCC patients compared with HS. | [78] | ||
| Plasma | miR-92a-1-5p, miR-149-3p, miR-424-3p | RNA sequencing, qRT-PCR | 5 RCC, 5 healthy controls. 22 RCC, 16 healthy controls | Significantly downregulated in the plasma exosomes of RCC. | [132] | |
| miR-15a | Microarray, qRT-PCR | ACHN cell | Upregulated in ccRCC cells. BTG2 gene is negatively correlated with miR-15a expression. | [123] | ||
| miR-let-7i-5p, miR-26a-1-3p, miR-615-3p | RNA-sequencing, qRT-PCR | A cohort of 44 metastatic RCC patients for screening and 65 validation controls | Low levels correlated with poor OS of metastatic RCC patients. | [145] | ||
| Serum | miR-1233, miR-210 | qRT-PCR | 82 ccRCC patients, 80 HS | Significantly higher in ccRCC patients than in HS | [131] | |
| miR-210 | Microarray, qRT-PCR | 45 pre-operative and 35 post-operative ccRCC patients, 30 HS | Significantly higher in ccRCC patients compared with HS, Significantly higher in pre-operative than post-operative samples. | [84] | ||
| miR-224 | qRT-PCR | 108 ccRCC patients | High levels are correlated with shorter PFS, CSS, and OS of ccRCC patients | [133] | ||
| Protein | Urine | MMP9, CP, PODXL, CAIX, and DKK4 | LC-MS/MS, western blotting | 29 RCC, 23 healthy controls | MMP9, CP, PODXL, CAIX, and DKK4 are higher in patients with RCC compared with HS. | [146] |
| CD10, EMMPRIN, DPEP1, syntenin 1, and AQP1 | CD10, EMMPRIN, DPEP1, syntenin 1, and AQP1 are higher in HS than in patients with RCC | |||||
| Serum | CD103 | Flow cytometry | 76 metastatic and 133 non-metastatic ccRCC patients | Higher ratio of CD103+ EVs to total EVs in samples from metastatic patients compared with samples from non-metastatic patients. | [77] | |
| Azurocidin | LC-MS/MS | 19 ccRCC patients, 10 HS | Significantly higher in ccRCC patients than in HS | [85] | ||
| Tissue | Azurocidin | LC-MS/MS | 20 paired tumors and adjacent normal tissues from ccRCC patients | Significantly higher in ccRCC patients than in HS | [85] | |
| Supernatants | RAB27B | ExoELISA-ULTRA CD63 kit | A498 cell | Oncogenic role in RCC and sunitinib resistance. | [110] | |
| IncRNA | Plasma | Circulating lncARSR | qRT-PCR | 71 advanced ccRCC patients, 32 HS | Poor sunitinib response, Sunitinib resistance via competitively binding miR-34/miR-449. | [90] |
| circRNA | Plasma | circ_400068 | CircRNA microarray | 28 RCC | Upregulated in RCC plasma exosomes, tissue samples, and cells. | [141] |
| Lipid | Urine | LysoPE | microLC-Q-TOF-MS | 8 ccRCC patients, 8 HS | 48 differential lipidome expression (22 upregulated and 26 downregulated) in ccRCC. | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussios, S.; Devo, P.; Goodall, I.C.A.; Sirlantzis, K.; Ghose, A.; Shinde, S.D.; Papadopoulos, V.; Sanchez, E.; Rassy, E.; Ovsepian, S.V. Exosomes in the Diagnosis and Treatment of Renal Cell Cancer. Int. J. Mol. Sci. 2023, 24, 14356. https://doi.org/10.3390/ijms241814356
Boussios S, Devo P, Goodall ICA, Sirlantzis K, Ghose A, Shinde SD, Papadopoulos V, Sanchez E, Rassy E, Ovsepian SV. Exosomes in the Diagnosis and Treatment of Renal Cell Cancer. International Journal of Molecular Sciences. 2023; 24(18):14356. https://doi.org/10.3390/ijms241814356
Chicago/Turabian StyleBoussios, Stergios, Perry Devo, Iain C. A. Goodall, Konstantinos Sirlantzis, Aruni Ghose, Sayali D. Shinde, Vasileios Papadopoulos, Elisabet Sanchez, Elie Rassy, and Saak V. Ovsepian. 2023. "Exosomes in the Diagnosis and Treatment of Renal Cell Cancer" International Journal of Molecular Sciences 24, no. 18: 14356. https://doi.org/10.3390/ijms241814356
APA StyleBoussios, S., Devo, P., Goodall, I. C. A., Sirlantzis, K., Ghose, A., Shinde, S. D., Papadopoulos, V., Sanchez, E., Rassy, E., & Ovsepian, S. V. (2023). Exosomes in the Diagnosis and Treatment of Renal Cell Cancer. International Journal of Molecular Sciences, 24(18), 14356. https://doi.org/10.3390/ijms241814356








